Abstract
Epithelial-mesenchymal transition (EMT) can directly contribute to some malignant phenotypes of tumor cells including invasion, metastasis and resistance to chemotherapy. Although EMT is widely demonstrated to play a critical role in chemoresistance and metastasis, the potential signaling network between EMT and drug resistance is still unclear. The distribution of drugs in the internal and external environment of the tumor cells is tightly linked with ATP-binding cassette (ABC) transporters. Recent studies have shown that ABC transporters expression changed continuously during EMT. We believe that EMT is an important regulator of ABC transporters. In this review, we discuss how EMT regulates ABC transporters and their potential linkages. And we hope the knowledge of EMT and ABC transporters will offer more effective targets to experimental research.
Keywords: ABC transporters, EMT, CSCs, resistance, TFs, miRNA
Introduction
Chemotherapy is one of the most effective methods in guiding therapy decisions. However, the abnormal activation of cell signaling and aberrant changing of some oncogenes often cause uncontrolled drug resistance, relapse and metastasis. Multidrug resistance (MDR) is responsible for the major causes of failure in cancer chemotherapy, by which tumor cells resistant to a single class of cytotoxic drugs can simultaneously become insensitived to other chemotherapy drugs that have similar structures and targets 1. Nearly three decades of research on MDR have revealed the mechanisms, including repairing of DNA damage, changing of drug target structure and the most important paradigm of increased energy-dependent efflux of anticancer agents by ABC transporters. ABC transporters often overexpressed in the majority of drug-resistant tumors 1, 2, and act as protectors of tumor cells by changing drug concentrations in both intracellular and extracellular environments. It has been reported that overexpression of ABC transporters proteins including P-glycoprotein (P-gp), multidrug resistance protein (MRP) and breast cancer resistance protein (BCRP) is one of the major reason account for MDR, which can reduce the ability of drugs by transporting them out of the cells through activating ATP hydrolysis 3. What`s more, ABC transporters can mediate the transport of a broad spectrum of drugs, such as doxorubicin, cisplatin, vincristine, methotrexate, and even the effective chemotherapy drugs Tyrosine kinase inhibitors are also substrates of them 4. Therefore, an important potential means of reversing drug resistance in tumors is by restraining ABC transporters 5.
EMT is an important biological process in the progression of malignant tumors. It cannot only enhance the ability of tumor cells to invade and metastasize 6, but also grant them stem cells properties 7, 8. During the malignancy progression, tumor cells tend to develop resistance characteristics 9, 10. The mechanisms that EMT induces resistance to chemotherapy drugs are complex and have received close attention. Recent studies suggest that EMT may cause overexpression of ABC transporters, thereby promoting drug resistance in tumor cells 11, 12.
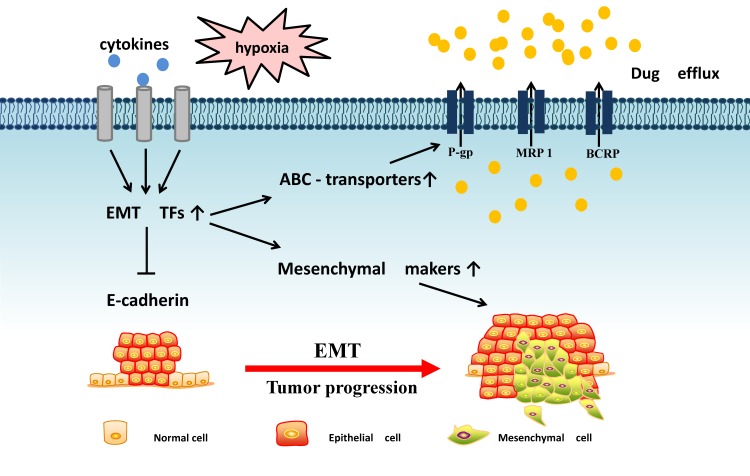
Through the dynamic biological conversion process of EMT, we explored the potential regulatory mechanisms of ABC transporters in EMT microenvironment, EMT transcription factors and EMT-related miRNA (Fig. 1). From these perspectives, we discuss the complex signals between EMT and ABC transporters, linking tumor invasion and metastasis with MDR characteristics. Thus, we are designed to provide a new insight for cancer research.
Figure 1.
The potential linkages between EMT and ABC transporters: EMT-related transcription factors often receive microenvironment signal that can inhibit E-cadherin expression and promote mensenchymal markers and ABC transporters expression, resulting in tumor metastasis and drug efflux.
1. ABC transporters and their roles in tumor progression
Chemotherapy drugs need to undergo complex pharmacokinetics processes, including absorption, distribution, metabolism and elimination, when they are playing a therapeutic role in the human body. However, ABC transporters have been identified as key determinants for pharmacokinetics processes of many antitumor drugs. They utilize ATP hydrolysis to stimulate the efflux of drugs from tumor cells, making this a challenging process in chemoresistance. Several ABC transporters can be accounted for the resistance to variety anticancer drug in tumor cells. Identification of the crystal structures of p-gp and some other efflux pumps have deeply facilitated ABC transporters inhibitors designing and chemoresistance research.
P-gp is the earliest and most typical ABC transporter that has a large variety of functions and substrates 13. It is encoded by ABCB1 gene, also known as the multidrug resistance gene (MDR1). P-gp has a broad-spectrum, multidrug efflux pump and its substrates include doxorubicin, docetaxel and etoposide, implying that a very special structure in its space 14. P-gp contains two transmembrane domains and two ATP-binding domains, and each transmembrane domain contains six fragments. The actions of transmembrane domains are believed to function as drug-binding sites of P-gp, which can stimulate ATP hydrolysis and drug efflux caused by conformational change 15. Tumor cells who exhibit high P-gp expression may have intrinsic or acquired resistance to chemotherapy. Determining whether a chemotherapy drug can be transported by P-gp may provide important information for predicting the drug efficacy. In addition, researchers also described the possibility that P-gp might play a critical role in regulating cell differentiation, proliferation and metastasis. However, sometimes a single block of ABC transporters cannot be a good reversal of drug resistance, because there are also other efflux pumps for tumor cells to employ.
Although P-gp-mediated MDR has been found in many tumor cell lines, not all drug resistance in tumors is mediated by P-gp, prompting further research on drug transporters. MRP has 13 members with sizes range from 1325 to 1545 amino acids, which is encoded by the ABCC gene family 15, 16. MRP1/ABCC1, considered as the second major efflux pump, is one of the MRP members and its capacity for drug efflux prevents effective treatment of a range of anticancer drugs such as methotrexate and vincristine 15. Overexpression of MRP1 has been found in many kinds of tumors where it plays a significant role in tumor defense, conferring a drastic relapse in patients. Some clinical investigation even showed the overexpression of MRP1 and treatment for MRP1 seem to be more effective than target P-gp.
Many chemotherapy drugs, such as mitoxantrone, are not good substrates for P-gp and MRP. For investigating the transport process mediating resistance to mitoxantrone, researchers discovered the third ABC transporters BCRP, which is encoded by ABCG2 17. BCRP is comprised of only one ATP-binding domain and a transmembrane domain, which are different from that of P-gp and MRP. BCRP`s broad substrate specificity has brought new challenges to the fight against MDR. Chemoresistance in breast cancer, lung cancer, leukaemia and other cancers was found to be significantly related to the presence of the ABCG2 gene, and many reports considered ABCG2 as an adverse prognostic risk factor in human cancer 18-20. This evidence could be summarized as the main course of drug transport and make it possible to predict the tumor progression in chemotherapy.
2. EMT and cancer stem cells in chemoresistance
With increasing awareness about tumor progression, researchers hypothesized that chemotherapy can kill most of tumor cells except for a small part, composed of cancer stem cells(CSCs), which are the root cause of malignant tumors 21, 22. CSCs can simultaneously lead to higher rates of invasion, metastasis and resistance to chemotherapy. The relationship between EMT and CSCs is critical for the development of tumors. Many molecular switches in EMT have been found to be associated with the generation and cross correlation properties of CSCs. EMT-triggered chemoresistance in CSCs has attracted much attention in recent years 10, 23. During tumor development, EMT induces a loss of polarity in epithelial cells, decreasing cells adhesion and changing from epithelial cells into mesenchymal cells. As a result of EMT, tumor cells show characteristic features of CSCs with superior tumorigenic effects, and develop more powerful drug resistant properties that promote tumor recurrence and metastasis 24, 25. There is growing evidence of the potential role of EMT in the development of drug resistance. Patients with EMT and resistance to chemotherapy often show a poorer quality of life than those who do not develop EMT 26.
CSCs can develop chemoresistance owing to their ability to repair DNA damage, resistance to apoptosis and aberrant expression of ABC transporters 27. We suggest that EMT-mediated CSCs may be associated with chemoresistance induced by overexpression of ABC transporters. SOX2 is well-established transcription factors in regulating EMT, which was also described to enhance CSCs properties in tumor cells. CSCs markers CD44, OCT4 and NANOG can be positively regulated by overexpression of SOX2 in head and neck squamous cell carcinoma(HNSCC). This study also shows that SOX2 can increase the number of cisplatin-resistant tumour cells by upregulating ABCG2 28. Doxorubicin-resistant Breast cancer cell MCF-7/ADR-1024 displayed a E-cadherin disappeared and N-cadherin, ZEB-1/2, Slug, Twist, vimentin increased phenotype as well as oncospheres formation with CD44 high/CD24 low CSCs surface marker expression 29. Compared with drug-sensitive cells, the increasing of EMT-associated mesenchymal proteins in colon cancer cells grant them more resistant to enzastaurin, whereas E-cadherin was absent and MDR1 was also highly expressed in these enzastaurin-resistant cells 30. Qu et al 31 observed that the expression of ABCG2 and P-gp were significantly higher than that of prechemotherapy tissues. ABCG2 and P-gp expressions were significantly positively correlated with vimentin expression but negatively correlated with E-cadherin expression, providing further important evidence for the association of EMT and MDR. Current views favor the model that the chemoresistant tumors often show a high expression of EMT markers and CSC properties. Although there are a lot of markers remain to be identified, it is reasonable to assume that EMT will not only play a single role in tumor invasion, but also involve in regulating drug transporters.
3. Underlying cell signaling network in regulating EMT and ABC transporters
Several cell signalings and molecules have been identified to participate in regulation of MDR and EMT, such as PI3K/AKT, TGF-β, HIF-1, NF-κB, EGF. Many of these elements have been identified in a complex community of tumor environment, then involved in the downstream cascade of signal transduction. This evidence may imply that the significance of the underlying cell signaling network in modulating EMT and expression of ABC transporters.
Hypoxia is an important feature of the tumor microenvironment which can induce EMT 32. Under hypoxia conditions, tumor angiogenesis is greatly stimulated to increase the supply of nutrients. Tumor cells can up-regulate ABC transporters under hypoxia environment 33, thereby altering the absorption profile of the drug. The initial response of cancer cells to hypoxia is the activation of HIFs that regulate a number of functional genes involved in cell survival, angiogenesis, EMT and chemoresistance 34, 35. HIF-α can stimulate the MDR1 gene expression through binding with the hypoxia response element (HREs) on MDR1 promoter 36, 37. Hypoxia can also cooperate with other oncogenic pathways such as NF-κB pathway to generate the chemoresistance in tumor cells. Bentires-Alj et al 38 found that NF-κB is activated and migrates to the nucleus, playing a regulatory role in P-gp expression. Interestingly, NF-κB is also critical for the induction and maintenance of EMT in mammary epithelial cells by up-regulating Ras 39, indicating the role of NF-κB as EMT and P-gp inducers. Hence, hypoxia tumor environment can synchronically drive EMT process as well as facilite chemoresistance, which highlight the potential surviving mechanism during tumor progression.
The microenvironment can also influence EMT by altering metabolism, secretion and other functions, thereby affecting the generation and development of malignancy. TGF-β is the most common EMT inducer and an important cytokine in the tumor microenvironment, and it can also increase ABC transporters expression 40. This phenomenon suggests that tumor cells can promote autocrine and paracrine leading to the development of tumor metastasis and drug resistance. The expression of proteins in the ABCC subfamily was up-regulated during EMT in the breast cancer cell line MDA-MB-468 using EGF, accompanied by a decrease in E-cadherin and an increase in mesenchymal molecules 41. However, Takano et al have 13 established RLE/Abca3 cells by introducing ABCA3 gene into wild-type RLE-6TN cells. But the effect of TGF-β displayed increased EMT markers and decreased ABCA3 mRNA in RLE/Abca3 cells. Compared with RLE/Abca3 cells, the expression of ABCA3 in A549 and H441 cells was not significantly affected by TGF-1. Yin et al 42 found that ABC transporters were reduced in TGF-β-induced EMT. This conclusion is contrary to our view, and the actions of TGF-β in regulating ABC transporters remains to be diversified. This may probably due to different regulatory effects of TGF-β on different ABC proteins or different experimental samples.
4. EMT transcription factors as regulators of ABC gene expression
EMT involves the loss of epithelial cell gene expression and the formation of a program of mesenchymal gene expression, in which E-cadherin reduction is the most important feature. Some EMT-related transcription factors (TFs) enter the nucleus and inhibit E-cadherin expression, whereas activate N-cadherin, vimentin and other mesenchymal gene expression 8, leading to changes in adhesion and polarity of epithelial cells. Once the generation of mesenchymal phenotype-related molecules starts, the ability of invasion, metastasis and resistance to chemotherapy can be greatly enhanced. Thus, EMT-related TFs play a major role in regulating ABC transporters expressions (Table 1).
Table 1.
Comparative analysis of the relationship between EMT TFs and ABC transporters in different tumor cells
| ABC transporters | EMT TFs | Tumor cell types | Referred anticancer drugs | Reference |
|---|---|---|---|---|
| P-gp/MDR1 | Twist1 | Cervical cancer cells, Hela | cisplatin | Zhu et al [43] |
| ZEB1/2, Slug, Twist | Breast cancer cells, MCF-7/ADR-1024 | doxorubicin | Tsou et al [29] | |
| Snail | Breast cancer cells, MCF-7/sanil treated with adriamycin | adriamycin | Li et al [44] | |
| BCRP/ABCG2 | Snail | Breast cancer cells, MCF-7 | mitoxantone | Chen et al [45] |
| MSX2 | Pancreatic Cancer Cells, B7 and B21 | 5-FU | Hamada et al [46] | |
| SOX2 | Head and neck squamous cell carcinoma cell line, SNU1041 | cisplatin | Lee et al [28] | |
| ZEB1 | Thyroid papillary carcinoma cell line, TPC-1 | mitoxantrone | Mato et al [47] | |
| MRP5/ABCC5 | Twist1 | Two 5-FU-resistant hepatocellular carcinoma cells, HLF-R4 and HLF-R10 | 5-FU | Uchibori et al [48] |
| ABCC2, ABCC4 | Snail, Slug, Twist | Glioblastoma multiforme cell lines, U87 and U373 treated with sFRP4 | temozolomide | Bhuvanalakshmi et al [49] |
EMT transcription factors and ABCB1 (P-gp). Zhu et al 43 found a positive correlation between upregulation of Twist1 and P-gp expression in cervical cancer. Twist1 silencing in Hela was accompanied with expression and function abatement of P-gp, and increased cell sensitivity to cisplatin. This result provides a new therapeutic target for the control of drug-resistant cervical cancer. Tsou et al 29 have observed that the expression of the EMT TFs ZEB1/2, Slug and Twist as well as MDR1 were increased in doxorubicin resistant breast cancer cells MCF-7/ADR-1024. EMT properties in MCF-7/ADR-1024 (treated with 1024 nM of doxorubicin) had higher migration ability than wild type of MCF-7, indicating a correlation between the EMT phenotypes and P-gp-mediated drug resistance. Overexpression of Snail in MCF-7 cells has exhibited insensitivity to adriamycin but not contribute to the expression of P-gp. Compared with MCF-7/pcDNA cells, MCF-7/Snail cells could express more P-gp protein when treated with adriamycin 44. Thus, a strategy for suppressing EMT TFs expression can lead to a significant reduction in P-gp production and induce the sensitivity to chemotherapy.
EMT transcription factors and ABCG2 (BCRP). Snail was highly correlated with BCRP expression in MCF-7/snail cells, and the transfer of Snail into MCF7 cells presented an increased in their resistance index to mitoxantone 45. Hamada et al 46 found that the transcription factor MSX2 can induce EMT, which then binds to the ABCG2 promoter and upregulates ABCG2 expression through SP1 recruitment. SOX2 has an abnormal expression in HNSCC, and it is recognized as an important EMT transcription factor. As we mentioned above, SOX2 is also known to be associated with CSCs trait. Screened EMT mediators found that snail was significantly down-regulated when using shSOX2 interference in HNSCC, and meanwhile, ABCG2 expression was also down-regulated thereby attenuating their cisplatin resistance 28. These data suggested that SOX2 plays a critical role in drug resistance and invasiveness of HNSCC. Furthermore, there is an increase in protein expression for ABCG2 in human thyroid papillary carcinoma TPC-1 mitoxantrone-resistant cell line compared with TPC-1. A significant up-regulated in ZEB1 and TWIST were detected in TPC-1 mitoxantrone-resistant cell, indicating a good correlation with ABCG2 expression level. After knockdown of the ZEB1 gene for 72 h, a significant reduction in ABCG2/BCRP gene expression was observed 47.
EMT transcription factors and ABCC (MRP). In a study of 5-FU resistant hepatocellular carcinoma cells (HCC), quantitative RT-PCR data showed that E-cadherin was down-regulated and Twist1 was up-regulated in the cells. In addition, ribonucleotide reductase expression decreased and MRP5 expression increased, indicating that EMT and MRP5 in 5-FU played an important regulatory role in resistant HCC 48. Bhuvanalakshmi et al 49 found that secreted frizzled-related protein 4 (sFRP4) worked as a tumor suppressor that can inhibit the expression of the EMT TFs Snail, Slug and Twist and the chemotherapy resistance genes ABCC2 and ABCC4.
5. MiRNA: Link EMT and ABC transporters
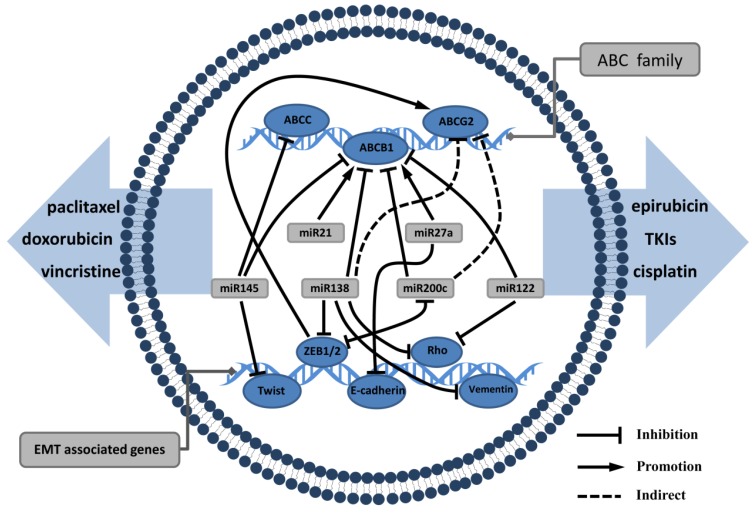
MiRNAs are small endogenous RNA molecules of 20-24 nucleotides and are involved in post-transcriptional gene expression in plants and animals. MiRNAs guide the binding of the RNA-induced silencing complex to the 3′-UTR region of the target mRNA, thereby inhibiting its translation and even inducing target mRNA degradation. MiRNAs have a complex regulatory network that can regulate the expression of multiple genes through a single miRNA. There is substantial evidence that miRNAs play an important role in EMT and drug resistance in tumors 50. Haenisch et al 51 have summarized examples of miRNA-regulated ABC transporters, particularly in the process of P-gp production, which is affected by multiple miRNAs 52. We believe that miRNA is an essential molecule linking EMT and ABC transporters (Fig. 2).
Figure 2.
miRNAs have a complex regulatory network that can regulate EMT and ABC genes: miRNAs such as miRNA122, miRNA 138, miRNA145, miRNA200 can not only inhibit mesenchymal markers, but also act as ABC transporters suppressor. miRNA21 and miRNA27a often promote EMT progression and upregulate ABC transporters expression that mediate the processes of drug distribution.
The miRNA200 family, which comprises five members, is a hotspot in cancer research and plays an important role in diagnosis and therapy of tumor metastasis 53. In EMT, the miR200 family can play a negative regulatory role to restrain tumor invasion and metastasis 54-56. MiR200 can directly target the 3′-UTR region of ZEB1 and ZEB2 and maintain the epithelial phenotype by upregulating E-cadherin production. However, ZEB1 and ZEB2 can reverse the upstream of the miR200 to form a negative feedback loop 57-59. Chen et al 60 found that miR200 inhibited MDR1 expression in breast cancer cells, thereby increasing the susceptibility of the cells to doxorubicin. This case indicated that miR200 is commonly down-regulated in patients with breast cancer resistant to chemotherapy. MiR138 upregulation in cells can reduce P-gp production to reverse MDR properties 61. In another study, miR138 can target ZEB2, vimentin, Rho and other mesenchymal proteins, leading to tumor cell invasion and motor function disorders then inhibiting EMT 62. MiR122 and miR145 can inhibit EMT and ABC transporters thus suppress the ability of metastasis and drug resistance 63-66. All these studies demonstrate that miRNAs participate in the crosstalk signalling network of EMT and ABC transporters expression, and mediate the processes of drug distribution and tumor metastasis.
Other miRNAs can upregulate EMT and ABC transporters. These miRNAs often play a role as oncogenes when they are highly expressed in tumor cells. miR21 has been shown to mediate tumor cell proliferation, apoptosis and autophagy, and its expression is associated with MDR. MDR1 gene expression can be significantly inhibited by suppressing miR21 67. Furthermore, miR21 can activate the AKT/ERK pathway to promote EMT biological processes by targeting PTEN 68, 69. miR27a inhibits E-cadherin and raf kinase inhibitor to induce EMT 70, 71, conferring resistance to gefitinib, doxorubicin and cisplatin. On the other hand, MDR1 gene expression has a positive correlation to miR27a; miR27a can bind to the MDR1 promoter, playing a similar role in transcription factor regulation 72. Hence, we can see that miRNA mediate complex cross-regulation mechanisms between EMT and ABC transporters. To inhibit metastasis and MDR of tumor cells can simultaneously proceed from the above miRNAs. It is anticipated that miRNAs will be used clinically as biomarkers of drug resistance.
6. Perspectives: The ABC transporters and EMT Paradigm
The invasion and metastasis of tumors are controlled by various regulating mechanisms. During EMT progression, the development of drug-resistance in tumors has a serious impact on the effectiveness of cancer drug therapy. Although there was little direct research on the relation between ABC transporters and EMT, previous studies have demonstrated some potential correlation between EMT and ABC transporters 11, 12. Hence, getting insight into the interaction between EMT and ABC transporters is critical for understanding the molecular and cellular mechanisms underlying chemoresistance development.
Several studies described EMT-related TFs are crucially participate in this complex network of ABC transporters regulating drug resistance and cell survival. The changing of p-gp expression can be due to the upstream cell signalings of EMT such as NF-κB pathway. Inhibition of NF-κB pathway displayed a decrease in P-gp and EMT-related TFs like Slug and Twist. Twist1 is a highly conserved transcription factor in EMT and have a novel function in maintaining cisplatin-resistant of Hela cells by its relationship with P-gp41. Previous study also depicted the aggravating effect of snail as an inducer of EMT. Positive correlations between Snail and BCRP were proved in the experiment, which further demonstrated an interaction between EMT and multidrug resistance 43. These results indicated a novel target to overcome tumor resistance through suppressing EMT upstream TFs. However, these TFs can be easily disturbed by extracellular signaling, which was derived from troublesome tumor microenvironment. Tumor local conditions often exist in hypoxia, which can trigger a cascade of cellular signaling and lead to tumor angiogenesis and a variety of nutrients supply. Thereinto, HIF-α, a direct production of hypoxia, is well-known to drug-resistance and also recognized to induce EMT by upregulating TFs. Taken together, these elements can stimulate the signal transduction processes in tumor cells, such as the TGF-beta pathway, the NF-κB pathway and the PI3K/AKT pathway, which not only induce EMT but also contribute to the proliferation of tumor cells and insensitivity of chemotherapy 40,48.
In addition, accumulating evidence showed that miRNAs play a major role in modulating ABC transporters and EMT biomarkers. Most of the miRNA research conducted to investigate their roles in regulating EMT and ABC transporters mainly focused on miR200 family 53. Most studies have identified miR200 as an inhibitor of EMT and MDR1 that can suppress cancer progression. Other miRNAs like miR138, miR122 and miR145 can reverse MDR and EMT properties through inhibiting the expression of their fundamental molecules. However, there are also some miRNAs develop a malignancy in tumor progression. MiR21 can inhibit PTEN thereby rouses PI3K/AKT pathway that affects tumor migration and upregulates MDR1 67, 69. Similarly, down-regulation of miR27a could confer an increase in E-cadherin and sensitivity of P-gp-related chemotherapy 72. Since miRNAs involve in multiple steps in tumor progression, a combination of inhibition of EMT and drug-resistance may be utilized by selectively targeting certain miRNAs. Recent studies also proved that miRNAs are differentially expressed in CSC subpopulation, which was described as the culprit of cancer malignancy. The identification of CSCs also attracted renewed attention on the role of EMT in drug resistance. Therefore, EMT is not only involved in CSCs initiating and maintaining, but also can contribute to CSCs insensitivity to chemotherapy. Based on the above analysis, a proposed strategy to overcome and circumvent EMT can probably block metastasis, ABC transporters and the maintenance of CSCs characteristics. What's more, it is unclear whether it can regulate the ABC transporters using the correlation between EMT and ABC transporters in normal tissues, so as to change the distribution of drugs and reduce the side effects of chemotherapy.
It should be noted that this study only concentrates on the positive relationship between EMT and ABC transporters. However, the reverse process of EMT - mesenchymal-epithelial transition (MET) - is believed to help tumor cells establish secondary lesions. During MET, active elements are corpuscles, adopting a loss of migratory with the changing of cell morphology and re-expressing hallmarks of epithelial cells. From this point of view, whether MET affects the interaction among miRNAs, TFs and ABC transporters remains unknown. Moreover, the tentative network is not appropriate for all the conditions. Its limitations notwithstanding, future study should be focused on the dynamic behavior of ABC transporters in the process of tumor metastasis.
The crosstalk of EMT property and tumor resistant has proved to be quite fruitful. Recent advances in tumor cytology and molecular biology have led to a better understanding of the relationship between EMT and ABC transporters. The above evidence and paradigm between EMT and ABC transporters can be applied to develop and design novel therapeutic targets, which not only help us to understand the development of tumors, but also play a critical role in the diagnosis, treatment and prognosis of tumors.
Acknowledgments
This project was supported in part by the Natural Science Foundation of China (No. 81273647), the Natural Science Foundation of Fujian Province (No.2013J01365), Young and middle-aged talent training project in fujian provincial health system (No.2015-ZQN-ZD-2), National health-education joint research project (No.WKJ-FJ-19).
References
- 1.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–57. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature reviews Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 3.Prasad R, Goffeau A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu Rev Microbiol. 2012;66:39–63. doi: 10.1146/annurev-micro-092611-150111. [DOI] [PubMed] [Google Scholar]
- 4.Wang XK, Fu LW. Interaction of tyrosine kinase inhibitors with the MDR- related ABC transporter proteins. Current drug metabolism. 2010;11:618–28. doi: 10.2174/138920010792927316. [DOI] [PubMed] [Google Scholar]
- 5.Darby RA, Callaghan R, McMahon RM. P-glycoprotein inhibition: the past, the present and the future. Current drug metabolism. 2011;12:722–31. doi: 10.2174/138920011798357006. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P, Sun Y, Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell cycle (Georgetown, Tex) 2015;14:481–7. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Seminars in cancer biology. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nature cell biology. 2014;16:488–94. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 9.Luo M, Brooks M, Wicha MS. Epithelial-mesenchymal plasticity of breast cancer stem cells: implications for metastasis and therapeutic resistance. Current pharmaceutical design. 2015;21:1301–10. doi: 10.2174/1381612821666141211120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Fan D. The epithelial-mesenchymal transition and cancer stem cells: functional and mechanistic links. Current pharmaceutical design. 2015;21:1279–91. doi: 10.2174/1381612821666141211115611. [DOI] [PubMed] [Google Scholar]
- 11.Saxena M, Stephens MA, Pathak H. et al. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell death & disease. 2011;2:e179. doi: 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takano M, Yamamoto C, Yamaguchi K. et al. Analysis of TGF-beta1- and drug-induced epithelial-mesenchymal transition in cultured alveolar epithelial cell line RLE/Abca3. Drug metabolism and pharmacokinetics. 2015;30:111–8. doi: 10.1016/j.dmpk.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et biophysica acta. 1976;455:152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 14.Jin MS, Oldham ML, Zhang Q. et al. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature. 2012;490:566–9. doi: 10.1038/nature11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong M. Biochemical studies on the structure-function relationship of major drug transporters in the ATP-binding cassette family and solute carrier family. Advanced drug delivery reviews; 2016. [DOI] [PubMed] [Google Scholar]
- 16.Cole SP, Bhardwaj G, Gerlach JH. et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science (New York, NY) 1992;258:1650–4. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 17.Doyle LA, Yang W, Abruzzo LV. et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galetti M, Petronini PG, Fumarola C. et al. Effect of ABCG2/BCRP Expression on Efflux and Uptake of Gefitinib in NSCLC Cell Lines. PloS one. 2015;10:e0141795. doi: 10.1371/journal.pone.0141795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Wang L, Zhu Y. et al. Breast cancer resistance protein (BCRP)-containing circulating microvesicles contribute to chemoresistance in breast cancer. Oncology letters. 2015;10:3742–8. doi: 10.3892/ol.2015.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maciejczyk A, Szelachowska J, Ekiert M. et al. [Analysis of BCRP expression in breast cancer patients] Ginekol Pol. 2012;83:681–7. [PubMed] [Google Scholar]
- 21.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6:10697–711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonagh L, Gray SG, Breen E. et al. Lung cancer stem cells: The root of resistance. Cancer letters. 2016;372:147–56. doi: 10.1016/j.canlet.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Espinoza I, Miele L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer letters. 2013;341:41–5. doi: 10.1016/j.canlet.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nature reviews Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- 25.Jordan NV, Johnson GL, Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell cycle (Georgetown, Tex) 2011;10:2865–73. doi: 10.4161/cc.10.17.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallini P, Lennard T, Kirby J. et al. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer treatment reviews. 2014;40:341–8. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Oh SY, Do SI. et al. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. British journal of cancer. 2014;111:2122–30. doi: 10.1038/bjc.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsou SH, Chen TM, Hsiao HT. et al. A critical dose of doxorubicin is required to alter the gene expression profiles in MCF-7 cells acquiring multidrug resistance. PloS one. 2015;10:e0116747. doi: 10.1371/journal.pone.0116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serova M, Astorgues-Xerri L, Bieche I. et al. Epithelial-to-mesenchymal transition and oncogenic Ras expression in resistance to the protein kinase Cbeta inhibitor enzastaurin in colon cancer cells. Molecular cancer therapeutics. 2010;9:1308–17. doi: 10.1158/1535-7163.MCT-10-0167. [DOI] [PubMed] [Google Scholar]
- 31.Qu H, Fang L, Duan L. et al. [Expression of ABCG2 and p-glycoprotein in residual breast cancer tissue after chemotherapy and their correlation with epithelial-mesenchymal transition] Zhonghua bing li xue za zhi Chinese journal of pathology. 2014;43:236–40. [PubMed] [Google Scholar]
- 32.Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer letters. 2013;341:63–72. doi: 10.1016/j.canlet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Li J, Wang R. et al. MDR1 will play a key role in pharmacokinetic changes under hypoxia at high altitude and its potential regulatory networks. Drug Metab Rev. 2015;47:191–8. doi: 10.3109/03602532.2015.1007012. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Liu Y, Yan X. et al. HIFs enhance the migratory and neoplastic capacities of hepatocellular carcinoma cells by promoting EMT. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:8103–14. doi: 10.1007/s13277-014-2056-0. [DOI] [PubMed] [Google Scholar]
- 35.Jiang J, Tang Y-l, Liang X-h. EMT: A new vision of hypoxia promoting cancer progression. Cancer biology & therapy. 2014;11:714–23. doi: 10.4161/cbt.11.8.15274. [DOI] [PubMed] [Google Scholar]
- 36.Callaghan R, Crowley E, Potter S. et al. P-glycoprotein: so many ways to turn it on. J Clin Pharmacol. 2008;48:365–78. doi: 10.1177/0091270007311568. [DOI] [PubMed] [Google Scholar]
- 37.Xie J, Li DW, Chen XW. et al. Expression and significance of hypoxia-inducible factor-1alpha and MDR1/P-glycoprotein in laryngeal carcinoma tissue and hypoxic Hep-2 cells. Oncology letters. 2013;6:232–8. doi: 10.3892/ol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bentires-Alj M, Barbu V, Fillet M. et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–7. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 39.Huber MA, Azoitei N, Baumann B. et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. Journal of Clinical Investigation. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panousis CG, Evans G, Zuckerman SH. TGF-beta increases cholesterol efflux and ABC-1 expression in macrophage-derived foam cells: opposing the effects of IFN-gamma. J Lipid Res. 2001;42:856–63. [PubMed] [Google Scholar]
- 41.Stewart TA, Azimi I, Thompson EW. et al. A role for calcium in the regulation of ATP-binding cassette, sub-family C, member 3 (ABCC3) gene expression in a model of epidermal growth factor-mediated breast cancer epithelial-mesenchymal transition. Biochemical and biophysical research communications. 2015;458:509–14. doi: 10.1016/j.bbrc.2015.01.141. [DOI] [PubMed] [Google Scholar]
- 42.Yin L, Castagnino P, Assoian RK. ABCG2 expression and side population abundance regulated by a transforming growth factor beta-directed epithelial-mesenchymal transition. Cancer research. 2008;68:800–7. doi: 10.1158/0008-5472.CAN-07-2545. [DOI] [PubMed] [Google Scholar]
- 43.Zhu K, Chen L, Han X. et al. Short hairpin RNA targeting Twist1 suppresses cell proliferation and improves chemosensitivity to cisplatin in HeLa human cervical cancer cells. Oncology reports. 2012;27:1027–34. doi: 10.3892/or.2012.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Liu C, Tang Y. et al. Overexpression of Snail accelerates adriamycin induction of multidrug resistance in breast cancer cells. Asian Pacific journal of cancer prevention: APJCP. 2011;12:2575–80. [PubMed] [Google Scholar]
- 45.Chen WJ, Wang H, Tang Y. et al. Multidrug resistance in breast cancer cells during epithelial-mesenchymal transition is modulated by breast cancer resistant protein. Chinese journal of cancer. 2010;29:151–7. doi: 10.5732/cjc.009.10447. [DOI] [PubMed] [Google Scholar]
- 46.Hamada S, Satoh K, Hirota M. et al. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. Journal of cellular physiology. 2012;227:729–38. doi: 10.1002/jcp.22781. [DOI] [PubMed] [Google Scholar]
- 47.Mato E, Gonzalez C, Moral A. et al. ABCG2/BCRP gene expression is related to epithelial-mesenchymal transition inducer genes in a papillary thyroid carcinoma cell line (TPC-1) Journal of molecular endocrinology. 2014;52:289–300. doi: 10.1530/JME-14-0051. [DOI] [PubMed] [Google Scholar]
- 48.Uchibori K, Kasamatsu A, Sunaga M. et al. Establishment and characterization of two 5-fluorouracil-resistant hepatocellular carcinoma cell lines. International journal of oncology. 2012;40:1005–10. doi: 10.3892/ijo.2011.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhuvanalakshmi G, Arfuso F, Millward M. et al. Secreted frizzled-related protein 4 inhibits glioma stem-like cells by reversing epithelial to mesenchymal transition, inducing apoptosis and decreasing cancer stem cell properties. PloS one. 2015;10:e0127517. doi: 10.1371/journal.pone.0127517. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Tang J, Li Y, Wang J. et al. Molecular mechanisms of microRNAs in regulating epithelial-mesenchymal transitions in human cancers. Cancer letters. 2016;371:301–13. doi: 10.1016/j.canlet.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 51.Haenisch S, Werk AN, Cascorbi I. MicroRNAs and their relevance to ABC transporters. Br J Clin Pharmacol. 2014;77:587–96. doi: 10.1111/bcp.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopes-Rodrigues V, Seca H, Sousa D. et al. The network of P-glycoprotein and microRNAs interactions. International journal of cancer Journal international du cancer. 2014;135:253–63. doi: 10.1002/ijc.28500. [DOI] [PubMed] [Google Scholar]
- 53.Feng X, Wang Z, Fillmore R. et al. MiR-200, a new star miRNA in human cancer. Cancer letters. 2014;344:166–73. doi: 10.1016/j.canlet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koutsaki M, Spandidos DA, Zaravinos A. Epithelial-mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: prognostic value and prospective role in ovarian cancer therapeutics. Cancer letters. 2014;351:173–81. doi: 10.1016/j.canlet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 55.Bracken CP, Khew-Goodall Y, Goodall GJ. Network-Based Approaches to Understand the Roles of miR-200 and Other microRNAs in Cancer. Cancer research. 2015;75:2594–9. doi: 10.1158/0008-5472.CAN-15-0287. [DOI] [PubMed] [Google Scholar]
- 56.Humphries B, Yang C. The microRNA-200 family: small molecules with novel roles in cancer development, progression and therapy. Oncotarget. 2015;6:6472–98. doi: 10.18632/oncotarget.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gregory PA, Bert AG, Paterson EL. et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature cell biology. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 58.Park SM, Gaur AB, Lengyel E. et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burk U, Schubert J, Wellner U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–9. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Tian W, Cai H. et al. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Medical oncology. 2012;29:2527–34. doi: 10.1007/s12032-011-0117-4. [DOI] [PubMed] [Google Scholar]
- 61.Zhao X, Yang L, Hu J. et al. miR-138 might reverse multidrug resistance of leukemia cells. Leuk Res. 2010;34:1078–82. doi: 10.1016/j.leukres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Wang C, Chen Z. et al. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang SC, Lin XL, Li J. et al. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PloS one. 2014;9:e101330. doi: 10.1371/journal.pone.0101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y, Xia F, Ma L. et al. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer letters. 2011;310:160–9. doi: 10.1016/j.canlet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 65.Hu J, Qiu M, Jiang F. et al. MiR-145 regulates cancer stem-like properties and epithelial-to-mesenchymal transition in lung adenocarcinoma-initiating cells. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:8953–61. doi: 10.1007/s13277-014-2158-8. [DOI] [PubMed] [Google Scholar]
- 66.Ikemura K, Yamamoto M, Miyazaki S. et al. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol Pharmacol. 2013;83:399–405. doi: 10.1124/mol.112.081844. [DOI] [PubMed] [Google Scholar]
- 67.Bourguignon LY, Spevak CC, Wong G. et al. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. The Journal of biological chemistry. 2009;284:26533–46. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bao L, Yan Y, Xu C. et al. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer letters. 2013;337:226–36. doi: 10.1016/j.canlet.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Lv C, Hao Y, Tu G. MicroRNA-21 promotes proliferation, invasion and suppresses apoptosis in human osteosarcoma line MG63 through PTEN/Akt pathway. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:9333–42. doi: 10.1007/s13277-016-4807-6. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Wang Y, Song Y. et al. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Molecular cancer. 2014;13:193. doi: 10.1186/1476-4598-13-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, Liu S, Shi R. et al. miR-27 promotes human gastric cancer cell metastasis by inducing epithelial-to-mesenchymal transition. Cancer genetics. 2011;204:486–91. doi: 10.1016/j.cancergen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Li M, Han Y. et al. Down-regulation of miR-27a might reverse multidrug resistance of esophageal squamous cell carcinoma. Digestive diseases and sciences. 2010;55:2545–51. doi: 10.1007/s10620-009-1051-6. [DOI] [PubMed] [Google Scholar]