Abstract
Purpose
The primary aim of this proposed study is to evaluate brain reorganization patterns in infants with perinatal stroke between 3 and 5 months of age using transcranial magnetic stimulation and magnetic resonance imaging, with the addition of the General Movements Assessment. A secondary aim is to demonstrate feasibility and safety of infant-appropriate brain assessment protocols.
Methods
Ten infants with perinatal stroke will be enrolled. In this exploratory study, infants will first receive magnetic resonance imaging scanning during natural sleep to examine their corticospinal tract integrity. Infants will then receive transcranial magnetic stimulation to assess their corticomotor excitability. A General Movements Assessment video of at least 5 minutes will also be recorded.
Discussion
Study results will enhance our understanding of brain reorganization in infants with perinatal stroke. We expect these results will also guide the development of early interventions designed to mitigate maladaptive neuroplastic changes and improve long-term motor outcomes.
Keywords: brain reorganization, cerebral palsy, general movements assessment, infant, magnetic resonance imaging, perinatal stroke, transcranial magnetic stimulation
INTRODUCTION
Perinatal stroke refers to ischemic stroke that occurs between the 20th week of gestation and 28 days of postnatal age.1 The incidence of perinatal stroke can be as high as 1 in 2300 newborns.1 Infants with perinatal stroke are at high risk of developing hemiparetic cerebral palsy (CP) with motor impairment on 1 side of the body,2 and the upper extremity being more affected than the lower extremity. Although estimates vary, approximately 50% of infants with perinatal stroke will eventually be diagnosed with CP2–4 Most often the diagnosis of CP is not made during early infancy5,6; however, the few months after birth may be a “window of opportunity” for infants with perinatal stroke to receive therapy, as this is both a critical period of development and a period of heightened neuroplasticity.7,8 There is limited information on how different designs of movement training for infants with perinatal stroke can potentially mitigate maladaptive neuroplastic changes of the brain, such as withdrawal of corticospinal pathways, projecting from the lesioned hemisphere to the contralesional limbs. To develop a rehabilitation intervention to prevent motor impairments or to minimize impairment, within this critical time window, we must first develop efficient and reliable assessments capable of identifying cortical reorganization.
The corticospinal tract (CST) represents the primary conduit for neural signals that control voluntary movements, primarily the trunk and limbs. Some functional CST projections are established at the prenatal stage of development.9 Postnatally in infants with typical development, concurrent growth of some CST axons, and elimination of others, takes place to refine function. Myelination is ongoing during this refinement process.10,11 Ipsilateral and contralateral axonal projections present at birth begin to progressively withdraw in response to activity-dependent competition, with the contralateral projections becoming more dominant.10,12 This developmental process has been revealed using transcranial magnetic stimulation (TMS) in a study by Eyre et al,13 who found evidence of progressively weakening ipsilateral motor responses in infants by 6 months of age. When a unilateral perinatal stroke occurs, the lesioned hemisphere may cease to “dominate” by losing its ability to develop functional crossed CST connections whereas the nonlesioned hemisphere may gain control of bilateral movement via both the crossed and uncrossed corticospinal projections. This maladaptation of the developing brain can have a negative effect on the development and quality of hand function14, 15 (eg, the development of undesired mirror movements). However, some infants with perinatal stroke do not show significant motor impairments nor can they be identified for early intervention needs in the first few months of life. A better understanding of the relationship between early brain reorganization and abnormal behavior after perinatal stroke is therefore warranted to improve prognosis and to provide targeted interventions for this population.
TMS is a painless and nonsurgical form of brain stimulation that uses the principle of electromagnetic induction applied over the scalp to excite cortical tissue. The use of TMS to examine and modulate corticomotor excitability in children with hemiparetic CP has been conducted without serious adverse events, such as seizure.11, 16–18 Some studies reported mild adverse events, such as dizziness and fatigue.17 Corticomotor excitability assessment using TMS has been conducted on infant populations and has provided information regarding early brain development. For example, Eyre at al9 performed TMS assessments in 223 preterm and term neonates and compared infant corticomotor excitability and physiological responses with those in adults. They found that infants had slower nerve conductivity. Santiago-Rodriguez et al19 recruited both infants with typical development and infants between 1.0 and 5.8 months of corrected age with periventricular leukomalacia (PVL). Using TMS, the authors found that infants with PVL had slower central and peripheral nerve conduction velocities compared with infants with typical development.19 Importantly, Eyre et al13 conducted a 2-year longitudinal analysis with infants with typical development and infants with unilateral and bilateral brain lesions. Their results supported that infants with unilateral brain injury gradually lose contralateral CST projections from the lesioned hemisphere and demonstrate hypertrophy of ipsilateral corticospinal projections from the nonlesioned hemisphere. These studies demonstrate the value of using TMS during early infancy and the effect of brain lesions on CST development.
Unlike children and adults, there is added complexity in infants for assessing tolerance to TMS assessment procedures. Obtaining more information about the responses to TMS in infants and details of TMS testing protocols and methodologies will guide the inclusion of TMS assessments for this unique population. Moreover, this information will enhance the future reproducibility of TMS assessment in infants. A detailed infant TMS assessment protocol in this study is described for the investigation of corticomotor excitability and brain reorganization in infants at 3 to 5 months of age following perinatal stroke.
Magnetic resonance imaging (MRI) assists with the early diagnosis of brain lesions in newborns or young infants and can provide details regarding structural changes or connectivity of the brain. For example, Arichi et al20 found that infants with PVL caused by hemorrhagic parenchymal infarct had suboptimal myelination in the posterior limb of the internal capsule compared with the nonlesioned hemisphere of infants at term equivalent age. With diffusion tensor imaging (DTI) analysis, using fractional anisotropy (FA) and radial diffusivity, they identified an asymmetry of CSTs between lesioned and nonlesioned hemispheres. MRI can also be used to predict future motor outcomes in infants with white matter injuries, brain hemorrhages, and perinatal stroke.21–24 In a retrospective study, Roze et al21 analyzed structural MRI and DTI data from 20 infants with focal brain lesions and demonstrated sensitivities of 73% and 91%, respectively, to predict future abnormal motor outcomes. Van der Aa et al22 also found that 3-month-old infants with perinatal arterial ischemic stroke, who later developed unilateral motor deficits, had asymmetric CSTs between lesioned and nonlesioned hemispheres. Combining neuroimaging techniques, specifically MRI/DTI, with TMS to assess corticomotor excitability and associated CST integrity may add valuable information on brain reorganization after perinatal stroke.
The General Movements Assessment (GMA) is a method to predict motor development for infants,25 and especially a future diagnosis of CP. General movements are spontaneous movements that can be observed in fetuses and infants. According to the classification of Prechtl’s Assessment of General Movements, different types of general movements can be observed during the 2 periods of writhing (appearing from preterm age to 6–9 weeks of postterm age) and fidgety movements, which are best observed between 9 and 20 weeks of postterm age. This is the age range, which includes the range chosen for the present study.25 Fidgety movements have small amplitudes and moderate speed observed from the whole body including the neck, trunk, and proximal and distal limb segments. These movements have variable acceleration and directions when observed in an awake infant. There are 3 categories of fidgety movement assessment: normal, abnormal, and absent. Importantly, if fidgety movements are absent, it is highly probable they will eventually be diagnosed with CP, including hemiparetic CP.25–27 Spittle et al28 found that, when general movements were assessed at 3 months of corrected age in 85 preterm infants, the outcomes were correlated with later diagnoses of CP, with 100% sensitivity, 81.3% specificity, and 82.4% accuracy. Furthermore, in a sample of 903 infants, Romeo et al29 also had 98% sensitivity and 94% specificity for predicting CP.
The main purpose of this study is therefore to use TMS and MRI to assess brain reorganization in infants with perinatal stroke. With the addition of the GMA, we will begin our study of the association of TMS and MRI measures with GMA measures to guide the design of future trials.
METHODS
Ethical Consideration
The Food and Drug Administration determined that an Investigational Device Exemption is not required for this proposed study. The University of Minnesota Institutional Review Board has approved this study Approvals by the Human Research Protection Program of University of Minnesota Clinical and Translational Science Institute through its Scientific Review Committee and University of Minnesota Center for Magnetic Resonance Research, as well as the recruitment support letter from Fairview Research Committees of Fairview Health Services, were obtained. This study is registered on clinicaltrials.gov (NCT02743728). Caregivers will be provided oral and written information about the study before meeting with investigators during which their questions will be answered. For example, information will be provided about possible infant responses during data collection. They will then be invited to provide their written consent.
Study Design
This is an exploratory cross-sectional study to collect evidence of brain reorganization patterns in infants with perinatal stroke between 3 and 5 months of corrected age and to demonstrate the feasibility and safety of noninvasive infant-appropriate assessment protocols.
Primary Objective
The primary objective is to compare the corticomotor excitability and CST integrity of the lesioned with the nonlesioned hemispheres in infants with perinatal stroke. Furthermore, different reorganization patterns between infants who are at higher or lower risk for developing hemiparetic CP will be considered. The hypotheses to be tested are:
The lesioned hemisphere has lower corticomotor excitability assessed by TMS than the nonlesioned hemisphere.
The integrity of the CST projection from the lesioned hemisphere assessed by FA is lower compared with the CST projection from the nonlesioned hemisphere.
Compared with the nonlesioned hemisphere, the relatively lower corticomotor excitability of the lesioned hemisphere is associated with lower FA values.
Infants with perinatal stroke who have absent fidgety movements have greater between-hemisphere asymmetries in corticomotor excitability and corticospinal integrity than infants with normal fidgety movements.
Secondary Objective
The secondary objective of this proposed study is to evaluate the feasibility and safety of the infant-appropriate TMS and MRI assessment protocols. We predict that seizures or other serious adverse events related to TMS or MRI will not occur in this study.
Enrollment and Recruitment
Infant participants will be recruited in collaboration with the neonatal intensive care unit and neonatal intensive care unit follow-up clinic at the University of Minnesota Masonic Childrens Hospital. The clinical research coordinator will discuss the study with caregivers, whose infants meet the research criteria. With permission from caregivers, 1 researcher from this study will contact the family to explain study details, answer questions, and obtain informed consent if the caregiver agrees for the infant to participate in this study.
Participants and Criteria
Ten infants born at term and 10 infants born preterm will be recruited between 3 and 5 months (12–20 weeks) of age, corrected for prematurity, who have a radiologically confirmed unilateral perinatal stroke. The age range proposed in this study was determined by considering the critical changes in the central nervous system and associated behavioral changes during this period including (1) differentiation of ipsilateral and contralateral CSTs that are detectable using TMS during this age range; (2) the onset of purposeful reaching behaviors combined with increased upper extremity movements that typically occur between 3 and 5 months of age30; and (3) general movements that occur during this age range that have been shown to have good predictability for CP in high-risk infants. Infants who have a history of neonatal seizure whose symptoms are well controlled will be eligible for this study. Infants with genetic disorders, metabolic disorders, neoplasm, disorders of cellular migration and proliferation, traumatic brain injury, MRI-incompatible indwelling medical devices, prior surgeries that constrain spontaneous movements, uncontrolled seizures, or other neurologic disorders unrelated to stroke will be excluded.
General Procedures
This study includes 2 visits. During visit 1, we will perform the MRI scanning at the Center for Magnetic Resonance Research. Within 7 days after the scanning, to avoid developmental changes of the brain, infant participants and their caregivers will attend the University of Minnesota Clinical and Translational Science Institute for the GMA and TMS assessments during visit 2.
MRI Assessment
During the first visit, the MRI scanning protocol will be conducted during natural sleep, without sedation, according to established protocols.31 One week before the day of visit, caregivers will receive a compact disc or digital file with a recording of the operating sound of the MRI scanner. The caregivers will be asked to play these sounds while the infants sleep to familiarize them with these noises. During the scanning day, the caregiver will feed the infant and then put the infant to sleep in a separate staging room from the MRI scanner, or rock the baby to sleep in the scanner suite. A memory foam mattress for infants will be used to support them firmly and comfortably on the scanner table. Silicone infant earplugs and customized MRI-compatible headphones will be used for ear protection and to diminish noise during scanning and to promote continued sleep. One investigator will stay with the infant throughout the scanning session to monitor the infants responses, with the caregiver observing the scanning through the control room window. Infant participants will be scanned on a Siemens Prisma 3T system (Waukesha, Wisconsin). The scan protocol will include structural MRI and diffusion-weighted MRI protocols. CST integrity will be quantified using established methods.32 The complete MRI data set will be obtained in less than 38 minutes.
TMS Assessment
TMS assessment will be performed during the second visit. Before the stimulation, infants will wear the same type of earplugs used during MRI scanning to protect their hearing from the sound of the stimulator discharging. During the assessment, vital signs, arousal state, and distress responses will be continuously observed and recorded by 1 of the investigators. We have incorporated previously published infant and toddler TMS protocols to develop our infant-appropriate protocol.13, 19, 33 Under the guidance of a frameless stereotactic neuronavigation system (Brainsight, Rogue Research, Montreal, Quebec, Canada), a TMS coil will be used to deliver the TMS pulses. Each infants MRI (T1-weighted structural MPRAGE) will be projected onto the neuronavigation system to assist with localization of the motor cortex, which includes targeting the “hand-knob” location of motor cortex as the potential hotspot. By stimulating the handknob or other excitable locations that control upper extremity muscle activity, single-pulse TMS (Bistim2, Magstim, UK) will be used to assess the responses of both the ipsilateral and contralateral projections from each hemisphere via electromyography (EMG) recording. The EMG signal will be recorded using surface EMG electrodes (EL254, BIOPAC System Inc, Aero Camino Goleta, California) attached over wrist flexors bilaterally while the caregiver interacts with the infants to facilitate their muscle contraction (Figure 1). We have incorporated a previously published EMG intensity threshold-triggered TMS technique in our protocol.34,35 Specifically, the delivery of TMS pulses is triggered when the EMG activity is higher than a predefined threshold. This threshold will be higher than the resting EMG activity observed from each infant. However, the threshold can vary due to electrode attachment, participants skin conductivity, skin preparation quality, and electrical environment noise level. This method in our protocol recognizes that resting motor threshold at this age is too high to determine and infants do not consistently contract their muscles during a task such as TMS assessment. Based on infant EMG observations from our pilot testing, we captured optimal resting EMG peak amplitude activity between ±10 and ±20 μV in the wrist flexor and biceps muscles, but we observed cardiac artifact in the biceps EMG recordings bilaterally (Figure 2). As a result, we chose the wrist flexor for TMS assessment in this study.
Fig. 1.
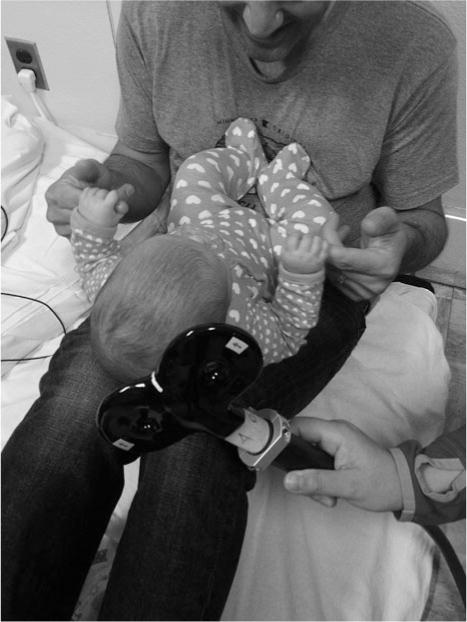
Caregivers will be asked to interact with infants by showing a toy or holding the infant’s hands to facilitate wrist flexor contraction and to trigger transcranial magnetic stimulation pulses. (Demonstration picture)
Fig. 2.
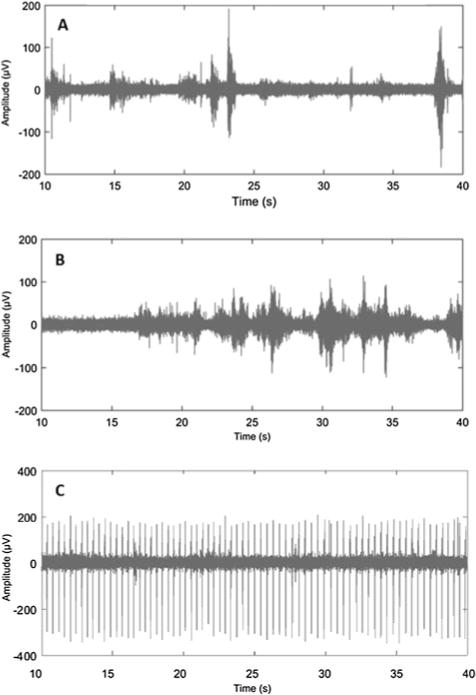
EMG activity when the infant’s arm was slightly constrained. (A) Biceps resting EMG activity between ±10 and ±20 μV; (B) wrist flexor with resting EMG activity between ±10 and ±20 μV; and (C) biceps EMG activity with cardiac artifact when bilateral signals were being collected simultaneously. EMG indicates electromyography.
The General Movements Assessment
At the beginning of the second visit before TMS assessment, infants will be videotaped for at least 5 minutes in a supine position wearing a diaper or onesie to allow for the clear observation of trunk and limb spontaneous movements. This video will be recorded when infants are in an awake but calm state. The video will be reviewed to evaluate fidgety movements by a certificated investigator who will be blinded to participants’ medical information and not involved in TMS and MRI assessments. Fidgety movements can be classified as normal, abnormal, or absent. Because the absence of fidgety movements is highly predictive of CP, TMS and MRI data from infants who do and do not have fidgety movements will be compared. TMS and MRI data from infants with abnormal fidgety movements will be assessed separately because abnormal movements are not predictive of CP, and prediction per se is not an outcome of this preliminary study.
Safety Monitoring Plan and Stopping Rules
We have developed an infant-based TMS and MRI risk mitigation plan. The plan takes into account the potential risks, monitoring, and risk mitigation strategies that are described in the “MRI Assessment” and “TMS Assessment” sections and listed in Table 1. We will incorporate a follow-up phone call 24 hours and 1 month after participation to continue the assessment of safety and to note any changes in medical status. Follow-up status for each infant will also be reviewed by the medical monitor.
TABLE 1.
Potential Risk Monitor and Mitigation Plan During TMS and MRI Assessments
| Assessments | Anticipated Risks | Risk Monitor and Mitigation |
|---|---|---|
| MRI | Dislodging of indwelling metals and disruption of medical devices | Exclude if metal/medical devices are incompatible |
| MRI | Metal projectiles inadvertently presented during MRI | Conduct on-site screen for removal of projectiles |
| MRI | Temporary mild hearing loss because of noise level of equipment | Infant participants will wear earplugs (~20 dB reduction) and headphones (~35 dB reduction) during MRI to protect against excessive noise |
| TMS | Seizure | EMG responses, continuous visual monitoring by assigned investigator |
| TMS | Stimulation over a tumor that may alter metabolic activity | Screen appropriately for exclusion criteria of neoplasm |
| TMS | Threshold altering pharmacologic agent | Physician review of each medical record for determination of appropriateness for study inclusion |
| TMS | Pain or discomforts | Age-appropriate vital signs (HR, RR, and BP), skin integrity, and distress responses will be monitored for determination of pausing or stopping |
| TMS | Fatigue and sleepiness | Infant arousal state will be monitored and recorded each minute during the TMS test |
| TMS | Temporary mild hearing loss because of noise level of equipment | Silicon infant-appropriate ear plugs will be inserted before TMS and continuously checked for placement |
| TMS | Temporary numbness or twitching of the face and difficulty with movement or motor control impairment | The researcher will observe and interact with the infant to observe/document any atypical facial or body movement throughout the TMS testing. |
Abbreviations: BP, blood pressure; EMG, electromyography; HR, heart rate; MRI, magnetic resonance imaging; RR, respiratory rate; TMS, transcranial magnetic stimulation.
Statistical Analysis
The corticomotor excitability of lesioned and nonlesioned hemispheres, along with CST integrity, through FA values, will be summarized and compared with paired t tests. The association of the cortical excitability with FA will be evaluated using generalized estimating equations (to account for correlation of paired measurements from each subject: excitability and FA from each hemisphere). Transformations and nonidentity link functions will be considered in exploratory analyses to evaluate nonlinear relationships. The association of movement quality (normal fidgety vs absent fidgety) with cortical excitability and relative tract integrity between hemispheres (ratio of FA values) will be summarized with odds ratios from logistic regression. Safety outcomes will describe adverse events, reporting the number and percentage along with seriousness, severity, frequency (within a subject), and relatedness.
DISCUSSION
The methodologies of TMS were established based on previous literature showing that TMS can detect changes in CST development after 3 months of age.13 The MRI scanning protocols during natural sleep were introduced by our study collaborator, whose team has successfully completed approximately 100 infant MRI sessions in a longitudinal study of 3- to 24-month-olds with typical development. Because the first few months after birth are a critical time for brain and motor development, we plan to assess measures associated with brain reorganization after perinatal stroke in infants between 3 and 5 months of corrected age.
This study will recruit infants who have perinatal stroke confirmed shortly after birth. This early diagnosis is usually made by brain imaging after an infant shows early neurological signs, such as a neonatal seizure, or an atypical birth history. We will not be able to recruit infants for our study if they have an undetected perinatal stroke and develop neurological impairments some months later. However, as our research pathway progresses, we aim to increase the sensitivity and selectivity of the detection of latent CP for infants with perinatal stroke, to a stage where infants with a questionable diagnosis could be reliably assessed using the combination of the proposed measures.
CONCLUSION
The significance of this study protocol is to progress the use of MRI and TMS, along with the GMA, to assess brain reorganization patterns in infant stroke that can lead to effective early therapeutic interventions. Upon completion of this study, we aim to conduct longitudinal studies to evaluate predictive data and develop interventions such as movement therapy, neuromodulation, or a combination of both, to mitigate maladaptive brain reorganization and improve developmental outcomes after perinatal stroke. The results of the present study will help establish a framework capable of evaluating the timing, type, and dosage of interventions to support brain development and enhance motor outcomes in this infant population. For the purpose of timely and accurate prediction of prognosis in highrisk infants, future studies will need to incorporate long-term follow-up to confirm predictors. Our findings will be integrated into future studies, identifying responders and nonresponders to treatments and to improve the effectiveness of clinical interventions. This new direction, combining neuroexcitability and neuroimaging, has the potential to advance current developmental support programs by reducing the effect of disability and improve the quality of life in children with CP caused by perinatal stroke and improve their quality of life.
Acknowledgments
Grant Support: This study was supported by grants from and Academic Health Center Seed Grant (2015) from the University of Minnesota and American Academy for Cerebral Palsy and Developmental Medicine (AACPDM) and the Cerebral Palsy Alliance, MnDRIVE Brain Conditions Fellowship.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Drs Chao-Ying Chen, Department of Physical Medicine and Rehabilitation, University of Minnesota, Minneapolis.
Dr. Michael Georgieff, Department of Pediatrics, University of Minnesota, Minneapolis.
Dr. Jed Elison, Institute of Child Development, University of Minnesota, Minneapolis.
Dr. Mo Chen, Institute for Engineering in Medicine, University of Minnesota, Minneapolis.
Dr. James Stinear, Department of Exercise Sciences, University of Auckland, New Zealand.
Dr. Bryon Mueller, Department of Psychiatry, University of Minnesota, Minneapolis.
Dr. Raghavendra Rao, Department of Pediatrics, University of Minnesota, Minneapolis.
Dr. Kyle Rudser, Clinical and Translational Science Institute, University of Minneapolis, Minnesota.
Dr. Bernadette Gillick, Department of Physical Medicine and Rehabilitation, University of Minnesota, Minneapolis.
References
- 1.Raju TN, Nelson KB, Ferriero D, et al. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007;120(3):609–616. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 2.Lehman LL, Rivkin MJ. Perinatal arterial ischemic stroke: presentation, risk factors, evaluation, and outcome. Pediatr Neurol. 2014;51(6):760–768. doi: 10.1016/j.pediatrneurol.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Chabrier S, Husson B, Dinomais M, et al. New insights (and new interrogations) in perinatal arterial ischemic stroke. Thromb Res. 2011;127(1):13–22. doi: 10.1016/j.thromres.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Golomb MR, Garg BP, Saha C, et al. Cerebral palsy after perinatal arterial ischemic stroke. J Child Neurol. 2008;23(3):279–286. doi: 10.1177/0883073807309246. [DOI] [PubMed] [Google Scholar]
- 5.Herskind A, Greisen G, Nielsen JB. Early identification and intervention in cerebral palsy. Dev Med Child Neurol. 2015;57(1):29–36. doi: 10.1111/dmcn.12531. [DOI] [PubMed] [Google Scholar]
- 6.Morgan C, Novak I, Dale RC, et al. Optimising motor learning in infants at high risk of cerebral palsy: a pilot study. BMC Pediatr. 2015;15(4):30. doi: 10.1186/s12887-015-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015;27(2):411–423. doi: 10.1017/S0954579415000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wachs TD, Georgieff M, Cusick S, et al. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci. 2014;1308(1):89–106. doi: 10.1111/nyas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyre JA, Miller S, Clowry GJ, et al. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123(pt 1):51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- 10.Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11(2):161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 11.Kirton A, Andersen J, Herrero M, et al. Brain stimulation and constraint for perinatal stroke hemiparesis: The PLASTIC CHAMPS Trial. Neurology. 2016;86(18):1659–1667. doi: 10.1212/WNL.0000000000002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyre JA, Taylor JP, Villagra F, et al. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57(9):1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 13.Eyre JA, Smith M, Dabydeen L, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62(5):493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- 14.Holmefur M, Krumlinde-Sundholm L, Bergstrom J, et al. Longitudinal development of hand function in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52(4):352–357. doi: 10.1111/j.1469-8749.2009.03364.x. [DOI] [PubMed] [Google Scholar]
- 15.Staudt M, Gerloff C, Grodd W, et al. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56(6):854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- 16.Gillick BT, Feyma T, Menk J, et al. Safety and feasibility of transcranial direct current stimulation in pediatric hemiparesis: randomized controlled preliminary study. Phys Ther. 2015;95(3):337–349. doi: 10.2522/ptj.20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillick BT, Krach LE, Feyma T, et al. Safety of Primed Repetitive Transcranial Magnetic Stimulation and Modified Constraint-Induced Movement Therapy in a Randomized Controlled Trial in Pediatric Hemiparesis. Arch Phys Med Rehabil. 2015;96(4 suppl):S104–S113. doi: 10.1016/j.apmr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillick BT, Krach LE, Feyma T, et al. Primed low-frequency repetitive transcranial magnetic stimulation and constraint-induced movement therapy in pediatric hemiparesis: a randomized controlled trial. Dev Med Child Neurol. 2014;56(1):44–52. doi: 10.1111/dmcn.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santiago-Rodriguez E, Leon-Castillo C, Harmony T, et al. Motor potentials by magnetic stimulation in periventricular leukomalacia. Pediatr Neurol. 2009;40(4):282–288. doi: 10.1016/j.pediatrneurol.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Arichi T, Counsell SJ, Allievi AG, et al. The effects of hemorrhagic parenchymal infarction on the establishment of sensori-motor structural and functional connectivity in early infancy. Neuroradiology. 2014;56(11):985–994. doi: 10.1007/s00234-014-1412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roze E, Harris PA, Ball G, et al. Tractography of the corticospinal tracts in infants with focal perinatal injury: comparison with normal controls and to motor development. Neuroradiology. 2012;54(5):507–516. doi: 10.1007/s00234-011-0969-5. [DOI] [PubMed] [Google Scholar]
- 22.van der Aa NE, Leemans A, Northington FJ, et al. Does diffusion tensor imaging-based tractography at 3 months of age contribute to the prediction of motor outcome after perinatal arterial ischemic stroke? Stroke. 2011;42(12):3410–3414. doi: 10.1161/STROKEAHA.111.624858. [DOI] [PubMed] [Google Scholar]
- 23.van der Aa NE, Verhage CH, Groenendaal F, et al. Neonatal neuroimaging predicts recruitment of contralesional corticospinal tracts following perinatal brain injury. Dev Med Child Neurol. 2013;55(8):707–712. doi: 10.1111/dmcn.12160. [DOI] [PubMed] [Google Scholar]
- 24.Van’t Hooft J, van der Lee JH, Opmeer BC, et al. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst Rev. 2015;4(5):71. doi: 10.1186/s13643-015-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Einspieler C, Prechtl HF. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev. 2005;11(1):61–67. doi: 10.1002/mrdd.20051. [DOI] [PubMed] [Google Scholar]
- 26.Guzzetta A, Mercuri E, Rapisardi G, et al. General movements detect early signs of hemiplegia in term infants with neonatal cerebral infarction. Neuropediatrics. 2003;34(2):61–66. doi: 10.1055/s-2003-39597. [DOI] [PubMed] [Google Scholar]
- 27.Guzzetta A, Pizzardi A, Belmonti V, et al. Hand movements at 3 months predict later hemiplegia in term infants with neonatal cerebral infarction. Dev Med Child Neurol. 2010;52(8):767–772. doi: 10.1111/j.1469-8749.2009.03497.x. [DOI] [PubMed] [Google Scholar]
- 28.Spittle AJ, Boyd RN, Inder TE, et al. Predicting motor development in very preterm infants at 12 months’ corrected age: the role of qualitative magnetic resonance imaging and general movements assessments. Pediatrics. 2009;123(2):512–517. doi: 10.1542/peds.2008-0590. [DOI] [PubMed] [Google Scholar]
- 29.Romeo DM, Guzzetta A, Scoto M, et al. Early neurologic assessment in preterm-infants: integration of traditional neurologic examination and observation of general movements. Eur J Paediatr Neurol. 2008;12(3):183–189. doi: 10.1016/j.ejpn.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Chen CY, Tafone S, Lo W, et al. Perinatal stroke causes abnormal trajectory and laterality in reaching during early infancy. Res Dev Disabil. 2015;38(3):301–308. doi: 10.1016/j.ridd.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Dean DC, 3rd, Dirks H, O’Muircheartaigh J, et al. Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatr Radiol. 2014;44(1):64–72. doi: 10.1007/s00247-013-2752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verde AR, Budin F, Berger JB, et al. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Front Neuroinform. 2014;7(1):51. doi: 10.3389/fninf.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayana S, Rezaie R, McAfee SS, et al. Assessing motor function in young children with transcranial magnetic stimulation. Pediatr Neurol. 2015;52(1):94–103. doi: 10.1016/j.pediatrneurol.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Kasai T, Yahagi S. Motor evoked potentials of the first dorsal interosseous muscle in step and ramp index finger abduction. Muscle Nerve. 1999;22(10):1419–1425. doi: 10.1002/(sici)1097-4598(199910)22:10<1419::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Sadnicka A, Kassavetis P, Saifee TA, et al. Cerebellar transcranial direct current stimulation does not alter motor surround inhibition. Int J Neurosci. 2013;123(6):425–432. doi: 10.3109/00207454.2012.763165. [DOI] [PubMed] [Google Scholar]


