Abstract
Purpose
We have previously reported on our technique to deliver intensity-modulated radiotherapy (IMRT) to the entire pleura while attempting to spare the lung in patients with malignant pleural mesothelioma (MPM). Herein, we report a detailed pattern-of-failure analysis in patients with MPM who were unresectable or underwent pleurectomy/decortications (P/D), uniformly treated with hemithoracic pleural IMRT.
Methods
Sixty-seven patients with MPM were treated with definitive or adjuvant hemithoracic pleural IMRT between 11/2004 and 5/2013. Pretreatment imaging, treatment plans, and post-treatment imaging were retrospectively reviewed to determine failure location(s). Failures were categorized as in-field (within the 90% isodose line), marginal (<90% and ≥50% isodose lines), out-of-field (outside the 50% isodose line), or distant.
Results
The median follow-up was 24 months from diagnosis and the median time to in-field local failure from the end of radiotherapy was 10 months. Forty-three in-field local failures (64%) were found with a 1- and 2-year actuarial failure rate of 56% and 74%, respectively. For patients who underwent P/D versus those who received a partial pleurectomy or were deemed unresectable, the median time to in-field local failure was 14 months versus 6 months, with 1- and 2-year actuarial in-field local failure rates of 43% and 60% versus 66% and 83%, respectively (p=0.03). There were 13 marginal failures (19%). Five of the marginal failures (38%) were located within the costomediastinal recess. Marginal failures decreased with increasing institutional experience (P = .04). Twenty-five patients (37%) had out-of-field failures. Distant failures occurred in 32 patients (48%).
Conclusion
After hemithoracic pleural IMRT, local failure remains the dominant form of failure pattern. Patients treated with adjuvant hemithoracic pleural IMRT after P/D experience a significantly longer time to local and distant failure than patients treated with definitive pleural IMRT. Increasing experience and improvement in target delineation minimize the incidence of avoidable marginal failures.
Keywords: Mesothelioma, Intensity-Modulated Radiotherapy (IMRT), Patterns of Failure
Introduction
Patients with malignant pleural mesothelioma (MPM) appear to have optimal outcomes with multimodality therapy (1). Historically, extrapleural pneumonectomy (EPP) has been the standard surgical procedure for MPM. A Phase II study performed at our institution demonstrated that adjuvant radiation therapy after EPP with conventional techniques could decrease locoregional recurrences down to 13% (2). However, EPP is associated with significant morbidity and mortality risks. Pleurectomy/decortication (P/D) is an alternative surgical approach that involves an organ-preservation approach and leaves uninvolved ipsilateral lung intact. Comparisons of overall survival rates from P/D to EPP have demonstrated reduced rates of death for patients treated with P/D (3, 4). This is theorized to be secondary to the decreased operative mortality, and the improved performance status postoperatively for P/D patients, which allows them to receive subsequent adjuvant therapies.
The use of P/D is increasing, which poses a difficult problem for delivering adjuvant radiation therapy. P/D is, by definition, a less complete resection than an EPP, and presumably carries a higher risk for locoregional recurrence. Therefore, additional local treatment is critical. We have previously reported using a matched photon-electron conventional hemithoracic radiation therapy technique, but found disappointing local control and excess toxicity (5). We, therefore, developed a novel, highly conformal IMRT technique to treat the ipsilateral pleura with maximal sparing of the ipsilateral and contralateral lung parenchyma (6). Our initial experience showed a 20% risk of grade ≥3 radiation pneumonitis, a reasonable rate in this high-risk patient population (7).
Unlike conventional radiotherapy, IMRT is more dependent on the treatment-planning team and the physician’s understanding of anatomy, patterns of spread, and risk for subclinical disease. It is therefore associated with significant inter-person variability (8). Unique patterns of failure are well documented with the implementation of IMRT in other organ sites, as sparing of involved normal tissue can result in unexpected marginal failures (9, 10). This is particularly true for a highly complex target area such as the entire hemithoracic pleura, extending from the lung apices to the diaphragmatic crura. For this reason, we report the first detailed and largest pattern-of-failure analysis for MPM patients with two intact lungs treated with IMRT.
Methods
An Institutional Review Board waiver was approved prior to conducting the present study. Patients treated between 11/2004 and 5/2013 were included. Eligibility criteria were as follows: patients were required to have both lungs intact prior to the initiation of radiotherapy (i.e., patients treated with EPP were excluded), to have been treated with IMRT as either definitive or adjuvant therapy, to have had a pretreatment fluorodeoxyglucose positron-emission tomography scan, and to not have been enrolled in our ongoing prospective multi-institutional Phase II protocol for MPM. A total of 67 patients with pathologically proven MPM met these eligibility criteria and were included in the current analysis.
The delivery of IMRT at our institution has previously been described.(11) Appendix A outlines our institution’s commonly used dose constraints for IMRT for patients with two intact lungs. Briefly, patients were immobilized prior to computed tomography (CT) simulation using a customized α-cradle in the supine position with their arms overhead. All patients had a pre-treatment positron-emission tomography-CT scan to aid with target delineation, and the gross tumor volume was modified to include any areas of gross fluorodeoxyglucose-avid disease. After four-dimensional (4D)-CT simulation became available at our institution, corrections for respiratory motion using a 4D-CT acquired at time of simulation were made in 46 patients (69%) to further improve target coverage. The initial clinical target volume (CTV) was defined as the involved pleura and/or postoperative site(s) and involved lymph node stations. The CTV generally spanned from the thoracic inlet superiorly until the insertion of diaphragm (approximately L2 vertebral body). A planning target volume (PTV) was generated using a 6 mm internal and 10 mm outer margin. This was modified to cover the entire thickness of the chest wall of the involved ipsilateral hemithorax where necessary. Treatments were delivered with 6-MV photons using the sliding window technique with Varian linear accelerators. Tissue inhomogeneity corrections were utilized in all patientshttp://www.sciencedirect.com/science/article/pii/S0360301611032251-bib10. The planning goal was to deliver the prescription dose to ≥95% of the PTV while respecting normal tissue constraints. For all patients, the goal prescription dose to the PTV was 50.4 Gy in 1.8 Gy per fraction, but was adapted to respect normal tissue constraints when necessary.
Based on the center of the recurrent tumor, treatment failures were categorized as in-field local failures (within the 90% isodose line), marginal failures (<90% and ≥50% isodose line) and out-of-field failures (outside the 50% isodose line). This categorization follows similar recently published guidelines from the International Thymic Malignancy Interest Group classification for describing failure patterns after radiation therapy for thymic malignancies, which also commonly fail in the pleural space (12, 13). In-field failures were further sub-classified to differentiate previous sites of gross disease and new sites within the pleural target volume. Failure location was determined based on a combined review of the post-treatment follow-up imaging (most commonly CT), and was correlated to the treatment plan and pretreatment imaging. Locoregional failure included in-field and marginal, and out-of-field ipsilateral thoracic failures. Distant failure was defined as hematogenous spread to another organ site (i.e., lung parenchyma, bone, peritoneum).
Subgroup analyses were based upon extent of surgical resection. Patients with complete P/D or an extended P/D were compared with those who were unresectable or were able to undergo only a partial pleurectomy. Actuarial likelihood estimates for failure outcomes were estimated using the Kaplan-Meier method with log-rank statistics. Treatment failure times were defined as time from the end of radiation treatment to treatment failure. Survival estimates were made from time of diagnosis until death or last follow-up. Two-sided P values of ≤ .05 were considered statistically significant. Statistical analysis was performed using SPSS version 21 (SPSS, Inc, USA).
Results
Patient and treatment characteristics
The median follow-up was 24 months from diagnosis. Baseline patient and treatment characteristics are listed in Table 1. At the time of diagnosis, most patients (85%) had clinical T1–3 disease, and no radiographic evidence of lymph node involvement (73%). Neoadjuvant chemotherapy was given to 51 (76%) patients, with most receiving cisplatin and pemetrexed. Surgical resection was attempted in 57 (85%) patients; however, at time of surgery, 15 of these patients were deemed unresectable. Thus, 25 patients (37%) in total did not undergo resection and, instead, underwent definitive hemithoracic pleural IMRT. Fourteen patients (21%) underwent only a partial P/D with gross residual disease. Patients were commonly upstaged during surgical exploration, with 26 (39%) patients having pT4 disease, compared with 10 (15%) with cT4 disease prior to surgery. IMRT was given to all patients with a median dose of 46.8 Gy (IQR 45.0–50.4 Gy). Two (3%) patients received an integrated boost to gross disease.
Table 1.
Baseline and patient characteristics
| N | % | |
|---|---|---|
| Gender | ||
| Male | 51 | 76 |
| Female | 16 | 24 |
| Age (years) | ||
| Median | 68 | — |
| Interquartile range | 62–72 | — |
| Race | ||
| White | 58 | 87 |
| Other | 9 | 13 |
| Histology | ||
| Epithelioid | 52 | 78 |
| Sarcomatoid or biphasic | 15 | 22 |
| Laterality | ||
| Right | 40 | 60 |
| Left | 27 | 40 |
| Clinical Stage | ||
| I | 6 | 9 |
| II | 26 | 39 |
| III | 22 | 33 |
| IV | 13 | 19 |
| Clinical T-stage | ||
| T1 | 7 | 10 |
| T2 | 37 | 55 |
| T3 | 13 | 19 |
| T4 | 10 | 15 |
| Clinical N-stage | ||
| N0 | 49 | 73 |
| N1 | 1 | 1 |
| N2 | 16 | 24 |
| N3 | 1 | 1 |
| Surgery | ||
| Extended pleurectomy/decortication | 2 | 3 |
| Pleurectomy/decortication | 26 | 39 |
| Partial pleurectomy | 14 | 21 |
| Unresectable at time of surgery | 15 | 22 |
| Surgery not attempted | 10 | 15 |
| Pathologic stage | (n=57) | |
| 0 | 2 | 3 |
| I | 2 | 3 |
| II | 12 | 18 |
| III | 14 | 21 |
| IV | 27 | 40 |
| Pathologic T-stage | (n=57) | |
| Tx | 2 | 3 |
| T1 | 3 | 4 |
| T2 | 17 | 25 |
| T3 | 9 | 13 |
| T4 | 26 | 39 |
| Pathologic N-stage | (n=57) | |
| Nx | 3 | 4 |
| N0 | 37 | 55 |
| N1 | 0 | 0 |
| N2 | 16 | 24 |
| N3 | 1 | 1 |
| Chemotherapy | ||
| Neoadjuvant | 51 | 76 |
| Adjuvant | 9 | 13 |
| None | 7 | 10 |
| RT Dose (Gy) | ||
| Median | 46.8 | - |
| Interquartile range | 45.0–50.40 | - |
Locoregional failure
In-field failures
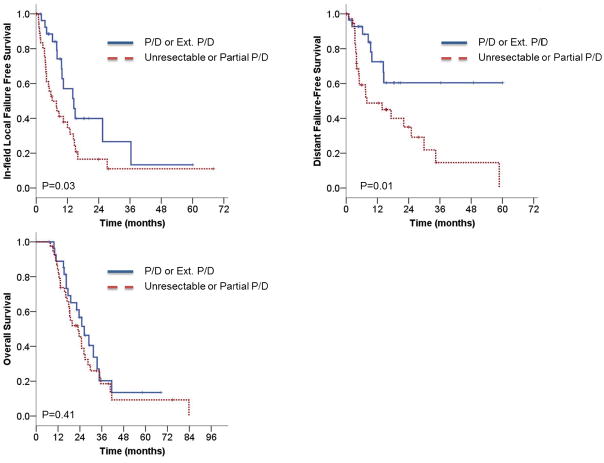
Forty-three patients (64%) experienced in-field local failures (Table 2). The median time to in-field local failure was 10 months, and the 1- and 2-year actuarial failure rates were 56% and 74%, respectively. Thirty-two (74%) of these patients experienced a local failure at sites of previous gross disease, most commonly in the pleural space. Nine (13%) patients failed within the radiation field. Three patients had failures within treated lymph nodes: two patients failed in subcarinal lymph nodes and one failed in an ipsilateral level 4 lymph node. For patients who underwent P/D or extended P/D versus those who received a partial pleurectomy or were deemed unresectable, the median time to in-field local failure was 14 months versus 6 months, with 1- and 2-year actuarial in-field local failure rates of 43% and 60% versus 66% and 83%, respectively (log-rank P = .03, Fig. 1a). In-field failures did not independently vary by year of treatment (P = .83).
Table 2.
Failure types and patterns
| All Patients | Surgery | Unresectable | ||||
|---|---|---|---|---|---|---|
| (n=67) | (%) | (n=42) | (%) | (n=25) | (%) | |
| Failure Type | ||||||
| Locoregional failures | ||||||
| Total* | 44 | 66 | 25 | 60 | 19 | 76 |
| In-field | 43 | 64 | 24 | 57 | 19 | 76 |
| Previous involved site | 32 | 48 | 14 | 33 | 18 | 72 |
| New site | 11 | 16 | 10 | 24 | 1 | 4 |
| Marginal | 13 | 19 | 5 | 12 | 8 | 32 |
| Out-of-field | 25 | 37 | 13 | 31 | 12 | 48 |
| Fissure | 11 | 16 | 6 | 14 | 5 | 20 |
| Distant | 32 | 48 | 18 | 43 | 14 | 56 |
| Failure Patterns | ||||||
| Local only | 9 | 13 | 6 | 14 | 3 | 12 |
| Local and regional | 8 | 12 | 6 | 14 | 2 | 8 |
| Local and distant | 10 | 15 | 6 | 14 | 4 | 16 |
| Local, regional, and distant | 16 | 24 | 6 | 14 | 10 | 40 |
| Regional only | 1 | 1 | 1 | 2 | 0 | 0 |
| Regional and distant | 0 | 0 | 0 | 0 | 0 | 0 |
| Distant only | 6 | 9 | 6 | 14 | 0 | 0 |
Fig. 1.
Unadjusted Kaplan-Meier curves comparing patients who received P/D or extended P/D versus unresectable patients or those who had a partial P/D. A) In-field local failure-free survival. B) Distant failure-free survival. C) Overall survival.
Marginal failures
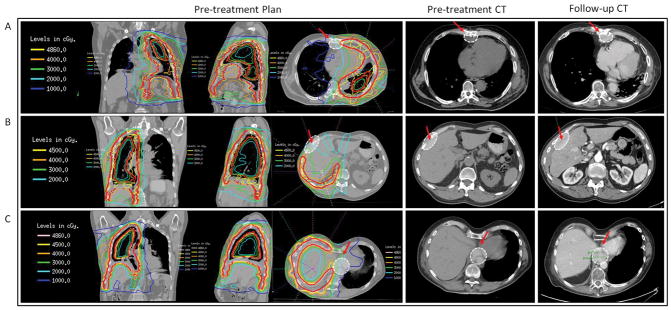
Thirteen patients (19%) experienced a marginal failure (Table 2). All patients who had a marginal failure had an in-field failure as well, so we did not observe any isolated marginal failures. It was determined that six of the marginal failures could have been included in the treatment field and perhaps avoided. Five of the marginal failures were located within the costomediastinal recess, while two marginal failures occurred in the costodiaphragmatic recess (a detailed list of the location of all margin failures is shown in Table 3). Three marginal failures had components of disease recurrence within a fissure. Three representative examples of marginal failures are shown in Fig. 2 (corresponding to Patients 1–3 in Table 3). When comparing patients treated from 2004 to 2008 versus 2009 to 2013 (both with equivalent median follow-up), nine of the 13 marginal failures occurred in patients treated in the first 5 years (P = .04).
Table 3.
Details of Marginal Failures
| Patient | Marginal Failure Site | Contouring Miss |
|---|---|---|
| 1 | Anterior diaphragmatic LN (costomediastinal recess) | No |
| 2 | Costodiaphragmatic recess | Yes |
| 3 | Paraesophageal/retrocrural (costodiaphragmatic recess) | Yes |
| 4 | Subcarinal LN | No |
| 5 | Anterior Diaphragmatic LN (costomediastinal recess) | Yes |
| 6 | Anterior diaphragmatic LN (costomediastinal recess) | Yes |
| 7 | Fissure | No |
| 8 | Subcarinal LN | No |
| 9 | Supraclavicular LN | No |
| 10 | Anterior diaphragmatic LN (costomediastinal recess) | Yes |
| 11 | Precarinal LN | No |
| 12 | Paraspinal | No |
| 13 | Adjacent to rib | Yes |
Abbreviations: LN = lymph node.
Fig. 2.
Example of three marginal failures, demonstrated each with a pretreatment planning CT in the coronal, sagittal, and axial planes with appropriate isodose lines, a baseline CT, and follow-up CT demonstrating the marginal failure. A) 62-year-old male treated with P/D with an R1 resection, no chemotherapy secondary to medical comorbidities, and 48.6 Gy to the left side for pathological stage III, p3N0M0 malignant pleural mesothelioma. He had a marginal failure in an anterior diaphragmatic lymph node. B) 70-year-old male treated with neoadjuvant cisplatin and pemetrexed, was deemed surgically inoperable due to cT4 disease, and had definitive radiotherapy to 45.0 Gy to the right side. He had a marginal failure along the lateral right pleura adjacent to the liver. C) 56-year-old male treated with neoadjuvant cisplatin and pemetrexed, P/D with an R1 resection, and 48.6 Gy for pathological stage II, pT2N0M0 MPM. He had a marginal failure at the paraesophageal/retrocrural interface. White dashed oval denotes site of failure, indicated by red arrow for clarity. Red solid line refers to the contoured PTV.
Out-of-field failure
Twenty-five patients (37%) had out-of-field failures (Table 2). Of these patients, 21 experienced a failure in the mediastinal lymph nodes. However, only one patient had an isolated regional failure. For all other patients with out-of-field failures, none of them were the first site of failure. Two patients experienced a pericardial effusion deemed to be malignant in nature, one patient failed within the ipsilateral right major fissure, and one patient failed in the ipsilateral lung with a malignant pleural effusion. The majority of patients (n=34; 51%) had widespread local, regional, and distant failures.
Fissure and incisional failures
Since the fissures are spared with our pleural IMRT technique, it was of particular concern whether a higher failure rate would be seen in those areas. Failure within the fissure could be defined as either in-field, marginal, or out-of-field depending on the proximity to the PTV. Fissural failures occurred in 11 patients (16%). Three of the fissural failures were marginal failures, while the remaining 8 patients were in-field fissural failures. One patient (1.5%) had a fissure-only failure, while the remaining 10 fissure failures all occurred in conjunction with failures within the pleura, regional, and/or distant sites.
Routine “boost” to the incision site is not standard at our institution, and only one patient (1.5%) experienced an incisional failure in the chest wall. The site of failure in this patient occurred within the included PTV, and this failure was classified as an in-field local failure.
Distant failure
Distant failure occurred in 32 patients (48%), with a median time to distant metastases of 17 months, and a 1- and 2-year actuarial distant failure rate of 40% and 55%, respectively. Ten patients failed below the diaphragm only, 9 patients failed within the lung parenchyma above the diaphragm only (four within the contralateral lung, and five in bilateral lung parenchyma), and 13 patients had distant failures above and below the diaphragm (Supplementary Table 1). Six (9%) patients relapsed in distant sites without local or regional recurrences. For patients who underwent P/D or extended P/D versus those who received a partial pleurectomy or were deemed unresectable, the median time to distant failure was not reached versus 8 months, with 1- and 2-year actuarial distant failure rates of 28% and 40% versus 51% and 65%, respectively (log-rank P = .01, Fig. 1b).
Overall survival
The median survival of the entire cohort was 24 months from the time of diagnosis, with 1- and 2-year actuarial OS rates of 85% and 50%, respectively. For patients who underwent P/D or extended P/D vs. partial pleurectomy or unresectable disease, the median OS was 26 months vs. 22 months, respectively. Similarly, the 1- and 2-year actuarial OS rates were 89% and 57% vs. 82% and 42% (log-rank 0.41, Fig. 1c).
Discussion
Herein we report the largest pattern-of-failure analysis in a cohort of MPM patients with two intact lungs uniformly treated with hemithoracic pleural IMRT. Pattern-of-failure analyses provide valuable information to optimize target delineation and planning of radiation treatments to maximize local control and minimize marginal failures that could have been obviated with minor therapeutic alterations (14–17). This is especially relevant when developing a new, complex technique such as hemithoracic pleural IMRT. Yajnik et al in 2003 reported a detailed pattern-of-failure analysis for patients treated with EPP followed by conventional matched photon-electron radiotherapy (18). The authors demonstrated that of the 35 patients in the series, 30 had failures along the diaphragmatic insertion from T12-L3. Importantly, 20 of these failures were at the level of L2. Notably the inferior field edge was demarcated at the L2 vertebral body, thereby receiving approximately 50% of the prescribed dose of 54 Gy, and thus would be defined as marginal failures. Data such as this was of critical importance for understanding the patterns of spread of this disease to adapt IMRT appropriately.
To the best of our knowledge, only one other institution has previously reported failure rates in a small series (n=20) with the use of IMRT post-P/D (19). Minatel et al reported a 20% local failure and a 35% distant failure rate, with remarkably low toxicity rates. However, the authors did not report detailed failure patterns in the context of their IMRT dose distribution. Our reported local failure rate of 74% at 2 years may appear to be discordant with the published local failures rate from Minatel et al of 20% (19). It should be noted that 39 patients (58%) in our series had gross residual disease after either a partial pleurectomy or not undergoing resection at all, where 100% of patients had surgery in the Minatel series. However, even patients who underwent P/D in our series had a 2-year local failure rate of 60%. The significant reduction in both local and distant failures with gross macroscopic resection is represented well in our series. For patients who undergo incomplete surgery and postoperative radiotherapy to 45–50 Gy, these radiation doses are clearly insufficient to provide long-term control of gross MPM.
We aim to further improve locoregional control rates by using upfront P/D, followed by adjuvant chemotherapy and dose-painting IMRT with an integrated boost of up to 60 Gy to grossly visible disease. This level of dose escalation is more consistent with Minatel et al, who treated patients up to 60 Gy and reported excellent long-term results. For unresectable MPM, improved patient selection based on novel radiographic or biomarkers is clearly needed to identify which patients may have long-term control with hemithoracic pleural IMRT.
A select number of series have reported failure patterns for patients undergoing pleurectomy (Supplementary Table 2). Three series primarily utilized P/D: Both Holsti et al and Gupta et al utilized conventional post-operative radiotherapy, and Minatel et al used IMRT (15, 19, 20). The definitions of failures vary by study, and no clear consensus appears to exist. We recommend using the isodose lines to determine whether a failure is located in-field, marginal, out-of-field, or distant. This would allow fair comparison of failure rates across series and more accurately determine if there are surgical or radiotherapeutic approaches that may prevent specific anatomic failure patterns.
As noted in other complex disease sites such as cancers of the head and neck that adopted IMRT to improve target coverage conformality and reduce toxicity, early adopters found unexpected marginal failures (9, 10). Reporting these failures was pivotal in the evolution of IMRT target delineation. We noted in the present series that, over time, marginal failure rates decreased significantly (P = .04). This demonstrates the impact of increasing experience and the ability to learn from recurring marginal failure sites over time, such as the costodiaphragmatic recesses, and redefine CTV definitions. Based on the present series we identified specific areas that appear at higher risk for contouring errors. These are principally located at the costophrenic, costodiaphragmatic, and cardiophrenic angles, and particular attention should be made for complete coverage. We do not recommend inclusion of fissures based on our data, as isolated fissural failures appear rare, and coverage of the fissures would negate the ability to spare the ipsilateral lung parenchyma. Based on the few incisional site failures, we do not recommend dedicated coverage to surgical incisions/drain sites, nor do we use bolus over the surgical scar to prevent excess skin toxicity. Lastly, we strongly recommend motion management corrections using a 4D-CT.
Our primary goal is transparency in reporting our failure rates, patterns, and locations to make other clinicians aware of where failures occur with the use of IMRT. However, limitations of our study are present. There is no current standard approach to report detailed local failures in relation to isodose lines, and the definitions we chose have intrinsic strengths and weaknesses. Therefore, comparisons with historical series should be interpreted cautiously. Recurrent tumors at the margins of the radiation field were often large, and peripheral aspects of the recurrence crossed into the higher isodose regions. In such cases, a judgment of the origin of the failure was subjective in nature. We used the center of the recurrent mass to determine categorization of a given mass as in-field, marginal, or out-of-field, but we erred on the conservative side to maximize the benefit for improving our target delineation based on this study.
Conclusion
After hemithoracic pleural IMRT, most local failures occur in sites of previous gross disease. Thus, macroscopic complete resection remains critical. Increasing experience and improvements in target delineation combined with dose escalation will likely decrease the incidence of in-field and marginal failure rates with this new technique. Standardization of reporting failure types in MPM is necessary.
Supplementary Material
Summary.
We have previously reported on our technique to deliver intensity-modulated radiotherapy (IMRT) to the entire pleura while attempting to spare the lung in patients with malignant pleural mesothelioma (MPM). The patterns of failure resulting from this new complex radiation technique are largely unknown. Herein, we report a detailed pattern-of-failure analysis in a cohort of patients with MPM who were unresectable or underwent pleurectomy/decortication, uniformly treated with hemithoracic pleural IMRT at a single institution.
Acknowledgments
This work was presented at the IMIG 2012 meeting, but has not been submitted for publication elsewhere.
We thank Lawrence A. Herman for editorial review of the manuscript.
Appendix A. Representative IMRT dose constraints
| Structure | Constraint | |
|---|---|---|
| PTV | D95% | ≥94% |
| PTV | V95% | ≥94% |
| PTV | D05% | ≤115% |
| Lung | V20Gy to contralateral lung | 7% of contralateral volume |
| V5Gy to contralateral lung | 25% of contralateral lung volume | |
| Mean contralateral lung dose | 8 Gy | |
| Combined lungs | V20Gy | 37–40% |
| NTCP | 25% | |
| Mean dose | 20 Gy | |
| Cord | Max point dose | 50 Gy |
| Heart | V30Gy | <50% of total volume |
| Esophagus | V60Gy | 17% |
| Mean dose | 34 Gy | |
| Bowel | Max point dose | 55 Gy |
| D05% | 45Gy | |
| Liver | V30Gy | 50% of volume |
| Stomach | Mean dose | 30 Gy |
Footnotes
Conflict of interest: none
References
- 1.Zauderer MG, Krug LM. Pleurectomy/decortication, chemotherapy and intensity modulated radiation therapy for malignant pleural mesothelioma: rationale for multimodality therapy incorporating lung-sparing surgery. Ann Cardiothorac Surg. 2012;1:487–490. doi: 10.3978/j.issn.2225-319X.2012.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant highdose hemithoracic radiation for malignant pleural mesothelioma. Journal of Thoracic and Cardiovascular Surgery. 2001;122:788–795. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 3.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626. 626 e621–623. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 4.Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. The Lancet Oncology. 2011;12:763–772. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta V, Mychalczak B, Krug L, et al. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. International Journal of Radiation Oncology Biology Physics. 2005;63:1045–1052. doi: 10.1016/j.ijrobp.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Rimner A, Rosenzweig KE. Novel radiation therapy approaches in malignant pleural mesothelioma. Ann Cardiothorac Surg. 2012;1:457–461. doi: 10.3978/j.issn.2225-319X.2012.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. International Journal of Radiation Oncology Biology Physics. 2012;83:1278–1283. doi: 10.1016/j.ijrobp.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van de Steene J, Linthout N, de Mey J, et al. Definition of gross tumor volume in lung cancer: inter-observer variability. Radiother Oncol. 2002;62:37–49. doi: 10.1016/s0167-8140(01)00453-4. [DOI] [PubMed] [Google Scholar]
- 9.Damast S, Wolden S, Lee N. Marginal recurrences after selective targeting with intensity-modulated radiotherapy for oral tongue cancer. Head Neck. 2012;34:900–906. doi: 10.1002/hed.21677. [DOI] [PubMed] [Google Scholar]
- 10.Schoenfeld GO, Amdur RJ, Morris CG, et al. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;71:377–385. doi: 10.1016/j.ijrobp.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2012;83:1278–1283. doi: 10.1016/j.ijrobp.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez D, Komaki R, Yu J, Ikushima H, Bezjak A. Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol. 2011;6:S1743–1748. doi: 10.1097/JTO.0b013e31821ea60c. [DOI] [PubMed] [Google Scholar]
- 13.Rimner A, Gomez DR, Wu AJ, et al. Failure Patterns Relative to Radiation Treatment Fields for Stage II–IV Thymoma. J Thorac Oncol. 2014;9:403–409. doi: 10.1097/JTO.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 14.Allen AM, Den R, Wong JS, et al. Influence of radiotherapy technique and dose on patterns of failure for mesothelioma patients after extrapleural pneumonectomy. Int J Radiat Oncol Biol Phys. 2007;68:1366–1374. doi: 10.1016/j.ijrobp.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Holsti LR, Pyrhonen S, Kajanti M, et al. Altered fractionation of hemithorax irradiation for pleural mesothelioma and failure patterns after treatment. Acta Oncol. 1997;36:397–405. doi: 10.3109/02841869709001287. [DOI] [PubMed] [Google Scholar]
- 16.Gomez DR, Hong DS, Allen PK, et al. Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol. 2013;8:238–245. doi: 10.1097/JTO.0b013e31827740f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta V, Krug LM, Laser B, et al. Patterns of local and nodal failure in malignant pleural mesothelioma after extrapleural pneumonectomy and photon-electron radiotherapy. J Thorac Oncol. 2009;4:746–750. doi: 10.1097/JTO.0b013e3181a5292c. [DOI] [PubMed] [Google Scholar]
- 18.Yajnik S, Rosenzweig KE, Mychalczak B, et al. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2003;56:1319–1326. doi: 10.1016/s0360-3016(03)00287-6. [DOI] [PubMed] [Google Scholar]
- 19.Minatel E, Trovo M, Polesel J, et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer. 2013 doi: 10.1016/j.lungcan.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Gupta V, Mychalczak B, Krug L, et al. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2005;63:1045–1052. doi: 10.1016/j.ijrobp.2005.03.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.