Abstract
Targeted therapies and immunotherapies have led to significant improvements in the treatment of advanced cancers, including metastatic melanoma. However, new strategies are desperately needed to overcome therapeutic resistance to these agents, as well as to identify effective treatment approaches for cancer patients that fall outside major targetable mutational subtypes (e.g. non-V600 BRAF melanoma). One such strategy is to extend the paradigm of individually tailored, molecularly targeted therapy into a broader spectrum of melanoma patients, particularly those bearing tumors without commonly recognized therapeutic targets, as well as having failed or were ineligible for immunotherapy. In this non-treatment pilot study, next-generation sequencing (NGS) technologies were utilized, including whole genome and whole transcriptome sequencing, to identify molecular aberrations in patients with non-V600 BRAF Metastatic Melanoma (MM). This information was then rationally matched to an appropriate clinical treatment from a defined pharmacopeia. Five patients with advanced non-V600 BRAF MM were enrolled. We demonstrated successful performance of the following during a clinically relevant time period: patient tumor biopsy, quality DNA/RNA extraction, DNA/RNA-based sequencing for gene expression analysis, analysis utilizing a series of data integration methodologies, report generation, and tumor board review with formulated treatment plan. Streamlining measures were conducted based on the experiences of enrolling, collecting specimens, and analyzing the molecular signatures of patients. We demonstrated the feasibility of using NGS to identify molecular aberrations and generate an individualized treatment plan in this patient population. A randomized treatment study utilizing lessons learned from the conduct of this pilot study is currently underway.
INTRODUCTION
Many common cancers are difficult to treat, in part, because they are heterogeneous, with each tumor subset having different molecular abnormalities. Identifying relevant molecular aberrations in genes encoding signaling proteins critical for cellular proliferation and survival within heterogeneous cancers is crucial to future progress in targeted therapeutics (1, 2). Key to the identification of targeted therapeutics for melanoma has been the discovery of common somatic events through deep molecular profiling. Several large scale studies using Next Generation Sequencing (NGS) (3–5) have very recently been expanded and corroborated by The Cancer Genome Atlas Network (TCGA) (4), providing a detailed landscape of genomic alterations in cutaneous melanomas. The TCGA’s results of whole-exome sequencing (WES) performed on 318 primary and/or metastatic cutaneous melanomas revealed a mean mutation rate of 16.8 mutations/Mb (the highest reported rate yet observed for any cancer analyzed by the TCGA)(6). Significantly mutated genes recognized as melanoma oncogenes and suppressor genes included BRAF (52%), NRAS (28%), TP53 (15%), NF1 (14%), CDKN2A (13%) and PTEN (8.5%) (4). Moreover, by molecular dissection we are beginning to recognize molecular subtypes in melanoma defined by specific driver mutations that may increase the likelihood of a tumor to respond to a specific targeted therapy (7, 8).
For decades, no single drug or drug combination demonstrated any appreciable impact on survival for patients with advanced metastatic melanoma (MM) (9). Nevertheless, the past few years have shown encouraging advances in the treatment of MM. One critical observation is the convergence of mutations in melanoma upon the RAS/RAF/MEK/ERK signaling pathway. Notably, BRAF inhibitors have demonstrated clinical efficacy in patients harboring oncogenic BRAF mutations and represent a major shift in the way we think about and treat melanoma (10, 11). As further improvement of this promising therapy continues, progress has begun in identifying therapeutic targets to treat patients that lack a BRAF V600E mutation, comprising approximately 50% of all MMs. Early studies of novel immunotherapies for MM, including the anti-PD-1 (programmed death-1) monoclonal antibody MK-3475, and combinations of ipilimumab and nivolumab, have recently shown great promise in the clinic (12, 13). In the area of targeted drug therapies, studies of the MEK inhibitor binimetinib (MEK162) in patients with NRAS-mutated melanoma, demonstrated evidence of response (14, 15). A subset of “pan-negative” MMs, which lack known driver mutations in BRAF, NRAS, KIT, GNAQ, and GNA11, and comprise more than 30% of melanomas, contain novel BRAF fusions that could make them responsive to MEK-directed therapy (16, 17). Importantly, a recognized subset of sun exposed cutaneous melanomas (termed “triple-wild type {Triple-WT}) were defined by the TCGA the as a heterogeneous subgroup characterized by a lack of hotspot BRAF, N/H/K-RAS or NF1 mutations. Additionally, the non-sun exposed melanomas (mucosal, acral and uveal) all have low frequency of BRAF hotspot mutations (7). This, along with the higher frequency of non-BRAF mutated melanomas among the elderly, combined with the aging population trend, as well as the need for additional treatments for patients that do not respond to immunotherapy, predicts a future shift in the prevalence of this molecular subtype and highlights the importance of identifying better targeted therapeutic approaches for these patients (18).
A challenge in the area of targeted cancer treatment is identifying optimal therapies to treat tumors that are both highly adaptive and exhibit significant tumor and patient heterogeneity (19–22). The terms “Personalized” as well as “Precision Medicine” have been used extensively to refer to the tailoring of medical treatment to the individual characteristics of each patient and represents an emerging paradigm in the treatment of cancer (23). Assigning therapy with drugs that target the specific molecular composition of a tumor — irrespective of tumor classification or anatomical origin — provides an alternative to the conventional approach that targets tumors of specific organs or tissues without consideration of the underlying tumor biology. Indeed, several preliminary studies utilizing real-time molecular profiling have demonstrated that results of tumor molecular analyses can successfully direct targeted cancer therapy (24–27).
The Melanoma Dream Team, funded by the Stand Up To Cancer (SU2C) and Melanoma Research Alliance (MRA), was formed to examine whether therapy selection based on systematic integration of large-scale genomic and pharmacopeia information will improve upon the current practice of the physician’s empiric choice for treatment of non-BRAF mutated MM (28). This approach uses the expanding knowledge of molecular networks and the mechanisms of action of a growing pharmacopeia to define matched drug therapies and to identify patients for which a target aligns with an existing agent in a pre-defined, melanoma-relevant portfolio (29, 30).
A Phase II, statistically-powered, randomized clinical trial (termed Genomics-Enabled Medicine for Melanoma, or G.E.M.M.) is currently being conducted to randomize patients to either molecularly-guided therapy or pre-defined, histologic physician interpretation for treatment option selection (clinicaltrials.gov #NCT02094872). The primary objective of this study is to examine the difference in best overall response rate (BORR) between the two treatment selection arms. Secondarily, differences in progression free survival (PFS) between the two study groups will be examined. The overarching hypothesis is that a precision medicine approach to non-V600 BRAF MM is more efficacious than empirically-selected therapy for this subset of patients who currently have limited hope for clinical benefit (31). Therefore, this trial is directly testing the merits of a specific data-driven predictive method through which any number of relevant drugs in a defined portfolio (including FDA-approved and investigational agents) has a chance of being recommended. However, prior to initiation of this large-scale efficacy trial, discussions with the United States Food and Drug Administration (FDA) led to the decision to conduct a pilot study, enrolling a small sample set of MM patients without the BRAF mutation, to assess the feasibility of using tumor molecular characterization to guide treatment selection and refine the study-related standard operating procedures (SOPs). This pilot trial was designed to mirror most aspects of the large-scale study, including the collection, processing, and analyzing of tumor tissue during a 5-week period from sample collection to review of an individualized treatment plan by a clinical tumor board. The pilot trial, described here, was not designed to include therapeutic intervention.
PATIENTS AND METHODS
Patient selection
The pilot study enrolled adult metastatic or advanced unresectable metastatic melanoma patients who were determined to be without a BRAF V600 mutation as evaluated using the Cobas® 4800 BRAF V600 Mutation Test. Participants were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 3; life expectancy of ≥4 months, as estimated by the treating oncologist; and adequate liver function defined as: total bilirubin ≤ 1.5 times the upper institutional limit reference range (ULRR) and alanine aminotransferase (ALT) < 5 times the ULRR. Prior chemotherapies, radiation therapies, immunotherapies, and experimental (non-FDA approved) therapies were allowed. Patients had to have surgical resection planned or biopsiable disease (defined as at least 1 cm3 tumor accessible for biopsy), and had to agree to blood and tissue acquisition for research purposes. Patients were excluded if a clinically significant medical condition was present which, in the opinion of the Investigator, made it undesirable for the patient to participate in the study, could jeopardize compliance with protocol requirements, or prevented a safe biopsy procedure. Institutional review board approval of the protocol and consent form were obtained and protocol design and conduct complied with all applicable regulations, guidance, and local polices.
Study Design
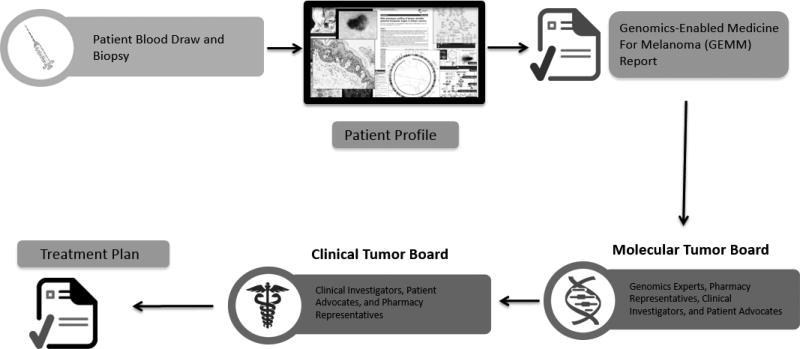
Figure 1 shows the process diagram for the clinical trial. This study was exploratory and therefore not statistically powered; five patients were chosen as a reasonable number to identify significant scenarios needing to be addressed prior to the conduct of the subsequent randomized study. Written informed consent was obtained from all patients enrolled on the study and eligibility screening evaluations were performed. Within 5 days of consent, patients underwent collection of tumor tissue and whole blood samples (collected for extraction of constitutional analytes). All specimens were de-identified and coded and shipped overnight to the Translational Genomics Research Institute (TGen; Phoenix, AZ) for processing and analysis. Clinical tissue custody, pathology evaluation, analyte generation, validation of prioritized biomarkers, and Next Generation Sequencing (NGS) of DNA/RNA occurred at TGen under Clinical Laboratory Improvement Amendments (CLIA) quality standards. Molecular information was analyzed by the TGen Genome Processing and Knowledge Recovery Team and a report listing identified somatic events were provided to a multi-disciplinary tumor board. The tumor board discussed the results of the molecular analysis and constructed an individualized treatment plan using a pre-assembled portfolio of therapeutic agents (both FDA-approved and investigational agents) selected to be considered for treatment of patients in the large-scale, randomized trial.
Figure 1.
Process diagram depicting key steps from patient consent through molecular profiling and treatment plan generation. The entire process from biopsy to treatment plan is designed to be performed in less than 5 weeks.
Feasibility was assessed based upon the completion of the following within a 5-week period: patient tumor biopsy; tissue pathologic evaluation; DNA/RNA extraction and quality control; molecular profiling; identification of DNA mutations and copy number alterations; RNA sequence and expression level alterations; integration of DNA and RNA information; knowledge mining and report generation; and tumor board review with formulated treatment plan.
Sequencing strategies and databases for target/drug matching and heuristics
The next-generation sequencing approaches employed in this trial identified somatic events from normal/tumor pairs at the genomic (DNA) level, including coding point mutations and small insertions/deletions, copy number changes, and structural events (intra-chromosomal rearrangements and translocations), and at the transcriptomic (RNA) level including differential gene expression and RNA fusions. These aggregate data sets were curated and annotated by the Genome Processing and Knowledge Recovery Team at TGen, and a molecular profile was generated for each patient. This molecular profile was collected along with the patient’s histopathology information and clinical history to form a patient profile. This profile was used to generate an individualized report, which was shared with a Molecular Tumor Board who assessed the weight of genomic evidence in support of particular target/drug matches. The matching of molecular alterations to drugs in study pharmacopeia was accomplished using a custom set of drug rules (see below).
Study pharmacopeia
Investigational agents in the study pharmacopeia were selected under the auspices of the assembled SU2C clinical melanoma expert team (Table 1a). Selection criteria included: mechanisms of action relevant to non-BRAF mutated melanoma biology; previous knowledge of drug and possible preclinical and clinical efficacy; prior determination of a recommended Phase 2 dose (RP2D) and/or maximally tolerated dose (MTD) of the drugs being administered in combination; and drug availability. Additionally, approved oncology agents were selected from broad therapeutic classes based on information available from the Melanoma Disease Model initiative from Cancer Commons and the Melanoma Molecular Map Project (Table 1b). Only those agents that overlapped with the drug list being evaluated in this clinical trial were considered by the Molecular and Clinical Tumor Boards.
Table 1.
| a. Investigational Agents Available in Study Pharmacopeia | |||
|---|---|---|---|
| Company Name: | Drug Name: | Target: | Route |
| GlaxoSmithKline | GSK1120212 (GSK212) | MEK 1/2 | Oral |
| GSK2141795 (GSK795) | AKT | Oral | |
| Millennium | MLN8237 | Aurora A kinase | Oral |
| TAK-733 | MEK | Oral | |
| MLN1117 | PI3Kα | Oral | |
| INK128 | TORC 1/2 | Oral | |
| MLN 2480 | PanRAF | Oral | |
| Pfizer | Axitinib (AG013736) | VEGFR | Oral |
| Bosutinib (SKI-606) | Abl, Src | Oral | |
| PF-00299804 | pan-erbB | Oral | |
| Amgen | AMG337 | cMET | Oral |
| Plexxikon | PLX3397 | FMS, Kit and Fit3-ITD | Oral |
| Astellas | OSI-027 | Torc1/2 | Oral |
| OSI-906 | IGF-R | Oral | |
| Bristol-Myers Squibb | BMS936558 | PD-1 | IV |
| BMS754807 | IGF | Oral | |
| BMS906024 | Notch | Oral | |
| Brivanib | FGFR/VEGF | Oral | |
| Exelixis | XL184 | Mulit-kinase (VEGFR2, Met, FLT3, Tie2, Kit and Ret) | Oral |
| b. Commercially available agents in Study Pharmacopeia | |||
|---|---|---|---|
| Company Name: | Drug Name: | Target: | Route |
| Eisai Inc | Alitretinoin | RARA, RXRA, RARB, RXRB, RARG, RXRG | topical |
| Janssen Products | Adriamycin | DNA, TOP2A | IV |
| Sanofi-Aventis | Fluorouracil | TYMS | IV, topical |
| Eli Lilly | Gemcitabine | RRM1, TYMS, CMPK, DNA | IV |
| Pemetrexed | TYMS, ATIC, DHFR, GART | IV | |
| Novartis | Imatinib | Multikinase (BCR-ABL, c-kit, PDGF-R, RET) | Oral |
| Merck | Temozolomide | DNA | Oral, IV |
| Flutamide | AR | Oral | |
| GlaxoSmithKline | Lapatinib | EGFR, ERBB2 | Oral |
| Millennium | Bortezomib | proteasomes | IV |
| Pfizer | Sunitinib | PDGFRa, PDGFRb, VEGFR1, VEGFR2, VEGFR3, KIT, FLT3, CSF1R, RET | Oral |
| Crizotinib | ALK, ROS1, MET | ||
| Irinotecan | topoisomerase-1 | IV | |
| Bristol-Myers Squibb | Etoposide | topoisomerase-2 | Oral/IV |
| Dasatinib | C-Src | Oral | |
| Paclitaxel | TUBB1, BCL2 | IV | |
| Carboplatin | DNA | IV | |
| AstraZeneca | Anastrozole | CYP19A1 | Oral |
| Gefitinib | EGFR | Oral | |
| Vandetanib | EGFR, VEGFR, RET | Oral | |
| Tamoxifen | ESR1 | Oral | |
| Bayer | Sorafenib | BRAF, RAF1, VEGFR2, VEGFR3, FLT3, PDGFRB, KIT, FLT4 | Oral |
| Genentech | Erlotinib | EGFR, NR1I2 | Oral |
| Trastuzumab | ERBB2, EGFR | IV | |
| Wyeth | Temsirolimus | FRAP1 | IV |
| Merck | Vorinostat | pan-histone deacetylase inhibitor | Oral |
| Abraxis | Vinblastine | TUBB2 | IV |
| Hoffman LaRoche | Interferon, Recombinant | IFNAR2, IFNAR1 | Sub-Q |
| Teva | Dacarbazine | POLA2 | IV |
Legend: IV, intravenous; Sub-Q, subcutaneous
Clinical Phenotype Data Collection
Both the patient clinical interface and demographics were collected through a GEMM Clinical Portal web interface, serving two purposes. First, the collection of the clinical data was used to create a structured PDF-type document for presentation prior to the genomics report during the tumor board, insuring uniformity in presentation through the course of the study. Second, the extensive clinical profile provided richer information to better understand how genomic alterations lead to phenotypic variability within melanoma patients in future studies. Collection of over 140 fields was broken into four tabbed sections: (i) Patient Information, (ii) Primary Diagnosis, (iii) Metastatic Disease, and (iv) Current Presentation. Within the first tabbed section on ‘Patient Information’ we obtained patient number, age, race, ethnicity, gender, and patient summary. Also within the first tabbed was information collected on adjuvant treatment including IFN, Vaccine, and other treatments. Finally, the first tabbed treatment included information on the sentinel lymph node biopsy, if available, describing the surgical resection and extent of lymph node involvement. Within the second tabbed section on ‘Primary Diagnosis’, we obtained extensive information on primary diagnosis information, including the type, metastatic sites of presentation, histology, Breslow depth, staging, initial mutation status. Within the third tab, information about ‘Metastatic Disease’ was collected describing the information on the type of treatment (surgery/radiation), and types of systemic treatment. Also obtained was information on up to two progressions, including treatment, number cycles, and response to treatment. Within the fourth tab, information on ‘Current Presentation,’ we obtained information on co-morbid medical conditions, other non-melanoma cancers, prior treatments, allergies, imaging and radiology, the results of lab work, physical examination, vital signs, and pathology.
Drug-Gene Matching
Treatments for a particular patient were recommended by the tumor board based on a report of genomic alterations. To aid in the process, genomic alterations previously linked to therapies were provided as a screening tool with the understanding that not all drug-gene links were relevant or fully supported by the data, and still required an expert panel to vet. The process of building such a report was fundamentally based on biomarker to gene rules. The content of the drug rule database was developed and curated by Ph.D. level domain experts. Each drug in the pharmacopeia was annotated with information on how specific genes and alterations in those genes may influence drug response from evidence in published literature sources. Information such as gene, genomic alteration such as variant, drug, direction of association (sensitive, resistant), publication link, and evidence text of the relationship. Annotated rule statements were captured in a custom database application using a controlled vocabulary. The application was implemented to store reproducible, rule-based lists linking genomic information with selected therapeutics. The application accepted genomic information that encompasses genes, measurement types, and values for measurements. The process of report generation began with genomic data, as described above. If the gene matches a gene in the database, then the aberration type was checked. If a match occurred, a rule statement was triggered and presented in table form stating the relationship and indication for the drug. If the aberration was not directly linked, but was an inferred rule, a statement was conveyed as such. The generated report also included additional evidence tables for triggered rules and included outbound hyperlinks to the original rule evidence source. An algorithmic method for ranking rules was not implemented, as that was considered to be the purpose of the tumor board.
The Molecular Tumor Board
Following molecular analysis of each patient’s tumor and normal specimens, a Molecular Tumor Board met via conference call/WebEx to assess the molecular profile generated for the individual patient, briefly discuss the patient’s medical history, and analyze the weight of evidence in support of a proposed target/drug match. The Molecular Tumor Board consisted of expert members of the team as follows: clinical investigators; genomics experts involved in sequencing and analysis, knowledge mining, and computational and systems biology; bioinformaticians; pharmacy representatives; medical geneticists, molecular biologists; and patient advocates. At least one Genomics expert/bioinformatician, one pharmacy representative, one patient advocate, and three clinical investigators were required for a proper quorum to hold a Molecular Tumor Board meeting. Each Molecular Tumor Board conference call was recorded in its entirety. Molecular profiling data was given to the Molecular Tumor Board members a minimum of 48 hours in advance of the meeting.
The Clinical Tumor Board
The information discussed by the Molecular Tumor Board was then passed along to the Clinical Tumor Board who determined an individualized treatment plan based on the available data. A final recommended treatment plan was generated by the Clinical Tumor Board utilizing the information contained in the knowledge mining analysis, information contained in the individualized patient report, and relevant patient clinical characteristics (e.g., history & physical, prior treatment history, comorbidities, etc.). Specific treatment details consisted of a regimen chosen from a guided list of agents implicated in relevant, critical molecular signaling pathways and/or from signature-based predictions of drug efficacy summarized in the individual molecular report. All agents listed in the pilot study pharmacopoeia were similarly planned for use in the randomized study, but the selected therapy could differ amongst individual patients depending on several factors, including results of their unique molecular profiling. Potential drug interactions between the targeted agents and the subject’s routine medications and supplements were considered by the Clinical Tumor Board, as well as each patient’s clinical characteristics and prior treatment history. Members of the Clinical Tumor Board underwent training prior to trial initiation to establish guidelines and to run through test cases associated with theoretical patients.
The decision-making process, including prioritization of treatments, was based on the following: (1) depth of knowledge for each individual agent included in the study pharmacopeia and its response in tumors with similar molecular characteristics including (but not limited to) peer-reviewed published in vivo data, in vitro data, and response profile differential; (2) safety considerations, including (but not limited to) review of the patient’s history & physical, prior treatments, concurrent medications, and potential drug/drug interactions; and (3) expertise of the team in evaluating each target.
The Clinical Tumor Board met by conference call/WebEx as needed following each Molecular Tumor Board. The Clinical Tumor Board consisted of expert members of the team as follows: at least three study clinical investigators; additional clinical investigators, as available; at least one patient advocate (non-voting); at least one pharmacy representative (non-voting). The final treatment recommendation was made through a majority vote of all clinical investigators in attendance at the Clinical Tumor Board meeting. If a majority vote in favor of a specific treatment was not reached, discussion would be made whether more information was needed, including the possible reconvening of the Molecular Tumor Board, or whether the patient should come off-study. Each Clinical Tumor Board conference call was recorded in its entirety and a summary of the meeting and treatment plan was written.
RESULTS
Patient experiences
Five patients between the ages of 45 – 73 were enrolled in this study between the period of November 12, 2012 and June 5, 2013 (Table 2). Four out of 5 patients had a primary diagnosis of cutaneous melanoma and 1 had a primary diagnosis of mucosal melanoma. All patients had previous treatment with some type of immunotherapy, with four patients receiving ipilumumab; one patient receiving interleukin-2; and two patients receiving a Programmed Death −1 (PD-1) inhibitor through a clinical trial. All patients were tested for BRAF mutation expression and were found to be non-V600 E mutant. If patients did not have BRAF expression tested previously using the Cobas® 4800 BRAF V600 Mutation Test, archival tissue was requested and analysis was performed by the Cobas® method. This resulted in a significant delay at times for biopsy, with an average of 14 days (range 0–30 days) delay to obtain tissue and results. With the assistance of multiple staff members, every effort was made to minimize this time frame. BRAF mutation status for patients 001 and 005 was initially verified using non-Cobas® methodology; their BRAF mutational status was not confirmed by the Cobas® method until after these patients had the biopsy procedure performed. This was due, in part, to the delay in obtaining archival tissue.
Table 2.
Summary of Patients
| Patient | Melanoma Type |
Biopsy site | Time from consent to biopsy (days) |
Time from biopsy to clinical tumor board (days) |
Available target for clinical treatment recommendation? |
|---|---|---|---|---|---|
| 001 | Cutaneous | Left inguinal lymph node | 1 | 59 | Yes |
| 002 | Cutaneous | Retroperitoneal lymph node | 2 | 37 | Yes |
| 003 | Cutaneous | Left inguinal mass | 2 | 37 | Yes |
| 004 | Mucosal | Liver mass | 1 | 30 | Yes |
| 005 | Cutaneous | Left iliac mass | 1 | 32 | Yes |
Once patients signed consent for the study and biopsy, patient’s eligibility was confirmed and biopsy obtained with 1–2 days of patient consent. Biopsies were obtained by Interventional Radiology and tissue was frozen within 30 minutes of the procedure. Three patients (001, 003 and 004) had ultrasound-guided biopsies while the other two patients (002 and 005) had CT-guided biopsies. All patients tolerated the procedure well; however one patient (003) experienced prolonged bleeding at the biopsy site after discharge. This required emergency treatment with lidocaine, epinephrine, and thrombin-gel foam. There was no significant drop in hematocrit in any patients. Tissue and whole blood samples were de-identified, coded with unique identifiers and successfully shipped overnight to TGen. Each patient’s tumor samples were analyzed for percent tumor content and were found to be adequate for analysis, ranging from samples containing 50% to 90% tumor content.
TGen staff performed all molecular analyses of tissue and blood samples. No patients experienced progression of their disease during the time from biopsy to discussion of genomic analysis, and the time to identification of targeted treatment improved significantly from patient 001 to patient 005. Initially, time between biopsy and molecular tumor board discussion for patient 001 was 59 days. Constant evaluation and process changes resulted in the improvement of efficiencies and a shortening of this time interval to 30 and 32 days for patients 004 and 005, respectively. This was within the goal of 35 days proposed in the protocol. Optimizations resulted from evaluation of protocol-specific and institutional SOPs to streamline sample handling, train personnel, and add process clarifications where needed. Utilization of project management application software (Trello; Trello, Inc.) was instituted to track the status of the samples at each stage of collection, processing, shipping, and analysis. Communication between clinical and research teams was assessed and enhanced, increasing efficiency.
Four amendments to the protocol were needed based on the experiences of enrolling, collecting specimens, and analyzing the molecular signature of individual patients. The first amendment addressed changes in tissue sample distribution. The second addressed issues with quality control analysis of the tumors and tissue collection procedures. The third and fourth amendment allowed for additional patients to be enrolled, if necessary, as well as to allow patients with non-cutaneous metastatic melanoma to be recruited.
Study feasibility assessments
Several benchmarks were evaluated during the conduct of this pilot study, including feasibility assessments for the completion of the following in a 5-week time period: patient tumor biopsy, quality DNA/RNA extraction, DNA/RNA-based sequencing and gene expression analysis, analysis utilizing a series of data integration methodologies, report generation, and tumor board review with formulated treatment plan. Table 3 includes a list of the assessments made and corrective actions that were performed if benchmarks were not met.
Table 3.
Study benchmarks
| Benchmark | Assessment | Benchmark met? |
Corrective Action Taken |
|---|---|---|---|
| Patient was properly enrolled | Patient was enrolled and eligible | 4/5 patients. | One patient did not have Cobas testing prior to enrollment; this patient had tissue sent out after enrollment which was subjected to Cobas testing, eventually meeting eligibility with a BRAF mutation indicated. Evaluation of protocol-specific and institutional SOPs. Modifications were made to streamline the procedure and add clarification. Increased training of responsible personnel was made. |
| Consent form was properly filled out and signed | 5/5 patients | Not applicable | |
| Blood/tissue samples were properly handled and shipped to their destination | Tissue and blood was obtained from the patient. | 5/5 patients | Not applicable |
| 100% of samples shipped | 5/5 patients | Not applicable | |
| Courier delivered 100% of samples | 5/5 patients | Not applicable | |
| Sample handling procedures were adequately followed | 5/5 patients | Not applicable | |
| Tissue samples (following shipment) show acceptable pathological qualification of tumor content | Tumor content was adequate in 80% of samples to provide sufficient quantity/quality to proceed with analyte extraction (≥ 25% tumor nuclei) | 4/5 patients | Guidelines for determining biopsy-able status of patients’ tumor were addressed and modified. A protocol amendment was prepared to allow an extra tissue core to be obtained to ensure proper pathology review, if one was needed. |
| Tissue/blood samples yield suitable DNA/RNA for sequencing | Sample quantity was adequate for obtaining molecular analytes for defined laboratory protocols. | 5/5 patients | Not applicable. |
| Sample DNA/RNA quality was adequate for defined laboratory protocols | 4/5 patients | Optimal quantity of tissue for RNA/DNA analyses was not obtained from first patient because internal threads on collection tube resulted in damaged tissue. Cryovials with internal threads were replaced with vials with external threads. | |
| Tumor samples yield assignment of molecular alterations deemed druggable to receive an assigned individualized treatment decision from the tumor board | Sample quantity, quality, and generation of molecular information performed in a timely fashion to integrate data in support of identifying druggable targets and associated therapy. | 3/5 patients | Processing and communication issues led to delays in the genomic analyses for two patients, resulting in exceeding the 5-week target timeframe. A meeting between clinical and scientific investigators was held to discuss issues contributing to the delays. Utilization of project management application software was instituted to track the status of the analyses at each stage of collection, processing, shipping, and analysis. |
Genetic findings and analytical validation
Medically actionable genetic aberrations were identified for all five patients. The primary molecular events and associated treatment recommendations are provided in Table 4. Interestingly, despite all five patients having BRAF nonV600E mutant melanoma, the results of their molecular profiling and discussions of the Clinical and Molecular Tumor Boards, led to the recommendation of 4 of 5 patients to receive a MEK inhibitor.
Table 4.
Molecular Events and Related Tumor Board Recommendations in Five Patients
| Patient ID | Number of Somatic Coding Mutations |
Key Somatic Events | Therapeutic Relationship |
|---|---|---|---|
| Patient 001 | 3,126 | BRAF(D594G), CDKN2A(Deletion), MAP2K1(E203K) | MEK162 |
| Patient 002 | 3,364 | NF1(R440X), RB1(S646F) | MEK162 |
| Patient 003 | 183 | NRAS(Q61R), CDKN2A(Deletion) | MEK162 |
| Patient 004 | 44 | PTEN(HmzDel), CSF1R(Over expression), FLT1(OExp), PDGFRB(Over Expression) | PLX3397 |
| Patient 005 | 369 | NRAS(Q61R), PTEN(Loss of function frameshift), CDKN2A(Deletion), TP53(Deletion) | MEK162 |
BRAF, v-Raf murine sarcoma viral oncogene homolog B; CDKN2A, cyclin-dependent kinase inhibitor 2A; MAP2K1, mitogen-activated protein kinase kinase 1; NF1, neurofibromin 1; RB1, retinoblastoma 1; NRAS, neuroblastoma RAS viral (v-ras) oncogene homolog; PTEN, phosphatase and tensin homolog, CSF1R, colony stimulating factor 1 receptor; FLT1, fms-related tyrosine kinase 1; PDGFRB, platelet-derived growth factor receptor, beta polypeptide; TP53, tumor protein p53
Extensive efforts were made to analytically validate the genomic profiling, including an evaluation of the potential effects of biopsy sampling variability, tumor heterogeneity, and potential inferring substances on downstream genomic interrogation, as well as an assessment of the sensitivity and precision (reproducibility and repeatability) of the next generation sequencing strategies to be employed in the GEMM Trial. The following narrative describes the analytical validation studies conducted and provides a summary of the results obtained.
Misinterpretation of inherited germline variants as tumor specific acquired mutations is a major concern, particularly when the therapies being selected were developed to address tumor specific acquired mutations. In every individual there are hundreds of new inherited germline events that with high predictive value be bioinformatically excluded without the sequencing of a germline or normal sample from healthy tissue. Within our study, sequencing of whole-blood is used to effectively determine and report on only those variants that are tumor specific. Clearly, germline variation could impact therapies, particularly in tumors that BRCA1/2 driven, for BRAF-wildtype melanoma this largely is not the case. Consequentially, the sequencing of whole-blood is largely impactful for insuring therapies selected on the basis of being relevant to tumor – specific mutations are indeed tumor specific and not inherited germline vairiation.
Likewise, another major impediment to detection of tumor specific mutations can be contamination of the tumor DNA with DNA isolated from surrounding stromal cells (32). To evaluate the sensitivity of the next generation sequencing strategy to be employed, we analyzed COLO829 and COLO829BL, two publicly available cell lines. COLO829 (ATCC CRL-1974) is an immortalized melanoma fibroblast line generated from a 45-year old Caucasian male, and COLO829BL (ATCC CRL-1980) is a lymphoblastoid line generated from peripheral blood collected from the same subject. These two cell lines were previously sequenced, and a detailed catalogue of all identified somatic events has been reported previously. Given the public availability of these data, libraries were generated from these cell lines in order to evaluate performance of our sequencing workflows.
COLO829 tumor/normal pair was sequenced across multiple platforms by multiple growths from multiple cell growths in order to establish a ‘gold-standard’ for analytical validation of somatic mutation calling. We conducted whole genome, PCR-free sequencing on an Illumina HISeq2500 to 90× depth at two sites using two different preparations of COL829 DNA, and sequencing was performed both at TGen and within the Illumina Translational Genomics Division. In addition, DNA was provided to Complete Genomics for sequencing. In order to establish a “truth set” of germline and tumor-specific point mutations and structural variants, we utilized the intersection of multiple variant callers on the Illumina 2500 and Complete Genomics calls sets. In the case of focal amplifications and focal deletions, we assayed the cell lines on Agilent CGH arrays. True negatives were established at positions where no variant detection indicated the existence of a variant and sufficient high quality reads uniquely mapped with expected percentages of anomalous reads, mismatches, and insertion/deletions. In those regions where there was disagreement with one of the calls, Sanger sequencing data was used to determine the truth set.
Variation in RNA expression data whether derived from RNA-seq or as in other studies microarrays can be attributed to a variety of factors including the quality of the starting material, the level of cellularity and RNA yield, and the platform employed. To control for these sources of possible variability, a common set of external RNA controls has been developed by the External RNA Controls Consortium (ERCC), an ad hoc group of academic, private, and public organizations hosted by the National Institute of Standards and Technology (NIST). The controls consist of a set of unlabeled, polyadenylated transcripts designed to be added to RNA analysis experiments after sample isolation in order to measure against defined performance criteria.
DISCUSSION
The emergence of BRAF inhibitors demonstrates that the efficacy of specific targeted treatment modalities is dependent upon the molecular constitution of the tumor, and that the observed variations in tumor response to current therapies are largely attributable to disease heterogeneity at the molecular level. Advances in informatics and molecular technologies, coupled with our expanding knowledge of molecular networks and mechanism of action of the existing pharmacopeia, provide increased opportunity to develop models that more accurately predicts tumor response. The clinical evaluations in the SU2C/MRA project, beginning with this feasibility study and the subsequent prospective randomized study, represent important steps in the development of precision oncology, in which each patient is treated individually based upon the systematic molecular analysis of their disease.
We propose that the sequencing of a patient’s cancer versus sequencing of their normal cells could enable a “deep dive” analysis of the patient’s cancer genomes. Coupled with innovations in knowledge engineering, such an analysis would provide sufficient depth and dimensionality of genomic measurements to properly interpret the molecular context of vulnerability to the point where we can truly understand the key oncogenic dependencies of an individual’s disease. It is this understanding, at an individual level, irrespective of other cases, that forms the foundation of our individualized approach called ‘Precision Medicine’.
Immunotherapy has rapidly emerged as a valuable therapeutic strategy for metastatic melanoma. The cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) antibody, ipilimumab, was FDA approved on the basis of improvement in overall survival and objective responses in approximately 11% of the patients (33). However, this agent also had significant toxicity in the majority of treated patients. Additionally, initial clinical trials did not evaluate the relevance of a potential molecular signature and patient subsets most likely to benefit. The immune checkpoint programmed death 1 (PD-1) receptor inhibitor, nivolumab subsequently demonstrated even greater therapeutic benefit against metastatic melanoma, with an improved objective response of 32% vs 11% for dacarbazine that translated as well into improvement in overall survival (40% vs 14%) (34). Robert and colleagues were able to subsequently demonstrate significant benefit against treatment naïve non-BRAF mutated metastatic melanoma, with a median progression free survival of 5.1 months and a 1 year overall survival of approximately 73%. Most recently, the combination of ipilimumab and nivolumab have been studied in combination. Among patients with BRAF wild-type tumors, the rate of confirmed objective response was 61% in the combination group vs 11% with ipilimumab alone with a complete response rate of 22% in the combination group vs 0% with ipilimumab alone. However, drug-related grade 3 or 4 adverse events occurred at a rate of 54% with this combination (35).
Despite aggressive treatment with immunotherapy, a significant percentage of BRAF wild-type patients go on to progress and die of their disease. Hence, these immunotherapeutic failures, the context of the patients we are recruiting to this trial, still represent an unmet medical need. An improved understanding of the molecular underpinnings of non-V600 BRAF mutant MM is urgently needed to advance the development of rational therapeutics for this disease. Critical to the success of the innovative translational study will be the creation of a framework for capturing, processing, analyzing, mining, and interpreting the data.
The emerging high-resolution molecular profiling that defines druggable targets represents a new paradigm in drug development. For our overall SU2C/MRA project, the current standard of investigating drug efficacy based solely on histopathologic classification will be challenged. Instead, we will investigate drug efficacy defined by the tumors’ molecular/genetic profiles. Even with the prospect of additional genomic information from large-scale retrospective studies of melanoma (e.g. TCGA), remarkably little information will be available to correlate the genomic make-up of mutationally-defined subtypes of disease with clinical outcome. Although not our primary aim, we will unveil significant genomic information relative to non-V600 BRAF mutant MM; we anticipate these data will define new targets for future drug discovery efforts. The studies outlined herein represent a step in the development of precision oncology, in which each patient is truly treated individually based upon the systematic molecular analysis of their disease. We believe the solid foundation built through the conduct of the SU2C/MRA project will begin to not only define appropriate therapeutic interventions for the non-V600 BRAF mutant MM patient population, but will also make significant strides in changing the treatment paradigm for all cancer patients.
Next steps
In the pilot trial described herein, patients were enrolled and proceeded through the entire workflow so as to evaluate every step from patient biopsy through report generation. This information assisted in the efficient design of the powered study as well as established acceptance criteria for reporting clinically actionable results to physicians in real time, identifying the logistical hurdles of completing genomic interrogation and delivering potentially druggable targets to the patient within 5 weeks.
This pilot study assisted us in developing a framework for therapeutic decision-making. Following trial completion, our team requested communication with the FDA and received guidance for our group to prepare the IND in tandem with a pre-submission to the FDA’s Center for Devices and Radiological Health (CDRH) to discuss the validation needed for a study of this type. We provided the validation information, using clinical samples from the pilot trial, blood and frozen metastatic biopsy materials, and interference studies with melanin and other substances.
The preliminary experience gained in the conduct of this pilot trial was invaluable in the preparation of an Investigational New Drug (IND) submission to the FDA detailing the large-scale, randomized, statistically-driven study (FDA IND#115,393; Clinicaltrials.gov #NCT02094872). The randomized study has subsequently been Institutional Review Board (IRB)-approved and has begun accruing patients (31).
Acknowledgments
GRANT SUPPORT:
All authors received research support from a Stand Up To Cancer – Melanoma Research Alliance / Melanoma Dream Team Translational Cancer Research Grant (SU2C-AACR-DT0612). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research (AACR).
The authors would like to acknowledge the patients who enrolled on this pilot study and agreed to undergo biopsies knowing that this was not a treatment trial. They would also like to thank the following patient advocates involved in the project: Jane Perlmutter, PhD; Mark Gorman; Sen. Cornelius Alexander McGillicuddy III; Derrick Hall, and Tracy Bame.
Footnotes
CONFLICT OF INTEREST STATEMENT: P. Chapman discloses service as a consultant to Genentech/Roche and GlaxoSmithKline. L. Fecher discloses service as a consultant to Genentech/Roche and Bristol-Myers Squibb.
References
- 1.Braiteh F, Kurzrock R. Uncommon tumors and exceptional therapies: paradox or paradigm? Mol Cancer Ther. 2007;6:1175–9. doi: 10.1158/1535-7163.MCT-06-0674. [DOI] [PubMed] [Google Scholar]
- 2.Stewart DJ, Kurzrock R. Cancer: the road to Amiens. J Clin Oncol. 2009;27:328–33. doi: 10.1200/JCO.2008.18.9621. [DOI] [PubMed] [Google Scholar]
- 3.Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–6. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–14. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 8.Lovly CM, Dahlman KB, Fohn LE, Su Z, Dias-Santagata D, Hicks DJ, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7:e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggermont AM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer. 2011;47:2150–7. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 10.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sznol M, Kluger HM, Callahan MK, Postow MA, Gordon RA, Segal NH, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) Clin Oncol. 2014;32:5s. (suppl; abstr LBA9003) [Google Scholar]
- 13.Ribas A, Hodi F, Kefford R, Hamid O, Daud A, Wolchok JD, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL) J Clin Oncol. 2014;32:5s. (suppl; abstr LBA9000) [Google Scholar]
- 14.Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. The lancet oncology. 2013;14:249–56. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 15.Sosman JA, Kittaneh M, Lolkema M, Postow MA, Schwartz G, Franklin C, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J Clin Oncol. 2014;32 abstr 9009. [Google Scholar]
- 16.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–46. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson KE, Lipson D, Stephens PJ, Otto G, Lehmann BD, Lyle PL, et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin Cancer Res. 2013;19:6696–702. doi: 10.1158/1078-0432.CCR-13-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer J, Buttner P, Murali R, Okamoto I, Kolaitis NA, Landi MT, et al. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res. 2011;24:345–51. doi: 10.1111/j.1755-148X.2011.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balakrishnan A, Bleeker FE, Lamba S, Rodolfo M, Daniotti M, Scarpa A, et al. Novel somatic and germline mutations in cancer candidate genes in glioblastoma, melanoma, and pancreatic carcinoma. Cancer Res. 2007;67:3545–50. doi: 10.1158/0008-5472.CAN-07-0065. [DOI] [PubMed] [Google Scholar]
- 21.Heng HH. Cancer genome sequencing: the challenges ahead. Bioessays. 2007;29:783–94. doi: 10.1002/bies.20610. [DOI] [PubMed] [Google Scholar]
- 22.Arnedos M, Vielh P, Soria JC, Andre F. The genetic complexity of common cancers and the promise of personalized medicine: is there any hope? J Pathol. 2014;232:274–82. doi: 10.1002/path.4276. [DOI] [PubMed] [Google Scholar]
- 23.Garraway LA. Genomics-driven oncology: framework for an emerging paradigm. J Clin Oncol. 2013;31:1806–14. doi: 10.1200/JCO.2012.46.8934. [DOI] [PubMed] [Google Scholar]
- 24.Von Hoff DD, Stephenson JJ, Jr, Rosen P, Loesch DM, Borad MJ, Anthony S, et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–83. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 25.Roychowdhury S, Iyer MK, Robinson DR, Lonigro RJ, Wu YM, Cao X, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med. 2011;3:111ra21. doi: 10.1126/scitranslmed.3003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsimberidou AM, Iskander NG, Hong DS, Wheler JJ, Falchook GS, Fu S, et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin Cancer Res. 2012;18:6373–83. doi: 10.1158/1078-0432.CCR-12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss GJ, Liang WS, Demeure MJ, Kiefer JA, Hostetter G, Izatt T, et al. A pilot study using next-generation sequencing in advanced cancers: feasibility and challenges. PLoS One. 2013;8:e76438. doi: 10.1371/journal.pone.0076438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herlyn M. New dream team for melanoma therapy. Pigment Cell Melanoma Res. 2012;25:279–80. doi: 10.1111/j.1755-148x.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- 29.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–6. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 30.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–6. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yale University. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2015. Molecularly Targeted Therapy in Treating Patients With Melanoma That is Metastatic or Cannot be Removed by Surgery. Available from: http://clinicaltrials.gov/ct2/show/NCT02094872?term=NCT02094872&rank=1 NLM Identifier: NCT02094872. [Google Scholar]
- 32.Jones S, Anagnostou V, Lytle K, Parpart-Li S, Nesselbush M, Riley DR, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med. 2015;7:283ra53. doi: 10.1126/scitranslmed.aaa7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 35.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414428. Epub 20 Apr 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]