Abstract
Complex transitions in chromatin structure produce changes in genome function during development in metazoa. Linker histones, the last component of nucleosomes to be assembled into chromatin, comprise considerably divergent subtypes as compared with core histones. In all metazoa studied, their composition changes dramatically during early embryogenesis concomitant with zygotic gene activation, leading to distinct functional changes that are still poorly understood. Here, we show that early embryonic linker histone B4, which is maternally expressed, is functionally different from somatic histone H1 in influencing chromatin structure and dynamics. We developed a chromatin assembly system with nucleosome assembly protein-1 as a linker histone chaperone. This assay system revealed that maternal histone B4 allows chromatin to be remodeled by ATP-dependent chromatin remodeling factor, whereas somatic histone H1 prevents this remodeling. Structural analysis shows that histone B4 does not significantly restrict the accessibility of linker DNA. These findings define the functional significance of developmental changes in linker histone variants. We propose a model that holds that maternally expressed linker histones are key molecules specifying nuclear dynamics with respect to embryonic totipotency.
Keywords: ATP-dependent chromatin remodeling, linker histone assembly
Epigenetic information recorded in chromatin plays crucial roles in producing the diversity of cellular patterns of gene expression in multicellular organisms. The targeted and global alterations in covalent modification of histones have been extensively studied as examples of epigenome code (1, 2). Equally significant, and recently revaluated, are changes in the composition of the chromosomes themselves (3). Histone H2A and H3 variants, which are regionally distributed in the nucleus, appear to have distinct functional properties, for example, in epigenetic silencing and gene expression (4).
An exceedingly well conserved, albeit poorly understood, example of such “regulation via replacement of a chromosomal component” is provided by linker histone dynamics during early metazoan development (5). The linker histone engages the nucleosomal fiber such that higher-order structures essential for proper gene regulation are formed (6). Their types change during early embryogenesis in higher organisms, including flies, sea urchins, frogs, and mice (5, 7). In Xenopus, maternally expressed histone B4 is the only linker histone found in eggs and is replaced by somatic histone H1 subtypes, primarily histone H1A, after the midblastula transition concomitant with zygotic gene activation (8, 9). Microinjection of targeted ribozymes into early embryos revealed that replacement of B4 with somatic H1 is required for selective gene repression and proper development (10). The previous study using model chromatin templates also suggested that the repressive effect of histone B4 on transcription is weaker than that of somatic H1 (11). It may be tempting to speculate that histone B4 contributes directly to dynamic chromatin in early embryos. In practice, however, a molecular study of this issue has been hampered because the addition of linker histone readily leads to chromatin aggregation. Thus, it was critical to develop a defined system for the assembly of homogeneous, soluble chromatin templates containing linker histones.
In this study, we resolved this issue by developing a chromatin assembly system using nucleosome assembly protein-1 (NAP-1), known to be a chaperone for core histones H2A and H2B (12, 13), as a linker histone chaperone to reveal functional differences between linker histone variants. We illuminate the molecular basis whereby the Xenopus oocyte-specific linker histone B4 mediates generalized genome competence during early development. Furthermore, we demonstrate that the repressive effects of somatic H1 on chromatin remodeling are abrogated by free linker histone chaperone, suggesting one mechanism to induce targeted chromatin remodeling in the somatic nucleus. These results provide direct molecular evidence for the functional significance of transitional changes in linker histone variants with respect to differential gene regulation.
Materials and Methods
Immunocytochemistry. Xenopus cDNAs encoding full-length histone B4 (8) were cloned into the vector TrcHisB (Invitrogen), and the resulting construct was transformed into Escherichia coli BL21. The His-6–B4 fusion protein was purified from E. coli lysates by using His binding resin according to the manufacturer's protocol. Xenopus linker histones H1A/B were purified from acid extracts of erythrocyte nuclei by RP-HPLC (9). Antisera to B4 and H1A/B were raised in rabbits and mice, respectively, and used for indirect immunofluorescence staining of embryonic cytological sections (14).
Recombinant Proteins. Xenopus cDNAs encoding full-length histone B4, H1A, and NAP-1 were cloned into the ApaI–XhoI, ApaI–NotI, and ApaI–XhoI sites of pGEX-6P-3 (Amersham Pharmacia) to generate plasmids pGEX-6P-B4, pGEX-6P-H1A, and pGEX-6P-NAP-1, respectively. The recombinant proteins were expressed in E. coli BL21-CodonPlus-RIL cells (Stratagene) and purified by using Glutathione-Sepharose-4B affinity beads and cleavage from the GST tags (Amersham Pharmacia). B4 and H1A were further purified and concentrated in two column chromatography steps by using Biorex70 (BioRad). Purified proteins were analyzed by 15% SDS/PAGE and stained with Coomassie blue. The recombinant ATP-utilizing chromatin assembling and remodeling factor (ACF) complex (Acf1 and ISWI) was prepared as described (15).
Preparation of Linker Histone/NAP-1 Complexes. Histone H1 was purified from HeLa cells by salt extraction. Linker histones B4, H1A, and HeLa H1 were mixed with NAP-1 in HN buffer (10 mM Hepes·KOH, pH 7.6/1 mM EDTA/10 mM KCl/1 mM DTT/0.25 mM PMSF) at various molar ratios and kept on ice for 1 h. After centrifugation, the supernatants were analyzed by 7.5% native riboflavin-photopolymerized PAGE and 15% SDS/PAGE to confirm complex formation. Linker histone complexes were frozen and stored at –80°C.
Linker Histone Assembly with Reconstituted Dinucleosomes. 5S dinuclesomes were reconstituted and purified as described (16). Dinucleosomes were incubated with linker histones or linker histone/NAP-1 complexes in binding buffer (10 mM Tris·HCl, pH 7.5/0.5 mM EDTA/50 mM KCl/1.0 μg/μl BSA/0.7 mM DTT/3.5 mM Mg-ATP/3 mM MgCl2) at room temperature for 30 min. Samples were mixed with an equal volume of loading solution (6% glycerol) and resolved on 0.7% nucleoprotein agarose gels in 0.5× TBE (44.5 mM Tris·HCl/44.5 mM boric acid/1 mM EDTA, pH 8.3). After electrophoresis, the gels were dried and autoradiographed.
Chromatin Remodeling Assays. End-radiolabeled dinucleosomes (35 fmol) containing linker histones were digested with 10 units of RsaI at 26°C for 1 h in 10 μl of reaction buffer (10 mM Tris·HCl, pH 7.5/3 mM Hepes, pH 7.9/0.4 mM EDTA/45 mM KCl/9 mM NaCl/1 μg/μl BSA/0.6 mM DTT/3 mM Mg-ATP/2.3 mM MgCl2/5.6% glycerol), in the presence or absence of ACF, and purified DNA was separated by 6% nondenaturing PAGE (16). The level of digestion was quantified with a BAS 2500 imaging analyzer (Fuji).
DNase I Footprinting. End-radiolabeled dinucleosomes (180 fmol) with linker histones were digested at 26°C for 1 min in 10 μl of reaction mixture with 0.5 or 1.0 units of DNaseI (Roche Diagnostics), and end-labeled nucleosomes without linker histones were digested at 26°C for 1 min in 10 μl of reaction mixture with 0.25 or 0.5 units of DNase I (Roche Diagnostics). Control naked DNA was digested with 0.125 or 0.25 units of enzyme. Dinucleosomes containing two molar equivalents of linker histones were isolated by 0.7% agarose gel electrophoresis, and purified DNA was analyzed by 6% denaturing PAGE (17). Quantitative analysis was carried out by using the BAS 2500 imaging analyzer (Fuji).
Purification of Linker Histone-Incorporated Dinucleosomes. Mixtures of dinucleosomes and B4/NAP-1 complexes were separated on a 9–21% sucrose gradient containing 10 mM Tris·Cl (pH 7.5), 10 mM KCl, 1 mM EDTA, 0.25 mM PMSF, and 1 mM 2-mercaptoethanol and centrifuged at 50,000 rpm for 3.5 h at 4°C in a Beckman TLS-55 rotor (Beckman). Each fraction was resolved by agarose gel electrophoresis to detect dinucleosomes containing two molar equivalents of linker histone. Fractions containing B4-incorporated dinucleosomes only were stored on ice.
Results and Discussion
A Physiological System for Assembly and Study of Linker Histone Function. Immunofluoresence staining of embryonic cytological sections revealed that histone B4, but not somatic histone H1A, is detectable in the nucleus at the morula stage (Fig. 1A, arrows). After the midblastula transition, histone B4 is gradually replaced by somatic H1 subtypes, and then completely disappears by the late-neurula stage (Fig. 1 A). In studies of the structural and functional effects of specific linker histones on chromatin, an important development was the recent discovery that NAP-1 forms a complex with linker histone B4 and functions as a B4 chaperone in Xenopus eggs (K. Shintomi, M. Iwabuchi, H.S., K.U., T. Kishimoto, and K.O., unpublished work). To test whether NAP-1 is also a somatic histone H1 chaperone, we prepared recombinant histones B4 and H1A and NAP-1 (Fig. 1B). Histones B4 and H1A were incubated with various amounts of NAP-1 under physiological conditions. The resulting linker histone/NAP-1 complexes were analyzed by native PAGE (Fig. 1C). NAP-1 alone migrated as at least two bands, suggesting that it forms oligomers, whereas B4 and H1A alone did not enter the gel, because of their strong positive charges (Fig. 1C, lanes 1, 2, and 7). We found that both B4/NAP-1 and H1A/NAP-1 complexes were formed efficiently when NAP-1 was mixed with the histones at a 1:1 stoichiometry (Fig. 1C, lanes 5 and 10, respectively). A decrease in the molar ratio of NAP-1 induced nonspecific precipitation of linker histones and NAP-1 and reduced the soluble fraction. In contrast, excess amounts of NAP-1 migrated as free NAP-1 (Fig. 1C, arrowheads).
Fig. 1.
Replacement of maternally expressed histone B4 with somatic H1A during early Xenopus embryogenesis and linker histone assembly using NAP-1 as a chaperone. (A) Cytological sections of Xenopus embryos at various developmental stages stained with B4 (red) and H1A (green) antibodies and DAPI. White arrows indicate nuclei. (Scale bar, 0.1 mm.) (B) Purified recombinant B4, H1A, and NAP-1 were analyzed by SDS/PAGE. M, molecular mass markers. (C) B4 and H1A form complexes with NAP-1 at 1:1 stoichiometry (dots). Molar ratios of NAP-1 to linker histones are indicated at top of the native riboflavin-photopolymerized gel. Arrowheads, positions of NAP-1. LDH, lactate dehydrogenase. (D) NAP-1 functions as a chaperone for linker histone H1A. Reconstituted dinucleosomes (4 nM) were incubated with H1A or H1A/NAP-1 complex and analyzed by agarose gel electrophoresis. Free DNA, dinucleosomes, and one and two molecules of H1A per dinucleosome are indicated. (E) The assembled chromatin template does not contain NAP-1. The mixture of dinucleosomes and H1A/NAP-1 complex was separated on a sucrose gradient. Each fraction was analyzed by agarose gel electrophoresis (Upper) and immunoblotted with NAP-1 antibody (Lower).
We next assessed the binding of recombinant histones B4 and H1A to radiolabeled reconstituted 5S dinucleosomes in the absence and presence of NAP-1. The 5S dinucleosome contains two positioned nucleosome cores and represents the minimal unit of model chromatin that contains intact linker DNA (17). NAP-1 improved H1A assembly as effectively as B4 assembly (Fig. 7, which is published as supporting information on the PNAS web site). In the absence of NAP-1, the resulting dinucleosome–H1A complexes migrated as smeared bands and were partially retained at the origin, depending on the amount of H1A provided (Fig. 1D, lanes 2–6). In contrast, when H1A/NAP-1 complexes were added, two sharp bands were observed, corresponding to dinucleosomes bound by one and two H1A molecules, respectively (Fig. 1D, lanes 7–11). Similar results were obtained with native histone H1 purified from HeLa cells (Fig. 2D). NAP-1 alone had no significant effects on dinucleosomes (Fig. 1D, lanes 1 and 12). To determine whether NAP-1 leaves the chromatin template after linker histone deposition, we fractionated the assembled chromatin templates on a sucrose gradient and observed a robust separation between the linker histone-incorporated dinucleosome and the chaperone (Fig. 1E). Collectively, these data indicated that use of a linker histone chaperone, NAP-1, allows physiological assembly of linker histone (either oocyte/embryonic or somatic type) into a model chromatin template.
Fig. 2.
Histone B4 allows chromatin remodeling, whereas somatic H1 represses it. (A) Schematic diagram of the locations of the RsaI sites on end-radiolabeled dinucleosome DNA. Arrows and ovals indicate the 5S RNA gene and the major (black) and minor (gray) positions of nucleosome cores, respectively (17). (B) Dinucleosomes (4 nM) were incubated with B4/NAP-1. B4 binding was checked by agarose gel electrophoresis (lanes 1–4). The remaining sample was analyzed for ACF-dependent chromatin remodeling by RsaI digestion (lanes 5–12). The percent digestion at the 5′ RsaI restriction site is shown at the bottom (lanes 5–12). (C and D) Dinucleosomes (4 nM) were incubated with H1A/NAP-1 (C) or HeLa H1/NAP-1 (D) and analyzed for chromatin remodeling as in B. (E) The relative remodeling activity of dinucleosomes containing stoichiometric amounts of linker histone variants is shown as a bar graph. Remodeling activity = [% digested (+ACF)–% digested (–ACF)]/[100–% digested (–ACF)]. The remodeling activity of dinucleosome templates without linker histones is taken as 100%. Results are the average of three independent experiments, and error bars represent standard deviations.
Chromatin Containing Oocyte/Embryonic Linker Histone Is Functionally Distinct. To reveal the functional differences between maternally expressed B4 and somatic H1, we first compared the effect of each linker histone on ATPase-driven chromatin remodeling. Alterations in histone–DNA and histone–histone contacts in an ATP-dependent manner is a primary mechanism for chromatin-based regulation of genome function, and a large number of conserved complexes function in this manner in the nuclei of all eukaryotes (18). For these experiments, we used the well characterized ACF (19). Recombinant ACF, consisting of Acf1 and the ISWI ATPase, functions by converting chemical energy into the sliding of core histones on DNA (15). We performed a sensitive and quantitative chromatin-remodeling assay that identifies structural transitions in chromatin by measuring accessibility to restriction enzymes (16). End-labeled dinucleosomes were digested with RsaI, which recognizes a site located at the edge of each nucleosome core (Fig. 2 A). In the absence of linker histones, ≈60% of the dinucleosome templates were digested at the 5′ RsaI site, producing 174-bp DNA fragments (Fig. 2 B–D, lanes 5). As expected, addition of ACF led to chromatin remodeling and near-complete digestion of the chromatin template (Fig. 2 B–D, lanes 6). The chromatin remodeling activity of ACF was reduced by incorporating increasing quantities of linker histone H1 into the chromatin (Fig. 2 C and D). Once two molecules of H1A were bound per dinucleosome, chromatin remodeling was essentially abolished (Fig. 2C). We observed a much stronger repression of remodeling by the addition of histone H1 purified from HeLa cells, which contain five somatic H1s and H10 (Fig. 2D).
In striking contrast to somatic histone H1, the oocyte/embryonic linker histone B4 had no effect on ATP-dependent chromatin remodeling even at maximal levels of B4 inclusion into the template (Fig. 2B). The effects of linker histone variants on chromatin remodeling are summarized in Fig. 2E. Notably, these functional differences in linker histone variants were not clear when linker histones were added without NAP-1 (Fig. 8, which is published as supporting information on the PNAS web site). This result provides molecular evidence that a maternally expressed linker histone establishes a much more dynamic chromatin context than does somatic H1.
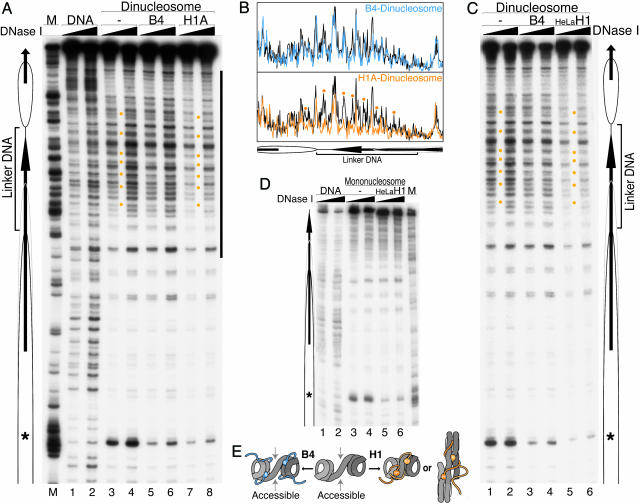
Oocyte/Embryonic Linker Histone Does Not Restrict the Accessibility of Linker DNA. Next, to assess the molecular mechanism that resulted in the differences in ACF-dependent chromatin remodeling, we compared the chromatin structures of dinucleosomes containing each linker histone by DNase I footprinting analysis. Cleavage of well positioned nucleosome cores gave the typical 10-base ladders outside of the linker DNA region (Fig. 3A, lanes 3 and 4). Incorporation of either histone B4 or H1A into dinucleosomes led to a strong reduction in DNase I cleavage at the position of the axis of dyad symmetry of the nucleosome, suggesting that both B4 and H1A bind around the nucleosomal dyad (Fig. 3A, *), as shown (20).
Fig. 3.
Incorporation of histone B4 and H1A leads to different structures in linker DNA of dinucleosomes. (A) End-radiolabeled dinucleosomes incubated alone, or with either histones B4/NAP-1 or H1A/NAP-1, were digested with increasing amounts of DNase I, as indicated. Dinucleosome containing two molar equivalents of linker histones were isolated by agarose gel electrophoresis and analyzed by denaturing PAGE. Arrows and ovals are as in Fig. 2 A. *, axis of dyad symmetry; M, G-specific cleavage marker. The major sites where DNase I cleavage is repressed in the linker DNA region (orange dots). (B) Quantitative scans of DNase I cleavage patterns around the linker DNA region (bar at right in A) from lanes 5 (blue) and 3 (black) (Upper) and lanes 7 (orange) and 3 (black) (Lower). (C and D) DNase I footprinting of chromatin templates incorporating histone H1 purified from HeLa cells. DNase I footprinting analysis of dinucleosomes (C) and mononucleosomes (D) was performed as in A. (E) Model of linker DNA structure of dinucleosomes containing maternal linker histone (blue) or somatic H1 (orange). Globular domains and long C-terminal tails of linker histones are shown by closed circles and ribbons, respectively.
Significant differences, however, were observed in the DNase I footprinting patterns at the linker DNA region. In contrast to the specific protection of linker DNA within H1A-incorporated dinucleosomes (H1A dinucleosomes), little change was observed in the cleavage of the linker DNA after association of histone B4 (Fig. 3A, lanes 3, 5, and 7). Careful comparison of DNase I cleavage patterns by phosphorimager scanning revealed that the cleavage pattern of linker DNA in the B4-incorporated dinucleosomes (B4 dinucleosomes) is almost the same as that in the free dinucleosome (Fig. 3B Upper). In contrast, certain specific sites within the linker DNA were protected from DNase I cleavage in the H1A dinucleosomes (Fig. 3B Lower, dots). The protection of linker DNA was observed more dramatically upon incorporation of HeLa H1 into dinucleosomes (Fig. 3C, lanes 1, 3, and 6), consistent with the much stronger repression of chromatin remodeling compared with histone H1A (Fig. 2 C and D). We also did not observe any structural changes in DNA protruding from the histone octamer after the incorporation of HeLa H1 into mononucleosomes as shown (21), although a reduction in DNase I cleavage at the nucleosomal dyad was seen (Fig. 3D).
These findings suggest that inclusion of histone H1 stabilizes two nucleosome cores, causing structural constraint in the linker DNA (Fig. 3E Right). In contrast, the incorporation of histone B4 does not reduce the accessibility of the linker DNA (Fig. 3E Left), thus allowing ATP-dependent chromatin remodeling. Consistent with this view, members of the ISWI ATPase family have been shown to bind stably to nucleosomes through a protruding free DNA and then change the histone–DNA interactions from the edge of the nucleosome core (22, 23). These histone variants differ drastically in the length and net charge of the C-terminal tail domains (5). The reduced basicity of oocyte/embryonic B4 compared with somatic H1 in the long C-terminal tail might contribute to the reduced interaction between adjacent nucleosomes.
Free NAP-1 Destabilizes Linker Histone Binding and Facilitates Chromatin Dynamics. We further dissected ATP-dependent chromatin remodeling activity in B4 dinucleosomes. A mixture of dinucleosomes and the B4/NAP-1 complex was separated by sucrose gradient centrifugation. The purified B4 dinucleosomes (Fig. 4A) were used for ACF-dependent chromatin remodeling analysis. As shown in Fig. 4B, we observed an enhancement in restriction digestion upon ACF addition, even after removal of B4/NAP-1 complexes and free NAP-1 produced after assembly, indicating that the B4 dinucleosome itself allows chromatin remodeling (lanes 5 and 6). In Xenopus eggs, huge excess amounts of B4/NAP-1 complex are stored and then gradually replaced with somatic H1A after the midblastula transition (9). The addition of B4/NAP-1 complexes to purified B4 dinucleosomes had no effect on chromatin remodeling (Fig. 4B, lanes 7 and 8), whereas addition of an H1A/NAP-1 complex severely repressed remodeling (Fig. 4B, lanes 9 and 10), demonstrating that the alteration in linker histone variants directly causes a functional transition in the chromatin.
Fig. 4.
Intrinsic properties of B4-incorporated dinucleosomes and effects of NAP-1 on chromatin remodeling. (A) Purification of B4 dinucleosomes. Each fraction was resolved by agarose gel electrophoresis (Upper) and Western blot analysis with an antibody against Xenopus NAP-1 (Lower). (B) Purified B4 dinucleosomes (fractions 7–9 in A) allow partial chromatin remodeling, and NAP-1 stimulates chromatin dynamics of the linker histones. Chromatin remodeling activity was analyzed as in Fig. 2, before and after purification of B4 dinucleosomes. Either B4/NAP-1 or H1A/NAP-1 complexes were added to purified B4 dinucleosomes as indicated at the top (lanes 7–10). These data are representative of data obtained from repeated trials of independently conducted experiments.
In addition, we found that the remodeling activity of ACF was significantly and reproducibly reduced in purified B4 dinucleosomes (Fig. 4B, lanes 4 and 6). These data suggest that free NAP-1, which is removed by the sucrose gradient purification, facilitates ATP-dependent chromatin remodeling. We further investigated the effects of NAP-1 on somatic H1 binding to dinucleosomes and subsequent ACF-dependent chromatin remodeling by increasing mole ratios of NAP-1 to H1/NAP-1 complexes. Interestingly, full activation of chromatin remodeling was observed even when histone H1 was only partially dissociated from dinucleosomes (Fig. 5 A and B), suggesting that NAP-1 induces dynamic exchange of linker histones in chromatin. It should be noted that excess amounts of free NAP-1 did not affect the accessibility of nucleosomal DNA in the absence of histone H1 (Fig. 5C, lanes 1 and 2), nor did it affect H2A/H2B binding to dinucleosomes (Fig. 1D, lane 12). Addition of free NAP-1 to purified H1 dinucleosomes abrogated the repressive effects of somatic H1 on ATP-dependent chromatin remodeling in a concentration-dependent manner (Fig. 5C, lanes 5–7). These results illuminate one of the possible pathways activating chromatin remodeling in the somatic nucleus, mediated by regionally concentrated histone chaperones.
Fig. 5.
Excess amounts of NAP-1 disassemble linker histones and abrogate the repressive effects of somatic H1 on chromatin remodeling. (A and B) The mixture of dinculesomes (4 nM) and H1A/NAP-1 (A) or HeLa H1/NAP-1 (B) was incubated with the indicated amounts of free NAP-1. H1 binding was checked (lanes 1–4) and analyzed for chromatin remodeling (lanes 5–12) as in Fig. 2. (C) Purified H1A dinucleosomes (3.5 nM) were incubated with increasing amounts of free NAP-1 and analyzed for chromatin remodeling. These data are representative of data obtained from repeated trials of independently conducted experiments.
Conclusion
We have developed a defined linker histone assembly system using NAP-1 as a chaperone. This system reveals, that in contrast to the effects of somatic H1, the association of oocyte/embryonic linker histone B4 with dinucleosomes has little effect on the accessibility of the linker DNA and allows ATP-dependent chromatin remodeling, even though it reduces accessibility around the nucleosomal dyad axis, as does somatic H1. These distinct properties of linker histone variants may explain why replacement of histone B4 with somatic H1 directly leads to gene repression and the loss of mesodermal competence observed in previous studies (10, 11).
Our results illuminate how maternally expressed linker histones create a fluid template that is permissive for remodeling and thus congruent in its totipotency to the cell that contains it (Fig. 6 Left). Replacement of early embryonic linker histones with somatic H1 establishes a repressive chromatin state that permits the striking chromatin remodeling required for selective gene expression during cellular differentiation (Fig. 6 Upper Right). As shown in Fig. 5, NAP-1 functions not only as an assembler but also as an exchanger to induce chromatin remodeling. Presumably, linker histone chaperones can be recruited by certain regulatory factors, allowing them to dynamically modulate somatic linker histone regionally in the nucleus (Fig. 6 Lower Right). The phosphorylation of somatic linker histones also reactivates chromatin remodeling (24). Chromatin structures containing phosphorylated linker histones might thus resemble those containing histone B4.
Fig. 6.
Proposed model for structural and functional transition of chromatin upon replacement of oocyte/embryonic linker histone with somatic H1 during early embryogenesis.
Mammalian oocytes lack somatic histone H1 but contain a Xenopus B4 homolog, histone H1foo (7). Our biochemical data suggest that the abnormalities observed in cloned animals might be caused by inadequate replacement of somatic linker histones with H1foo (25, 26). Recent studies have demonstrated distinct functional properties for each somatic linker histone variant in vivo (27, 28). The modification of histone H1 also seems to be regulated dynamically (29). The characteristics of the oocyte/embryonic linker histone variant described here highlight the emerging role of early embryonic chromatin remodeling, which promotes the dynamic reorganization of the nucleus.
Supplementary Material
Acknowledgments
We thank Dr. S. Dimitrov (Institut Albert Bonniot, Grenoble, France) for the B4 plasmid, Drs. A. Uchida, C. Masutani, and F. Hanaoka (Osaka University, Osaka) for purified HeLa histone H1 and technical advice, and Drs. G. Almouzni, J. Hayes, and F. Urnov for valuable comments on the manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Hayashi Memorial Foundation for Female Natural Scientists.
Author contributions: H.S. and K.U. designed research; H.S. and H.A. performed research; H.S., K.O., T.I., and K.U. contributed new reagents/analytic tools; H.S. analyzed data; H.S. and K.U. wrote the paper; and S.H. and Y.K. provided financial support.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NAP-1, nucleosome assembly protein-1; ACF, ATP-utilizing chromatin assembling and remodeling factor.
References
- 1.Felsenfeld, G. & Groudine, M. (2003) Nature 421, 448–453. [DOI] [PubMed] [Google Scholar]
- 2.Jenuwein, T. & Allis, C. D. (2001) Science 293, 1074–1080. [DOI] [PubMed] [Google Scholar]
- 3.van Holde, K. E. (1988) Chromatin (Springer, New York).
- 4.Malik, H. S. & Henikoff, S. (2003) Nat. Struct. Biol. 10, 882–891. [DOI] [PubMed] [Google Scholar]
- 5.Wolffe, A. P., Khochbin, S. & Dimitrov, S. (1997) BioEssays 19, 249–255. [DOI] [PubMed] [Google Scholar]
- 6.Wolffe, A. P. (2000) Chromatin: Structure and Function (Academic, San Diego).
- 7.Tanaka, M., Hennebold, J. D., Macfarlane, J. & Adashi, E. Y. (2001) Development (Cambridge, U.K.) 128, 655–664. [DOI] [PubMed] [Google Scholar]
- 8.Smith, R. C., Dworkin-Rastl, E. & Dworkin, M. B. (1988) Genes Dev. 2, 1284–1295. [DOI] [PubMed] [Google Scholar]
- 9.Ohsumi, K. & Katagiri, C. (1991) Dev. Biol. 147, 110–120. [DOI] [PubMed] [Google Scholar]
- 10.Steinbach, O. C., Wolffe, A. P. & Rupp, R. A. (1997) Nature 389, 395–399. [DOI] [PubMed] [Google Scholar]
- 11.Ura, K., Nightingale, K. & Wolffe, A. P. (1996) EMBO J. 15, 4959–4969. [PMC free article] [PubMed] [Google Scholar]
- 12.Ishimi, Y., Hirosumi, J., Sato, W., Sugasawa, K., Yokota, S., Hanaoka, F. & Yamada, M. (1984) Eur. J. Biochem. 142, 431–439. [DOI] [PubMed] [Google Scholar]
- 13.Ito, T., Bulger, M., Kobayashi, R. & Kadonaga, J. T. (1996) Mol. Cell. Biol. 16, 3112–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohsumi, K. & Katagiri, C. (1991) Dev. Biol. 148, 295–305. [DOI] [PubMed] [Google Scholar]
- 15.Ito, T., Levenstein, M. E., Fyodorov, D. V., Kutach, A. K., Kobayashi, R. & Kadonaga, J. T. (1999) Genes Dev. 13, 1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ura, K., Araki, M., Saeki, H., Masutani, C., Ito, T., Iwai, S., Mizukoshi, T., Kaneda, Y. & Hanaoka, F. (2001) EMBO J. 20, 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ura, K., Hayes, J. J. & Wolffe, A. P. (1995) EMBO J. 14, 3752–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker, P. B. & Horz, W. (2002) Annu. Rev. Biochem. 71, 247–273. [DOI] [PubMed] [Google Scholar]
- 19.Ito, T., Bulger, M., Pazin, M. J., Kobayashi, R. & Kadonaga, J. T. (1997) Cell 90, 145–155. [DOI] [PubMed] [Google Scholar]
- 20.Zhou, Y. B., Gerchman, S. E., Ramakrishnan, V., Travers, A. & Muyldermans, S. (1998) Nature 395, 402–405. [DOI] [PubMed] [Google Scholar]
- 21.Hayes, J. J. & Wolffe, A. P. (1993) Proc. Natl. Acad. Sci. USA 90, 6415–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brehm, A., Langst, G., Kehle, J., Clapier, C. R., Imhof, A., Eberharter, A., Muller, J. & Becker, P. B. (2000) EMBO J. 19, 4332–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan, H. Y., He, X., Kingston, R. E. & Narlikar, G. J. (2003) Mol. Cell 11, 1311–1322. [DOI] [PubMed] [Google Scholar]
- 24.Horn, P. J., Carruthers, L. M., Logie, C., Hill, D. A., Solomon, M. J., Wade, P. A., Imbalzano, A. N., Hansen, J. C. & Peterson, C. L. (2002) Nat. Struct. Biol. 9, 263–267. [DOI] [PubMed] [Google Scholar]
- 25.Gurdon, J. B., Byrne, J. A. & Simonsson, S. (2003) Proc. Natl. Acad. Sci. USA 100, 11819–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teranishi, T., Tanaka, M., Kimoto, S., Ono, Y., Miyakoshi, K., Kono, T. & Yoshimura, Y. (2004) Dev. Biol. 266, 76–86. [DOI] [PubMed] [Google Scholar]
- 27.Konishi, A., Shimizu, S., Hirota, J., Takao, T., Fan, Y., Matsuoka, Y., Zhang, L., Yoneda, Y., Fujii, Y., Skoultchi, A. I. & Tsujimoto, Y. (2003) Cell 114, 673–688. [DOI] [PubMed] [Google Scholar]
- 28.Lee, H., Habas, R. & Abate-Shen, C. (2004) Science 304, 1675–1678. [DOI] [PubMed] [Google Scholar]
- 29.Vaquero, A., Scher, M., Lee, D., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. (2004) Mol. Cell 16, 93–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.