SUMMARY
Asymptomatic infections often proceed undetected, yet can still prime the host to be sensitive to secondary environmental stress. While the mechanisms underlying disease caused by asymptomatic infections are unknown, it is believed that productive pathogen replication is required. We report that the environmental stress of carbon dioxide (CO2) anesthesia converts an asymptomatic rhabdovirus infection in Drosophila to one that is lethal. This lethality results from a pool of infectious virus in glial cells and is regulated by the antiviral RNAi pathway of the host. CO2 sensitivity is caused by the fusogenic activity of the viral glycoprotein, which results in fusion of neurons and glia. Expression of the viral glycoprotein, but not a fusion defective mutant, is sufficient to cause CO2 sensitivity, which can occur even in the absence of productive viral replication. These findings highlight how viral proteins, independent of pathogen replication, may predispose hosts to life-threatening environmental stress.
Graphical Abstract

Asymptomatic infections can severely impact host health. Chow et al. delineate a deadly outcome from asymptomatic VSV infection. CO2, a standard anesthetic in fly laboratories, induces VSV glycoprotein (G) to mediate cell-cell fusion in the nervous system. The results demonstrate that non-pathological proteins can be dangerous in the right environment.
INTRODUCTION
Pathogens kill because they cause cellular stresses that exceed what is viable. Sometimes, as in asymptomatic infections, no changes in host health are observable. However, considerable effort by the host may be required to maintain this asymptomatic state, suggesting that stresses on the host may be difficult to detect. These changes in cellular homeostasis are not without significance, as asymptomatic infections can render the host sensitive to a variety of secondary environmental stresses. Secondary stresses can come in the form of a subsequent microbial encounter, such as bacterial infections that lead to the death of influenza virus infected patients (Rynda-Apple et al., 2015). While neither of these infections alone is lethal, influenza virus alters lung homeostasis such that subsequent bacterial encounters incite massive tissue damage and death. Non-infectious stresses (e.g. environmental chemicals) can also provoke disease symptoms in an infected individual. An example of this principle can be found from studies of subclinical hepatitis C virus infections, which result in life-threatening liver pathology when combined with alcohol consumption (Novo-Veleiro et al., 2016). While we have an increasing understanding of how virulent pathogens elicit homeostatic disruptions that cause disease directly, our knowledge of how asymptomatic infections poise a host for sensitivities to secondary stress is limited.
Despite our lack of understanding of how infections provoke sensitivity to secondary stress, it is widely assumed that these sensitivities are the result of active pathogen replication in a specific tissue (e.g. influenza infection). Thus, under conditions where pathogen replication is limited, there should be no sensitivity to a secondary stress. However, there are reports that viral infections cause sensitivities to the environment, even under conditions of minimal pathogen replication, such as during Epstein-Barr virus latency (Takeda et al., 2014). These observations suggest that stress sensitivity may not necessarily be the consequence of tissue damage associated with pathogen replication, but rather may result from an activity encoded by a viral protein, even in the absence of replication. Little is known of how viral proteins, independent of pathogen replication, can cause disease. In this study, we sought to address how viral infection influences sensitivities to the environment.
To understand how viral infections could provoke sensitivity to a secondary stress, we sought an experimental system that would allow us to dissect mechanisms within the host and pathogen. The fruit fly Drosophila melanogaster is an ideal organism to study due to its genetic tractability and its ability to be infected by a variety of human pathogens (Schneider et al., 2007). This host has been used extensively to identify host pathways that determine the outcome of virulent infections (Buchon et al., 2014), yet the use of the fruit fly to study asymptomatic infections is more limited. The rhabdovirus sigma naturally infects fruit flies, yet infected animals exhibit no apparent symptoms of disease. Studies dating to the 1930s reported that sigma virus causes flies to become sensitive to carbon dioxide (CO2) anesthesia (L’Heritier and Teissier, 1937, 1938a, 1938b), in that infected flies die upon exposure to CO2. Sigma virus infection therefore alters some aspects of fly physiology that renders these organisms susceptible to an otherwise non-lethal stress of CO2 anesthesia. Using this experimental model, we sought to identify mechanisms underlying infection-associated disease susceptibility.
RESULTS
Vesicular stomatitis virus infection elicits a rapid sensitivity to CO2 anesthesia
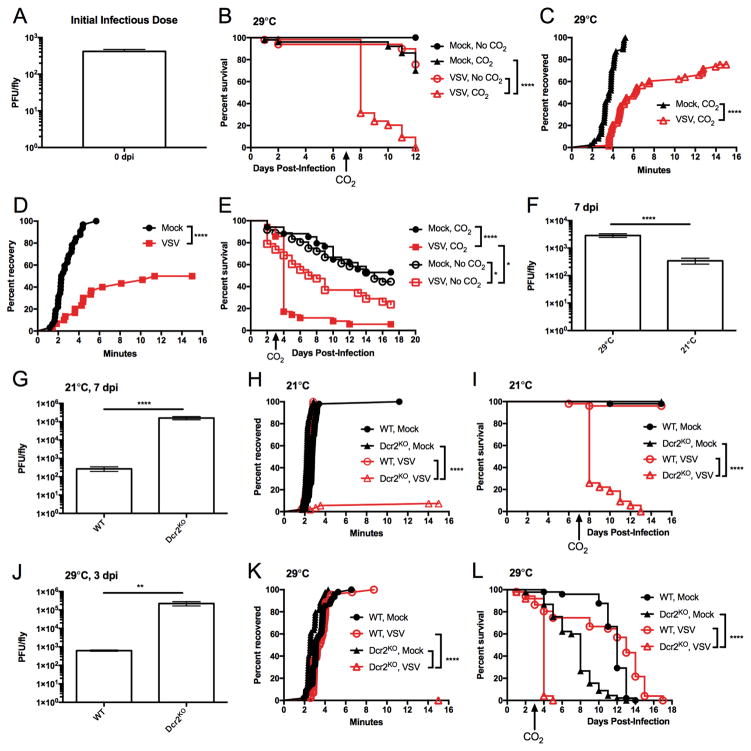
Vesicular stomatitis virus (VSV) is the best-studied rhabdovirus. A study from the early 1970s suggested that, like sigma virus, VSV-infected Drosophila are sensitive to CO2 (Bussereau, 1973). To confirm these findings, we induced a systemic infection by pricking flies in the thorax using a pin that had been swabbed in liquid stock of virus containing 1011 plaque forming units (PFU)/mL. This method resulted in the delivery of several hundred PFU of virus into each fly, as assessed by plaque assay immediately following infection (Figure 1A). When infected flies were incubated for 7 days at 29°C an d then anesthetized with CO2 for 30 seconds, only 30% of VSV-infected flies remained viable the next day (Figure 1B). Uninfected flies and flies not exposed to CO2 did not display this pronounced loss in viability after CO2 anesthesia (Figure 1B). Thus, VSV-infection of Drosophila provides a model to study infection-induced sensitivities to environmental stress.
Figure 1. VSV-infected animals have immediate, life-threatening sensitivity to CO2 anesthesia depending on multiple parameters.
(A) Drosophila melanogaster were infected using standard method of pricking the thorax with a pin dipped in VSV. Flies were immediately collected following infection and homogenates were titrated on Vero cells to measure plaque forming units (PFU) (N=3). (B, C) Adult flies were pricked with a sterile pin or infected with VSV. Survival was monitored during the course of infection (B), and acute recovery to 30 seconds of CO2 anesthesia was monitored 7 dpi (C). One experiment representative of three is shown. (D, E) Anopheles gambiae mosquitos were injected with buffer or VSV (107 PFU) in the thorax. After 3 days, animals were anesthetized for 30 seconds with CO2. Acute recovery after anesthesia (D) and survival (E) during the course of infection were monitored. One of three representative experiments is shown. (F) VSV-infected WT flies were incubated at 29°C or 21°C. At 7 dpi, flies were collected to measure PFU. (G) VSV titers were compared between 21°C infections of WT and Dcr2 KO flies collected 7 dpi (N=3). (H, I) WT and Dcr2KO flies were infected with VSV or received mock treatment. Acute recovery from CO2 anesthesia was monitored 7 dpi (H), and survival was tracked during the course of infection (I). One experiment representative of three is shown. (J) VSV titers were compared between 29°C infections of WT and Dcr2KO flies collected 3 dpi (N=3). (K, L) VSV infected and mock treated WT and Dcr2KO flies were incubated at 29°C. At 3 dpi, flies were subjected to CO2 anesthesia and monitored for acute recovery (K). Flies were monitored for survival during infection (L). One experiment representative of three is shown. **p<0.01; ***p<0.001; ****p<0.0001; ns, not significant
In considering how VSV could elicit a life-threatening sensitivity to CO2, the length of time between CO2 exposure and death assessment (1 day) yielded multiple explanations. For example, CO2 exposure could have rendered flies temporarily immunodeficient (Helenius et al., 2009), which would result in enhanced viral replication and death over the subsequent 24 hours. Alternatively, CO2 exposure could result in some form of acute trauma, independent of subsequent viral replication. These models can be distinguished kinetically, as the former model depends on enhanced viral replication after CO2 exposure. If correct, several hours would likely need to pass before any phenotypes associated with CO2 sensitivity would be revealed. In contrast, the latter model of acute trauma may manifest itself in a short period of time. We therefore examined the earliest possible phenotype associated with CO2 exposure, the ability of flies to wake and stand on their legs. To recover from CO2 anesthesia, as defined by returning to an upright position on their legs, uninfected flies required 3.7 minutes (median recovery time) (Figure 1C). In contrast, VSV-infected flies exhibited a delayed recovery from anesthesia with a 6.2 minute median recovery time (Figure 1C). Thus, in addition to the long-term consequence of CO2 exposure (lethality), an acute sensitivity to this stress is evident. The speed by which this phenotype is revealed following CO2 anesthesia (minutes) eliminates the possibility that an increase in viral replication is responsible. We therefore conclude that CO2 causes some acute change in the physiology of VSV-infected flies that leads to death.
To determine if VSV could elicit similar disease phenotypes in other hosts, we performed infections of the clinically significant insect vector Anopheles gambiae. Similar to Drosophila, the combination of VSV infection and CO2 anesthesia elicited a significant delay in recovery from CO2 anesthesia (p<0.0001, Figure 1D), with life-threatening consequences observed 24 hours later (Figure 1E). CO2 sensitivity may therefore be a common feature of VSV infections of insects. In contrast to other models of infection-induced sensitivities to subsequent stress, which are revealed over the course of several days (Jamieson et al., 2013), the speed of disease manifestation in VSV-infected flies provides a unique model to understand acute sensitivities to infection-induced stress.
The fly innate immune system regulates CO2 sensitivity during asymptomatic VSV infection
To better understand how VSV causes CO2 sensitivity, we sought methods to modulate the productivity of the viral infection. This was addressed in two ways. First, we took advantage of the fact that infected flies incubated at 21°C a re more resistant to VSV infection than those incubated at 29°C. Consequently, flies incubated at 21°C have decreased viral titers than their counterparts incubated at 29°C (p<0.0001, Figure 1F ). A second means of altering infection productivity is to manipulate the antiviral RNA interference (RNAi) pathway in flies. Using a loss of function mutant of the RNAi regulatory factor Dicer-2 (Dcr2KO), we observed more than a 100-fold increase in the production of infectious viral particles, as compared to wild type (WT) flies (p<0.0001, Figure 1G). Thus, temperature changes and RNAi manipulation can be used to assess the influence of viral replication on CO2 sensitivity. In contrast to infections at 29°C, VSV-infected WT flies incubated at the lower temperature of 21°C displayed no immediate sensitivity to CO2 anesthesia (Figure 1H). Under these conditions, no lethality was observed in VSV-infected flies 24 hours after CO2 exposure (Figure 1I). These data suggest that limiting VSV replication renders the host insensitive to CO2. If this prediction is correct, then flies lacking the antiviral RNAi pathway should be sensitive to CO2, even at 21°C. This hypothesis was tested by performing VSV infections of Dcr2KO flies at 21°C. VSV-infected Dcr2 KO flies were highly sensitive to CO2 (Figure 1H). Indeed, less than 5% of Dcr2KO flies recovered from CO2 anesthesia, and these flies exhibited a precipitous drop in survival in the day following treatment (Figure 1I). Thus, two means of modulating viral replication (temperature and RNAi inactivation) resulted in differential sensitivity to CO2 exposure. When high temperature was combined with Dcr2 deficiency, a severe sensitivity to CO2 was observed. At 29°C, more than a 100-fold increase in viral titers could be observed in Dcr2KO flies compared to WT flies (p<0.01, Figure 1J). Under these conditions, acute and lethal CO2 sensitivity was observed in Dcr2KO flies 3 days post-infection (dpi) (Figure 1K, L). Taken together, these data establish that the productivity of viral infection positively correlates with CO2 sensitivity.
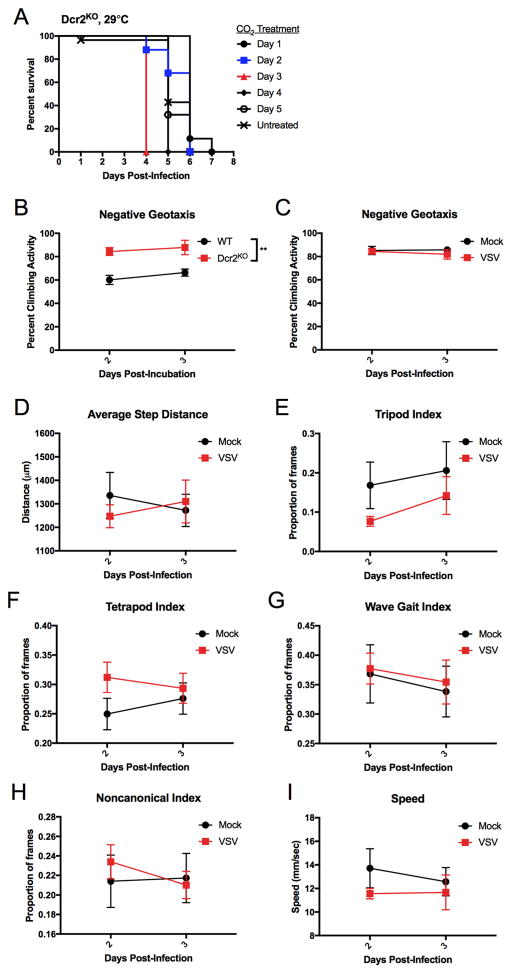
The observed sensitivity to CO2 was most severe in Dcr2KO flies incubated at 29°C. This sensitivity to CO2 anesthesia was evident at 3 dpi, whereas anesthesia applied 1 or 2 dpi had no effects on survival (Figure 2A). Thus, a clear division in CO2 sensitivity exists between 2 and 3 dpi of Dcr2KO flies. We utilized this distinct threshold to determine if Dcr2KO flies displayed symptoms of disease before and after onset of CO2 sensitivity, but before CO2 treatment was administered. The startle-induced negative geotaxis assay assesses climbing behavior after flies have been tapped down to the bottom of a vial (Barone and Bohmann, 2013). We first determined if uninfected Dcr2KO had any climbing deficiencies compared to WT flies. Uninfected Dcr2KO were not defective for climbing activity, and actually performed better than WT flies after being incubated at 29°C for 2 and 3 days (two-way A NOVA, Figure 2B). We proceeded to test VSV-infected Dcr2KO flies for climbing activity 2 and 3 dpi at 29°C an d compared them to mock treated flies. No significant differences were observed between infected and uninfected flies, and no changes in climbing activity were observed from 2 dpi to 3 dpi (two-way ANOVA, Figure 2C). These results suggest that prior to CO2 anesthesia, VSV infection in Drosophila is asymptomatic.
Figure 2. Climbing behavior and gait of infected animals are unaffected by VSV infection.
(A) Dcr2KO flies were infected with VSV and kept at 29°C. Flies were anesthetized at indicated days with CO2 for 30 seconds or left untreated. Survival was monitored. (B) WT and Dcr2KO flies were pricked on the thorax and then incubated at 29°C. On days 2 and 3, flies were assessed for their ability to climb up the vial after being startled. No statistical significance was observed between the two time points assessed (two-way ANOVA). A statistically significant difference in climbing activity was observed across the two genotypes (**p<0.01, two-way ANOVA, N=3). (C) Dcr2KO flies were infected with VSV or mock treated and incubated at 29°C. Flies were assessed for climbing activity after being startled. No statistically significant differences were observed between infected and mock-treated flies nor between days 2 and 3 post-infection (two-way ANOVA, N=3). (D–I) Dcr2KO flies were all pricked on the same side of the thorax and were either infected with VSV or mock treated. Flies were incubated at 29°C and tracked longitudinally 2 and 3 dpi. Video recordings of fly gait behavior were analyzed using FlyWalker software. No statistical differences were observed between mock (N=8) and VSV (N=11) infected groups at each time point for step distances, gait indices, and speed (t-test).
To complement these behavioral analyses, an optical recording method and FlyWalker software were used to rigorously test the possibility that VSV infection alone alters coordinative function (Mendes et al., 2013). The experimental setup detects refracted light from footsteps of flies as they walk across a lighted platform. The data is recorded as videos at 300 frames per second and further processed to measure parameters such as speed and leg positioning in relation to the body (Video S1). We compared mock and VSV-infected Dcr2KO flies at the crucial time points of 2 and 3 dpi, when infections change from anesthesia insensitivity to sensitivity. In comparison to mock treatment at 2 and 3 dpi, VSV infections resulted in no changes in footstep distances, gait of animals, and walking speed (Figure 2D–I). These behavioral analyses therefore support the idea that VSV causes asymptomatic infections in Drosophila, and that CO2 treatment converts these infections into a life-threatening risk to the host.
An inability to tolerate a state of hypercapnia underlies VSV-induced CO2 sensitivity
Having established the contribution of viral infection to the sensitivity to an environmental stress, we sought to understand the specific role of CO2 in these phenotypes. CO2 anesthesia has two simultaneous effects. Blowing pure CO2 into a fly vial creates a hypercapnic environment and simultaneously depletes oxygen, creating a hypoxic environment. To differentiate between the effects of hypercapnia and hypoxia, nitrogen gas (N2) was used as an alternative anesthetic. Blowing pure N2 into the fly vial reduces oxygen levels without increasing CO2 levels. Thus, N2 induces hypoxia but not hypercapnia, whereas CO2 induces both. The comparison of N2 and CO2 anesthesia therefore allows us to distinguish the role of hypoxia from hypercapnia in infection-induced sensitivity to stress. As we have observed, VSV-infected WT flies that were incubated at 21°C recovered from CO 2 anesthesia without delay (Figure 3A). In contrast, Dcr2KO flies that were infected with VSV were highly sensitive to CO2, with greater than 90% of flies being unable to recover from this anesthesia (p<0.0001, Figure 3A). Under these conditions, the median recovery time shifted from 2.5 minutes (WT) to >15 minutes (Dcr2KO). When flies were anesthetized with N2, the median recovery times for WT and Dcr2KO flies differed by 1 minute. While statistically significant (p<0.0001), this difference was modest (Figure 3A). In addition, a 70% drop in survival the day following anesthesia was only observed for Dcr2KO flies treated with CO2 (Figure 3B). Dcr2KO flies treated with N2 lived for several more days before finally succumbing to infection (Figure 3B) (Galiana-Arnoux et al., 2006). Because flies displayed little acute or long-term sensitivity to N2 anesthesia, hypoxia is not likely responsible for the disease phenotypes associated with infection. We therefore conclude that the disease associated with CO2 sensitivity results from infected flies being unable to tolerate a state of hypercapnia.
Figure 3. Anesthesia sensitivity is specific to hypercapnia.
WT and Dcr2KO flies were infected with VSV and kept at 21°C. At 8 dpi, flies were anesthetized with CO2 or N2. Acute recovery (A) and survival (B) are shown. One experiment representative of three is shown. ****p<0.0001
VSV must infect glial cells to elicit CO2 sensitivity
By modulating infection temperature and host immune defenses, a correlation was observed, suggesting that high viral titers on the day of CO2 administration causes lethality (Figure 1). To determine if high viral titers at the onset of infection are required for CO2-induced lethality, we used an injector to deliver precise volumes into the thorax of flies with concentrations of VSV that spanned 1000-fold (Figure 4A). Results 2 dpi provided the most insightful data. The group with the lowest infectious dose was lower in titer compared to the other groups at 2 dpi (p<0.01, multiple t-tests). Most of these flies were not CO2 sensitive. Groups receiving higher virus doses had higher viral titers 2 dpi and were mostly CO2 sensitive. Despite having high viral titers, some flies did not display CO2 sensitivity (Figure 4A). By 3 dpi, virtually all flies, regardless of infectious dose, were CO2 sensitive. These results indicate that the initial dose of infectious virus influences disease progression, but viral titers in whole flies cannot fully explain CO2 sensitivity. We therefore considered the possibility that VSV must infect a specific region in the fly in order to elicit CO2 sensitivity.
Figure 4. VSV infection of the nervous system causes CO2 sensitivity.
(A) Nanoject II was used to inject 9.2nL volumes of VSV into the thorax of Dcr2KO flies at specified PFU. Infections were incubated at 29°C and anesthetized with 30 seconds of CO2 at 1, 2, 3, and 4 dpi. Flies were observed for 5 minutes to determine which had delayed CO2 anesthesia recovery before being collected to measure virus titers. (B) Ago2KO flies were infected with VSV-GFP via pricking of the thorax. Groups of infected flies were anesthetized with CO2 on the indicated days post-infection and survival was recorded. One of three experiments is shown. (C) Ago2KO flies were infected with VSV-GFP via pricking of the thorax. At indicated times post-infection, flies were segmented as heads, thoraxes, and abdomens, homogenized, and blotted for GFP protein expression. Each lane represents a single fly. One of three representative experiments is shown. (D) WT and RNAi knockout flies were infected with VSV-GFP and incubated at 21°C for 11 days. Brains were dissected and stained for GFP expression. One of three representative experiments is shown. (E) Dcr2KO flies expressing GFP under the control of a glial, neuronal, or tracheal specific Gal4 were infected with VSV-Luc. After 11 days at 21°C, brains were dissected and stained for GFP and luciferase expression. DRAQ5 staining was used to detect nuclear DNA. Confocal images were taken with a 40X oil objective lens. Insets are 4X magnifications of selected areas. (F, G) Dcr2KO flies were bred to express functional Dcr2 in neurons or glia. Flies were infected with VSV and kept at 21°C. Flies were anesthetized with CO2 at 8 dpi. One experiment representative of three is shown. (H) VSV titers were measured 8 dpi from animals incubated at 21°C (N=3). (I, J) Ago2 expression was knocked down ubiquitously or in specific cell types. VSV infected flies were incubated at 21°C. At 12 dpi, flies were assessed for acute recovery from CO2 anesthesia (I) and survival (J) throughout the experiment. One experiment representative of three is shown. (K) Infected animals kept at 21°C for 12 days were homogenized to measure VSV titers (N=3). *p<0.01; ***p<0.001; ****p<0.0001; ns, not significant. See also Figure S1.
To identify sites within infected flies that support VSV replication, we monitored viral protein production in specific anatomical locations over time. A recombinant VSV-green fluorescent protein (GFP) reporter virus was used to infect Argonaute 2 (Ago2KO) mutants, another RNAi gene required for antiviral defense (van Rij et al., 2006). At 29°C, Ago2 KO flies began showing signs of CO2 sensitivity as early as 3 dpi, as assessed by a decrease in survival 24 hours after CO2 anesthesia (Figure 4B). About 30% of animals were CO2-sensitive 3 dpi, and by 4 dpi more than 90% of flies were CO2-sensitive (Figure 4B). Using the GFP reporter to track VSV within infected flies, we assayed three body segments (head, thorax and abdomen) for GFP expression via Western analysis. We found that the timing of CO2 sensitivity onset correlated with strong GFP expression (Figure 4C). However, GFP did not preferentially accumulate in one body segment (Figure 4C), suggesting simultaneous virus spread throughout the body of the fly. To identify specific tissues affecting CO2 sensitivity, we focused on the nervous system because many rhabdoviruses are neurotropic and CO2 anesthesia acts directly on the nervous system (Nicolas and Sillans, 1989). Fluorescent microscopy confirmed that VSV could be detected in the brains of CO2 sensitive flies (Figure 4D). To gain further clarity of the cell types infected, we used the Gal4/UAS overexpression system to drive expression of GFP in specific cell types of Dcr2KO flies (Brand and Perrimon, 1993). Flies were infected with a VSV-luciferase (VSV-Luc) reporter virus and explanted brains were stained with a luciferase-specific antibody. While VSV-Luc colocalized with GFP expressed by glia or neurons, no colocalization was observed with tracheal cells (Figure 4E). Thus, VSV infects the two cell types of the nervous system, neurons and glia.
We reasoned that if CO2 sensitivity required infection of the nervous system, then preventing viral replication in the nervous system should render flies resistant to CO2. To test this idea, Dcr2KO flies were genetically altered such that Dcr2 function was rescued in either glia or neurons. Under these conditions, at 21°C, these cell types should restrict viral replication. In contrast, all other cells in the fly should support viral replication. When we restored Dcr2 function in neurons, 20% of neuronal Dcr2 rescue flies recovered within 5 minutes of anesthesia, whereas 10% of Dcr2 knockout flies recovered (p<0.05, Figure 4F). Restoring Dcr2 function to glial cells resulted in 70% of flies recovering within 5 minutes of CO2 removal (p<0.0001, Figure 4F). In the day following CO2 anesthesia, over 80% of Dcr2 knockouts were deceased, whereas over 80% flies with Dcr2 function rescued in glia or neurons remained viable (Figure 4G). VSV titers in flies were high for the three genotypes, indicating that VSV was still capable of replicating in other cell types despite being restricted from neurons or glia (Figure 4H). These data indicate that VSV replication in the nervous system is required to elicit sensitivity to CO2.
To determine if VSV replication in the nervous system is sufficient to elicit CO2 sensitivity, we inactivated the antiviral RNAi pathway in glial cells or neurons. This was accomplished by overexpressing a hairpin RNA to knock down expression of Ago2. Knocking down Ago2 in specific cell types renders these cells susceptible to VSV infection, while the remaining host tissues are resistant to infection at 21°C. Flies with Ago2 knocked down specifically in neurons behaved like WT flies, recovering from CO2 anesthesia within 5 minutes (Figure 4I). Although a statistically significant difference was observed between WT and neuronal knockdown groups (p<0.001), the median recovery times were negligible (2.52 and 2.354 minutes, respectively). Knockdown of Ago2 in glia caused a delay in recovery, and a combination knockdown of Ago2 in glia and neurons resulted in recovery kinetics comparable to flies with Ago2 knocked down throughout the entire body using the actin driver (Figure 4I). Compared to control flies expressing Ago2, survival was decreased following CO2 anesthesia for flies with Ago2 knocked down ubiquitously and in neurons and glia (p<0.0001, Figure 4J). When viral titers were measured at the time point when flies were anesthetized with CO2, low levels of infectious virus were detected in WT flies and flies with Ago2 knocked down in neurons (Figure 4K). In contrast, viral titers were increased in flies with Ago2 knocked down in glia and were further increased in flies with Ago2 knocked down in neurons and glia (p<0.001, Figure 4K). These collective data support a model whereby VSV infection of neurons and glia is sufficient to cause complete sensitivity of flies to CO2, and that relative to neurons, infection of glia is more important in causing CO2 sensitivity.
Not all neurotropic viruses elicit CO2 sensitivity
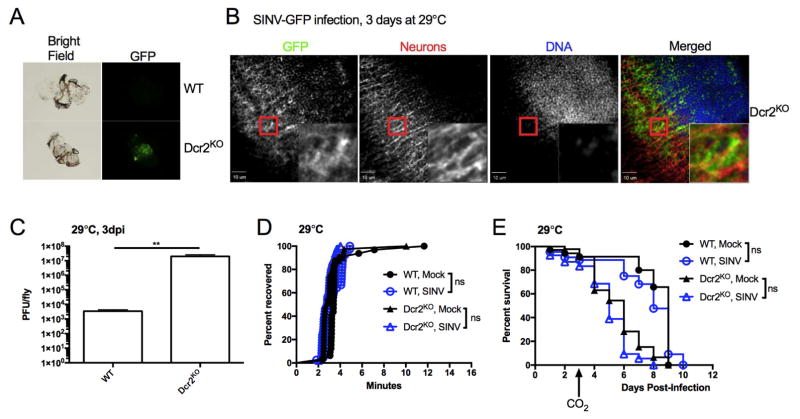
Sensitivity to CO2 anesthesia was caused by VSV infection of the nervous system. Thus, perturbation of the nervous system could be the root cause of CO2 sensitivity. VSV, the inducer of nervous system perturbation, could potentially be replaced by a similar stressor. To determine the relative significance of nervous system perturbation, we considered whether another neurotropic pathogen would elicit a similar inability to tolerate CO2. To address this possibility, flies were infected with a recombinant togavirus, Sindbis virus (SINV) encoding a GFP reporter gene. After 3 dpi, viral expression of GFP was detected in brains dissected from infected Dcr2KO flies but not in WT flies (Figure 5A). Confocal imaging of infected Dcr2KO brains detected virus in neurons and additional cell types in the brain (Figure 5B). Dcr2KO flies exhibited a 1000-fold increase in viral titers compared to WT flies (p<0.01, Figure 5C). Thus, like VSV, SINV replication is restricted by the RNAi pathway and can disseminate to the brain. However, Dcr2KO flies did not exhibit any signs of CO2 sensitivity when infected with SINV (Figure 5D, E). Median recovery time of SINV-infected Dcr2KO flies was within 1 minute of all other groups (Figure 5D). Additionally, SINV-infected Dcr2KO flies did not exhibit increased lethality 24 hours after anesthesia (Figure 5E). Thus, despite SINV also infecting the nervous system, CO2 sensitivity is specific to rhabdoviruses such as VSV and sigma (L’Heritier, 1948). Thus, CO2 sensitivity is unlikely to be the result of perturbation of the nervous system. Instead, CO2 sensitivity is pathogen specific.
Figure 5. Sindbis virus infection does not cause CO2 sensitivity.
(A) Flies were pricked in the thorax with a pin swabbed in a concentrated stock of reporter virus, SINV-GFP (1011 PFU/mL), and infected flies were incubated at 29°C. At 3 dpi, brains were dissected and imaged for viral GFP expression. Representative dissections from one of three experiments are shown. (B) Dcr2KO flies were infected with SINV-GFP for 3 days at 29°C. Explanted brains were fixed and stained with antibodies against GFP and HRP to detect virus and neurons, respectively. DRAQ5 staining was used to detect nuclear DNA. Insets are 4X magnifications of selected areas. (C) Flies were injected in the thorax with 106 PFU of SINV. After 3 days at 29°C, viral titers were measured from infected flies (N=3). (D, E) Flies were injected with buffer or 106 PFU of SINV and incubated at 29°C. At 3 dpi, flies were subjected to CO2 anesthesia and acute recovery (D) and survival (E) were assessed. One of three representative experiments is shown. **p<0.01; ns, not significant
A single viral protein, independent of infection, causes a lethal sensitivity to CO2
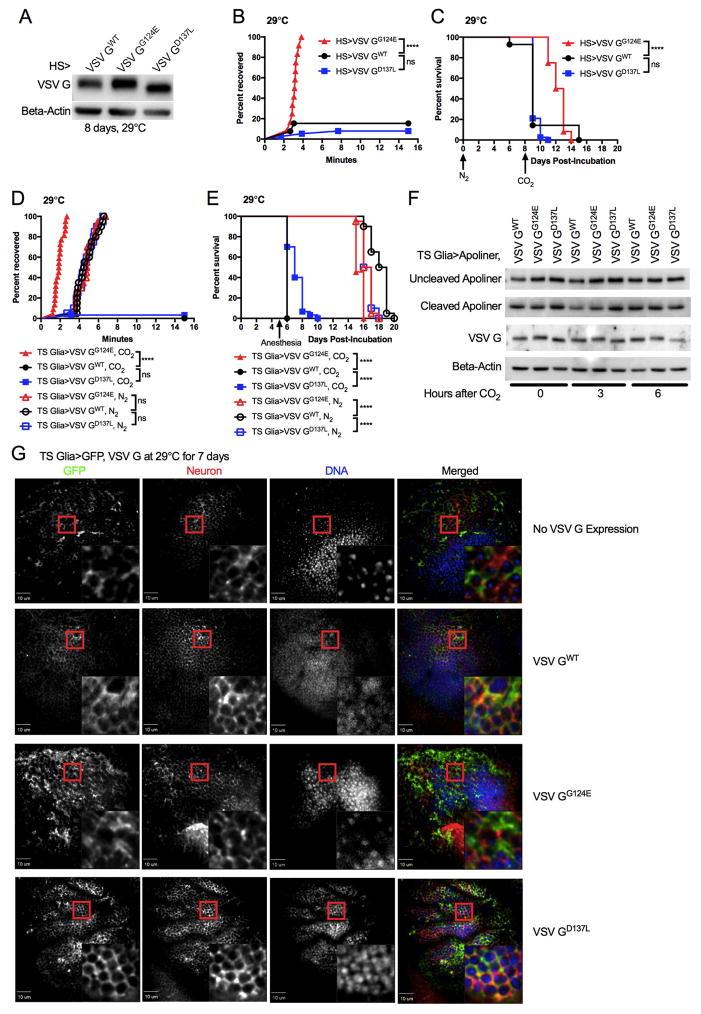
The specificity of CO2 sensitivity to VSV could be explained by a unique pathology associated with the VSV infectious cycle, but it could also be explained by the actions of a protein that is common to rhabdoviruses, independent of infection. This latter idea is unusual, as models of virus-induced disease sensitivity are thought to result from a productive viral infection that induces pathology (Jamieson et al., 2013; Novo-Veleiro et al., 2016). It should therefore be impossible to dissociate VSV infection from sensitivity to CO2. The simplicity of the VSV genome provides an opportunity to test this hypothesis: that a single viral protein may cause disease sensitivity. Transgenic lines of flies were therefore generated, each of which encode a single gene from the VSV genome. Because they were left untagged, detection of protein production by western analysis would not provide consistent comparisons of each protein. Instead, we used qPCR analysis to confirm transcription of the viral transgenes driven by heat shock-Gal4. After 8 days of incubation at 29°C, gene specific mRNAs were quantified. This analysis revealed that VSV G and VSV L transgenes were expressed at comparable levels. In contrast, the other three VSV transgenes are expressed at levels that differ from that of VSV G and VSV L (p<0.05, Figure 6A). Western analysis of VSV G and VSV M demonstrated that these proteins could be detected in transgenic and infected flies, but transgene expression was low compared to viral infection (Figure 6B). Despite low expression relative to viral infection, VSV G expressing transgenic flies exhibited delayed acute recovery from CO2 anesthesia, and these flies had a 70% decrease in survival 24 hours later (Figure 6C, D). In contrast, expression of the L, M, N, and P genes from VSV in transgenic flies did not induce characteristic acute or lethal phenotypes upon exposure to CO2 (Figure 6C, D). Expression of L is perhaps the best comparison to G since no significant difference in mRNA expression was found by qPCR (p=0.4666, Figure 6A). A single viral protein (VSV G) is therefore sufficient to induce the same sensitivity to CO2 that is observed following VSV infection.
Figure 6. VSV G expression in glia is sufficient to cause CO2 sensitivity.
Flies in this figure were reared at 18°C. Flies were collected after eclosion by anesthetizing with N2 and sorting them on an ice-cold glass dish. (A) VSV transgene expression was driven by a heat shock-Gal4 (HS). After 8 days at 29°C, RNA was purified from f lies and gene-specific mRNA was measured by qPCR (N=3). (B) Dcr2KO were infected with VSV and incubated for 3 days at 29°C. VSV G and M transgene expression was driven by HS by incubating flies at 29°C for 8 days. Protein lysates of samples were western blotted for VSV G and M expression. Beta-actin was used as a loading control. (C, D) Flies were incubated at 29°C for 8 days to drive expression of VSV genes. Flies were subjected to CO2 anesthesia to assess acute recovery (C) and then were returned to 29°C to continue monitoring survival (D ). One experiment representative of three is shown. (E) Glia and fat body-specific Gal4 transgenes drove VSV G expression. Flies were compared to ones containing only a Gal4 driver or VSV G transgene as controls. One of three representative experiments is shown for each blot. (F, G) A temperature sensitive transcriptional inhibitory protein (TS) repressed VSV G expression in glial and fat body cells until flies were incubated at 29°C. After 5 days at 29°C, flies were subjected to CO2 anesthesia. Acute recovery to anesthesia (F) was assessed and survival (G) during the course of heat shock was monitored. One experiment representative of three is shown. *p<0.05; ****p<0.0001; ns, not significant
VSV infection of glial cells is necessary and sufficient to induce CO2 sensitivity (Figure 4). To determine if VSV G transgene expression recapitulated these attributes of infection-induced CO2 sensitivity, we expressed VSV G in glial cells. VSV G protein was readily produced in glia (Figure 6E), and expression of VSV G in glia was sufficient to induce acute recovery delays and death following CO2 anesthesia (Figure 6F, G). To determine the specificity of these phenotypes, VSV G was also expressed in the fat body (Figure 6E). Fat body expression of VSV G did not result in acute recovery delays to CO2 or survival defects (Figure 6F, G). Thus, CO2 sensitivity specifically arises due to expression of VSV G in glial cells.
pH-dependent fusogenic activity of VSV G promotes glia-neuron fusion and acute neuro-trauma
To understand how VSV G results in CO2 sensitivity, we returned to the observation that CO2 anesthesia causes hypercapnia. Hypercapnia is defined as an increase in CO2, which results in acidification of the exposed tissues and/or fluids. This property was of interest because VSV G causes cell-cell fusion (syncytia formation) at acidic pH (Florkiewicz and Rose, 1984; Riedel et al., 1984). CO2 can have an acidifying effect, whereas N2 anesthesia does not. We therefore hypothesized that the acute sensitivity to CO2 was the result of syncytia formation induced by VSV G. To test this hypothesis functionally, we compared wild type VSV G (GWT), which has a fusion threshold at a pH of 6.3, to two fusion defective VSV G mutants. These mutants were chosen because they encode full-length proteins that display no defect in folding or transport to the plasma membrane (Fredericksen and Whitt, 1995, 1996). A G→E mutation at amino acid position 124 (VSV GG124E) causes fusion to occur at a pH of 5.5 or lower (Fredericksen and Whitt, 1995). A milder mutation, VSV GD137L allows fusion at pH of 5.7 or lower (Fredericksen and Whitt, 1996). Transgenic flies were engineered to express VSV GWT or the mutant alleles as a single copy from the same recombinogenic site on chromosome 2. The alleles were expressed at comparable levels using a heat shock-Gal4 (Figure 7A). We then tested whether VSV G fusion activity correlated with CO2 sensitivity. As expected, VSV GWT flies displayed acute recovery and survival defects in response to CO2 anesthesia (Figure 7B, C). While VSV GD137L mutants were also CO2 sensitive, VSV GG124E expressing flies did not display any CO2 sensitivity (Figure 7B, C). The fusogenic activity of VSV G is therefore responsible for CO2 sensitivity, and the difference observed between the two mutants suggests that CO2 anesthesia acidifies pH between 5.7 and 5.5.
Figure 7. Fusogenic activity of VSV G causes immediate neuro-trauma in the nervous system.
Flies in this figure were reared at 18°C. Flies were collected after eclosion by anesthetizing with N2 and sorting them on an ice-cold glass dish. (A) Expression of WT and fusogenic mutants of VSV G were induced via 29°C incubation for 8 days. Flies were collected and VSV G expression was detected by Western blotting. One of three representative experiments is shown. (B, C) Flies were heat shocked at 29°C for 8 days to induce expression of VSV GWT, GG124E, or GD137L before being assessed for acute recovery to CO2 anesthesia (B). Flies were then returned to the 29°C incubator to continue measuring viability (C). One experiment representative of three is shown. (D, E) Flies were incubated for 5 days at 29°C to induce VSV G expression. Flies were subjected to 30 seconds of CO2 or 60 seconds of N2 to assess recovery rate (D). Flies were then returned to 29°C incubation to continue tracking survival (E). One experiment representative of three is shown. (F) Apoliner and VSV G variants were expressed in glial cells by incubating flies at 29°C for 7 days. Flies were anesthetized with CO2 for 30 seconds and collected immediately, 3 hours later, or 6 hours later. Western blotting of fly protein lysates was used to assess Apoliner cleavage by GFP blotting. One experiment representative of three is shown. (G) VSV G and GFP expression was induced in glial cells by placing flies at 29°C for 7 days. Flies were anesthetized with CO2, and dissected brains were stained with GFP and HRP antibodies to stain for glia and neurons, respectively. DRAQ5 staining was used to stain for DNA. Confocal imaging was done using a 40X oil objective lens. Insets are 4X magnifications of selected areas. See also Figure S1 for additional staining control. ****p<0.0001; ns, not significant
As infection of glial cells is critical in causing recovery delays and death following CO2 anesthesia, we examined the influence of the fusion-defective VSV G mutants in these cells. VSV GWT, GG124E, and GD137L expression were induced in glia for 5 days. While GWT and GD137L were sufficient to induce CO2 sensitivity, the GG124E fusion mutant expressed in glia could not cause sensitivity to CO2 (Figure 7D, E). N2 anesthesia, which does not have acidifying effects, did not result in acute recovery delays and precipitous death within a day following anesthesia (Figure 7D, E). Thus, expression of a fusion competent VSV G is sufficient to recapitulate the hallmarks of infection-induced CO2 sensitivity, in that expression in glia is sufficient and the disease is not elicited by N2 anesthesia.
Our functional analysis indicates that upon CO2 treatment, VSV G is likely influencing some aspect of glial cell physiology that is detrimental to host viability. One explanation is that CO2-induced membrane fusion initiates changes in glial physiology that result in apoptotic cell death. Alternatively, CO2-induced fusion could cause immediate, non-regulated trauma by disrupting glial cell function. To distinguish these possibilities, the apoptosis reporter transgene, Apoliner, was utilized (Bardet et al., 2008). Apoliner encodes a red fluorescent protein connected to GFP via an amino acid sequence that can be cleaved by caspase enzymes that mediate apoptosis. VSV G and Apoliner were expressed in glial cells to induce CO2 sensitivity. Flies were exposed to 30 seconds of CO2 anesthesia and collected immediately, 3 hours later, and 6 hours later. Apoptosis was assayed by western analysis for size shifts in GFP. No changes were observed in cleaved Apoliner abundance for VSV GWT, GG124E, and GD137L over the course of the six hours (Figure 7F). As acute recovery phenotypes are evident within minutes of CO2 exposure, yet Apoliner cleavage was not observed over the time course, apoptosis of glia cannot account for the disease phenotypes associated with CO2 exposure.
As glia and neurons are tightly associated, and glial cells support neuronal function (Freeman, 2015), another possibility is that glia fuse with each other and/or with neurons upon exposure to CO2. Either event would be expected to acutely interfere with nervous system operation. To determine if there was any evidence of these fusion events in flies, the glia-specific Gal4 driver was used to co-express GFP and VSV G. A horseradish peroxidase (HRP) antibody was used to label neurons in dissected brains (Jan and Jan, 1982). Our labeling strategy was specific, as demonstrated by micrographs of the fly ommatidium where neurons and glia are in a highly patterned formation (Figure S1). When VSV GWT or GD137L were expressed, colocalization of glial and neuronal labelling was observed, suggesting fusion between neurons and glia, or at least changes in the organization of the glia-neuron interface (Figure 7G). When no VSV G was expressed, and when VSV GG124E was expressed, the neuronal and glial markers were closely associated, but colocalization was not observed (Figure 7G). This analysis suggests that CO2 anesthesia induces fusion events between neurons and glia, and probably between glia, resulting in immediate neuro-trauma. These data therefore provide a molecular explanation for how VSV infection causes sensitivity to CO2 and provide an example of how a single viral protein, independent of productive infection, can precipitate susceptibility to environmental stress.
DISCUSSION
In this study, we explored how an asymptomatic viral infection can be rendered lethal upon exposure to an environmental stress. The most notable feature of this disease state is that it is not elicited by either infection or the stress. Only the combination of infection and stress causes disease. This experimental model therefore provided a unique perspective into the means by which asymptomatic infections can convert to symptomatic infections, through the exposure of the host to a secondary stress. Moreover, the speed which CO2 associated symptoms is observed (minutes) and the specificity of disease to VSV strongly supports the conclusion that a mechanism of disease tolerance has been uncovered. This conclusion does not, however, preclude the discussion of resistance mechanisms in disease progression, as our analysis supports the need for viral spread to the nervous system for VSV G to be expressed in glia. Thus, while the spread of the virus through the body is largely controlled by resistance mechanisms (e.g. RNAi), the mere presence of virus in the nervous system is not sufficient to cause lethality. Only upon exposure to an environmental stress is disease observed. For this reason, we propose that insufficient resistance mechanisms render the host highly dependent on context-dependent tolerance mechanisms to survive.
While our infections with VSV resulted in sensitivity to a secondary stress, the opposite outcome is also possible. For example, chronic infection with herpesviruses renders mice resistant to Listeria and Yersinia infections (Barton et al., 2007). Presumably, the viral infection raises basal interferon gamma expression, priming the immune system for resistance to subsequent bacterial infections. These host-pathogen relationships become increasingly more complex when considering that organisms outside of controlled experimental conditions are faced with many infectious challenges over the course of life. As others have noted, the history of previous immune challenges can affect outcomes to the next stress (Foxman and Iwasaki, 2011; MacDuff et al., 2015; Reese et al., 2016; Stelekati et al., 2014). Thus, we risk losing important information if we focus only on the direct, destructive effects of a pathogen in isolation.
Our analysis of the host and viral regulators of CO2 sensitivity revealed several important determinants of disease progression. We found that productive viral infection is necessary to allow for sensitivity to CO2 anesthesia to arise, as revealed by manipulations of temperature and the RNAi pathway. We defined glial cells as a principal cell type that must be infected to render Drosophila sensitive to CO2, as restricting VSV replication from these cells prevented disease development. Under these conditions, VSV was capable of replicating in other cell types in the fly. These data therefore establish that even for a broadly tropic infection, catastrophic consequences on viability stem from changes to a specific tissue. A deeper understanding of pathogenicity will require studies that focus on crucial cell types and mechanistic changes that that influence disease progression.
Our mechanistic analysis of how VSV induces CO2 sensitivity revealed that we can completely dissociate infection from the onset of disease, in that transgenic flies expressing only VSV G sufficiently reproduce the phenotype. Application of CO2 acidifies the nervous system, inducing VSV G to mediate cell-cell fusion. Using two VSV G mutants allowed us to approximate the pH acidification induced by CO2 anesthesia to be between 5.5 and 5.7. SINV fusion is also pH dependent, but it has been noted to require an optimal pH between 5.3 and 5.5. This observation may explain why SINV does not cause CO2 sensitivity (Mann et al., 1983).
Our results highlight how single viral proteins, not active pathogen replication, can alter the host in ways that create sensitivities to the environment. These ideas may be most important when considering latent viral infections, such as those caused by herpesviruses, where latency associated physiological changes in the host may occur (Bhattacharjee et al., 2016; DiMaio et al., 2014). Examples of latency associated changes include Kaposi’s sarcoma-associated herpesvirus (KSHV) infections, where viral replication does not occur, but angiogenesis of the endothelium is induced through unclear mechanisms (DiMaio et al., 2014). In addition, Epstein Bar virus encodes proteins that promote lymphoma development, even under conditions of latency (Bhattacharjee et al., 2016). Whether these or other latent viral infections also cause sensitivities to secondary environmental stresses is unclear, but our findings provide a mandate to consider such possibilities in experimental and clinical settings.
STAR METHODS
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jonathan C. Kagan (jonathan.kagan@childrens.harvard.edu).
Experimental model and subject details
Drosophila melanogaster
Flies were fed a standard cornmeal medium made by the Norbert Perrimon laboratory. Flies were incubated at 25°C with a 12-hour light cycle. Unless otherwise noted, flies were anesthetized with CO2 for collection and breeding. For ease of maintenance, only adult males were used for studies although CO2 sensitivity was observed in females as well. Flies were infected for experiments within 10 days of eclosion. Fly genotypes used in experiments are listed in Key Resources Table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Monoclonal mouse anti-GFP (JL-8) | Clontech | Cat#632381 |
| Polyclonal rabbit anti-beta actin | Cell Signaling | Cat#4967 |
| Monoclonal mouse anti-VSV G | Sigma | Cat#5507 |
| Monoclonal mouse anti-GFP | Abcam | Cat#ab1218 |
| Polyclonal rabbit anti-GFP | Clontech | Cat#632592 |
| Polyclonal rabbit anti-firefly luciferase | Abcam | Cat#ab21176 |
| Polyclonal goat anti-horseradish peroxidase | Jackson Immunoresearch | Cat#123-005-021 |
| Bacterial and Virus Strains | ||
| VSV | Sean P. Whelan | N/A |
| VSV-GFP | (Whelan et al., 2000) | N/A |
| VSV-Luc (rVSV-LUC) | (Cureton et al., 2009) | N/A |
| SINV-GFP (Sindbis-GFP) | (Saleh et al., 2009) | N/A |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Critical Commercial Assays | ||
| iScript cDNA synthesis kit | Bio-Rad | Cat#1708890 |
| iQ SYBR Green Supermix | Bio-Rad | Cat#1708882 |
| mMessage mMachine | Ambion | Cat#AM1340 |
| Nucleofector Kit L | Lonza | Cat#VCA-1005 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Fly: WT: w1118 | Source unknown | N/A |
| Fly: Dcr2KO: Dcr2L811fsX | This paper | N/A |
| Fly: Ago2KO: Ago2414 | This paper | N/A |
| Fly: Dcr2KO, Glia>GFP: Dcr2L811fsX, UAS-GFP/Dcr2L811fsX; Repo-Gal4/+ | This paper | N/A |
| Fly: Dcr2KO, Neuron>GFP: Dcr2L811fsX, Elav-Gal4/Dcr2L811fsX, UAS-GFP | This paper | N/A |
| Fly: Dcr2KO, Trachea>GFP: Dcr2L811fsX, Breathless-Gal4/Dcr2L811fsX, UAS-GFP | This paper | N/A |
| Fly: Dcr2KO, Control: UAS-Dcr2, w1118; Dcr2L811fsX/Dcr2L811fsX | This paper | N/A |
| Fly: Dcr2KO, Glia>Dcr2: UAS-Dcr2, w1118; Dcr2L811fsX/Dcr2L811fsX; Repo-Gal4/+ | This paper | N/A |
| Fly: Dcr2KO, Neuron>Dcr2: UAS-Dcr2, w1118; Elav-Gal, Dcr2L811fsX/Dcr2L811fsX | This paper | N/A |
| Fly: Control>Ago2 RNAi: UAS-Ago2-IR/+ | This paper | N/A |
| Fly: Actin>Ago2 RNAi: Actin5C-Gal4/UAS-Ago2-IR | This paper | N/A |
| Fly: Neuron>Ago2 RNAi: Elav-Gal4/UAS-Ago2-IR | This paper | N/A |
| Fly: Glia>Ago2 RNAi: UAS-Ago2-IR/+; Repo-Gal4/+ | This paper | N/A |
| Fly: Neuron + Glia>Ago2 RNAi: Elav-Gal4/UAS-Ago2-IR; Repo-Gal4/+ | This paper | N/A |
| Fly: HS>VSV G: Heat Shock-Gal4/+; UAS-VSV G/+ | This paper | N/A |
| Fly: HS>VSV M: Heat Shock-Gal4/+; UAS-VSV M/+ | This paper | N/A |
| Fly: HS>VSV P: Heat Shock-Gal4/UAS-VSV P | This paper | N/A |
| Fly: HS>VSV N: Heat Shock-Gal4/UAS-VSV N | This paper | N/A |
| Fly: HS>VSV L: Heat Shock-Gal4/+; UAS-VSV L/+ | This paper | N/A |
| Fly: Glia>VSV G: Repo-Gal4/UAS-VSV G | This paper | N/A |
| Fly: Control>VSV G: UAS-VSV G/+ | This paper | N/A |
| Fly: Glia>Control: Repo-Gal4/+ | This paper | N/A |
| Fly: Fat body>Control: R4-Gal4/+ | This paper | N/A |
| Fly: Fat body>VSV G: R4-Gal4/UAS-VSV G | This paper | N/A |
| Fly: TS Control>VSV G: +/UAS-VSV G, TubP-Gal80ts | This paper | N/A |
| Fly: TS Glia>VSV G: Repo-Gal4/UAS-VSV G, TubP-Gal80ts | This paper | N/A |
| Fly: TS Fat body>VSV G: R4-Gal4/UAS-VSV G, TubP-Gal80ts | This paper | N/A |
| Fly: HS>VSV GWT: Heat shock-Gal4/UAS-VSV GWT | This paper | N/A |
| Fly: HS>VSV GG124E: Heat shock-Gal4/UAS-VSV GG124E | This paper | N/A |
| Fly: HS>VSVGD137L: Heat shock-Gal4/UAS-VSV GD137L | This paper | N/A |
| Fly: TS Glia>VSV GWT: UAS-VSV GWT/+; Repo-Gal4, TubP-Gal80ts/+ | This paper | N/A |
| Fly: TS Glia>VSV GG124E: UAS-VSV GG124E/+; Repo-Gal4, TubP-Gal80ts/+ | This paper | N/A |
| Fly: TS Glia>VSV GD137L: UAS-VSV GD137L/+; Repo-Gal4, TubP-Gal80ts/+ | This paper | N/A |
| Fly: TS Glia>Apoliner, VSV GWT: UAS-VSV GWT/+; Repo-Gal4, TubP-Gal80ts, UAS-Apoliner/+ | This paper | N/A |
| Fly: TS Glia>Apoliner, VSV GG124E: UAS-VSV GG124E/+; Repo-Gal4, TubP-Gal80ts, UAS-Apoliner/+ | This paper | N/A |
| Fly: TS Glia>Apoliner, VSV GD137L: UAS-VSV GD137L/+; Repo-Gal4, TubP-Gal80ts, UAS-Apoliner/+ | This paper | N/A |
| Fly: TS Glia>GFP: UAS-GFP/+; Repo-Gal4, TubP-Gal80ts/+ | This paper | N/A |
| Fly: TS Glia>GFP, VSV GWT: UAS-GFP, UAS-VSV GWT/+; Repo-Gal4, TubP-Gal80ts/+ | This paper | N/A |
| Fly: TS Glia>GFP, VSV GG124: UAS-GFP, UAS-VSV GG124E/+; Repo-Gal4, TubP-Gal80ts/+ | This paper | N/A |
| Fly: TS Glia>GFP, VSV GD137L: UAS-GFP, UAS-VSV GD137L/+; Repo-Gal4, TubP-Gal80ts/+ | This paper | N/A |
| Anopheles gambiae: strain G3 | Mark Q. Benedict | BEI Resources, Cat#MRA-112 |
| Oligonucleotides | ||
| See Table S1 for qPCR primers | ||
| Recombinant DNA | ||
| pUASP | (Rørth, 1998) | Drosophila Genomics Resource Center, Cat#1189 |
| pValium10-moe | (Perkins et al., 2015) | http://www.ncbi.nlm.nih.gov/nuccore/GU931387 |
| TE:Sindbis:3’eGFP | (Saleh et al., 2009) | N/A |
| pVSV1(+)GFP | (Whelan et al., 2000) | N/A |
| Software and Algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Zoom in Images and Stacks (macro for ImageJ) | Gilles Carpentier | https://imagej.nih.gov/ij/macros/tools/Zoom_in_Images_and_Stacks.txt |
| JWatcher, v1.0 | Macquarie University and UCLA | N/A |
| FlyWalker | (Mendes et al., 2013) | http://biooptics.markalab.org/FlyWalker/ |
| Other | ||
Anopheles gambiae
Anopheles gambiae G3 eggs were obtained from MR4 (contributed by Mark Q. Benedict). Mosquito were reared at 24°C with a 12-h our light cycle. Larvae were grown in distilled water and fed pulverized fish meal. Eclosed adults were then transferred to cages using netting and a light suction vacuum. Adults were fed 10% sucrose diluted in distilled water. Sheep blood was given to females to maintain stocks. For experiments, animals were infected within three days of eclosion and were not blood-fed. Both males and females were used for experiments.
Vesicular stomatitis virus
Vesicular stomatitis viruses (VSV, VSV-GFP, and VSV-Luc) were obtained for Sean P. Whelan. Viruses were derived from a chimera composed of multiple Indiana strains (Orsay, San Juan, etc.). Sequences can be provided upon request.
VSV stocks were produced by infecting BHK-21 cells. BHK-21 cells were grown in complete Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. BHK-21 cells were grown to 90% confluence in T175 tissue-culture treated flasks (Corning 353112). Roughly 2X107 cells were in a flask. Media was removed from flasks and cells were infected with virus diluted in serum-free DMEM. Cells were infected at MOI 0.1. Virus was adsorbed in 5 mL culture volume for 1 hour at 37°C with intermittent agitation. After incubation, media was removed and cells were grown in 15 mL of complete DMEM at 37°C. Cells would exhibit cytopath ic effects at 18–24 hours post-infection. At which point, culturing media was collected and cell debris was pelleted by spinning at 1500 rpm for 10 minutes. Supernatant was passed through a 0.4 um syringe filter.
To concentrate VSV, filtered virus supernatant was spun at 21,000 rpm for 90 minutes at 4°C in a Beckman Ty50.2 Ti rotor. Supernatant was removed and virus was resuspended by incubating virus pellet in NTE buffer (0.1 M sodium chloride, 1 mM EDTA, and 0.01 M Tris, pH 7.4) overnight at 4°C. Fifteen percent sucrose (w/v ) was dissolved in NTE buffer and chilled on ice. Nine milliliters sucrose solution was aliquoted into 10.4 mL polycarbonate tube (Beckman Coulter 355603), and resuspended virus was gently layered on top. Virus was pelleted through the sucrose cushion by spinning at 47,000 rpm for 1 hour at 4°C using Beckman rotor Type 70.1Ti. Pelleted virus was slowly resuspended by incubating pellets in NTE buffer overnight at 4°C. Virus titers were measured by plaque assay and stocks of virus were stored at −80°C.
Sindbis-GFP virus
SINV-GFP was derived from the TE:Sindbis:3’eGFP plasmid, a gift from Raul Andino. Sequence can be provided upon request.
To grow SINV-GFP, plasmid was first linearized using XhoI enzyme. Linear plasmid was then in vitro transcribed using the mMessage mMachine kit (Ambion AM1340) as per manufacturer protocol. Purified mRNA was then electroporated into BHK-21 cells using the Lonza Nucleofector Kit L (VCA-1005). Complete DMEM was collected from cell culture 1 day after nucleofection. Virus in supernatant was adsorbed to BHK-21 cells at 90% confluency in tissue-cultured treated flasks by incubating at 37°C for 30 minutes with intermittent rocking. Infected cells were grown in 3% FBS DMEM for 24 hours. Viral supernatant was concentrated and subsequent batches were grown from this stock by infecting BHK-21 cells at MOI 0.1.
To concentrate SINV-GFP, virus was first precipitated by increasing sodium chloride concentration to 0.5 M and adding 10% (w/v) polyethylene glycol. Virus supernatant was rocked at 4°C for at least 2 hours. Virus was then pellete d by spinning at 15,000 g for 15 minutes in Ty50.2 Ti rotor. Pellet was allowed to slowly resuspend in TNE buffer (0.1 M sodium chloride, 1 mM EDTA, 0.05 M Tris, pH 7.4) overnight at 4°C. Resuspended virus was layered over ice-cold TNE buffer containing 15% sucrose in a polypropylene tube (Beckman Coulter 355603). Virus on sucrose cushion was spun at 40,000 rpm in a 70.1 Ti rotor for 2 hours. Pelleted virus was resuspended slowly by incubating overnight at 4°C with TNE buffer. Virus titers were measured by plaque assay using BHK-21 cells, and virus stocks were stored at −80°C.
Method Details
Antibodies and plasmids
Mouse anti-GFP (JL-8, Clontech) was diluted 1:2000 for Western blotting. Rabbit anti-Beta Actin (4967, Cell Signaling) was used at 1:2000 dilution. Mouse anti-VSV G (V5507, Sigma) was diluted 1:10,000. For microscopy, mouse anti-GFP (ab1218, Abcam) at 1:1000 dilution and rabbit anti-GFP (632592, Clontech) at 1:200 dilution were used depending on which other primary antibodies were used. Anti-luciferase antibody (ab21176, Abcam) was diluted 1:1000, and anti-horseradish peroxidase (123-005-021, Jackson Immunoresearch) was diluted 1:200. DRAQ5 (Thermofisher) was diluted 1:2000.
Using Phusion polymerase (New England Biolabs), VSV genes were amplified from the +1 VSV GFP plasmid (Whelan et al., 2000) with restriction sites added to the 5′ and 3′ ends and consensus Kozak sequence (GCCGCCACC) added before the translation start codons. For VSV G, L, M, N, and P, 5′ and 3′ restriction sites were respectively used as follows: NotI/BamHI, KpnI/NotI, KpnI/XbaI, KpnI/XbaI, and KpnI/BamHI. Restriction enzyme digested DNA was ligated into corresponding restriction sites of the pUASP vector plasmid (Drosophila Genomics Resource Center).
VSV G wild type and mutant genes were cloned into the pValium10-moe vector (Transgenic RNAi Project). VSV GG124E and GD137L were made by site directed mutagenesis. Kozak sequence was added to the translational start site and restriction enzyme sites EcoRI and BglII were respectively added to the 5′ and 3′ ends.
Generation of transgenic animals
pUASP and pValium10 plasmids were injected into embryos to make transgenic flies by Rainbow Transgenic Inc. (Camarillo, CA). pUASP vectors were injected into a W1118 fly background. pValium10 plasmids were injected into flies containing P{nos-phiC31/int.NLS}X, P{CaryP}AttP40 on the X chromosome and 25C6 respectively. Transformants were balanced using yv, wgGla-1/CyO flies.
Dicer2L811fsX and Ago2414 mutant flies were a gift from Richard Carthew. Heat shock-Gal4, Actin5C-Gal4, Breathless-Gal4, and UAS-GFP were gifts from Norbert Perrimon. Elav-Gal4 (8765), Repo-Gal4 (7415), r4-Gal4 (33832), UAS-Dicer2 (25756), TubP-Gal80ts (7018) and UAS-Apoliner (32123) were obtained from Bloomington fly stocks. UAS-Ago2-IR (100356) was obtained from the Vienna Drosophila Resource Center.
Infections and CO2 recovery assay
Virus stocks were grown in BHK-21 cells and purified to a concentration greater than 1011 PFU/mL. For infection, flies were anesthetized using CO2. A minutien pin (Fine Science Tools) was swabbed in the concentrated stock of virus, and flies were pricked on one side of the thorax. Flies were returned to a normal air environment in vials containing standard cornmeal fly media. Survival was measured 3 hours later, and non-viable flies were excluded from the experiment. Alternatively, flies or Anopheles gambiae were injected with virus using Nanoject II (Drummond) in the thorax. Mosquitoes which did not survive 24 hours after injection were removed from the experiment.
For CO2 recovery assay, JWatcher software was used to quantify the recovery rate of anesthetized flies. Recovery was defined by the ability of flies to stand upright on their legs. Simultaneously with the start of the timer, a blowgun (Genesee Scientific) was used to fill fly vials with pure CO2 for 30 seconds. Flies were then transferred into fresh food vials and laid out evenly along the vial wall for observation. Flies were observed for a maximum of 15 minutes. The same procedure was used for N2 anesthesia, except anesthesia lasted for 60 seconds to accommodate for the longer time required for flies to become anesthetized. More than 20 flies were used for each experimental condition for recovery assays and survival data.
Behavioral assays
Flies were assessed for behavioral abnormalities using the startle induced negative geotaxis assay. The method used has been previously described (Barone and Bohmann, 2013). Briefly, flies were collected using CO2 anesthesia and infected with VSV or mock treated. For each experimental condition ≥15 flies were transferred to fresh food vials and were incubated at 29°C. Two and three days later, flies were transfer red to two empty vials taped together. Flies were acclimated to the environment for thirty minutes before negative geotaxis assay began. Flies were startled by tapping vials against the bench top for 10 seconds while being filmed. The films were used to calculate what percentage crawled at least 2 cm above the bottom of the vial 10 seconds after startling ended. After a 30 second rest, the assay was repeated 14 more times.
Dcr2KO flies were tested for gait dysfunction using FlyWalker analysis (Mendes et al., 2013). Flies were infected with VSV by consistently pricking one side of the thorax. Infections were incubated at 29°C. At 2 and 3 dpi, flies were placed in a chamber and filmed to detect light scatter of frustrated Total Internal Reflection caused by footsteps contacting the surface of illuminated optical glass. FlyWalker software was used to measure multiple gait parameters.
Plaque assay
Flies were homogenized in serum-free DMEM (Life Technologies) and filtered through 0.45um filter spin columns (Millipore). Ten-fold serial dilutions were made of each sample and plated onto confluent Vero cells for VSV and BHK-21 cells for SINV in 6-well plates. After a 1 hour incubation at 37°C with gentle shaking every 1 0 minutes, inoculum was removed and Minimal Essential Media (MEM) (ThermoFisher 11700-077) supplemented with 0.12% sodium bicarbonate and 25mM HEPES, pH 6.7 was added onto cells. MEM contained 0.25% agarose for VSV plaque assays and 1% agarose for SINV plaque assays. After 18 hours of incubation Vero cells were fixed using buffered formalin (Sigma HT501128-4L), and BHK cells were fixed after 48 hours. Overlay was removed and cells were stained with 10% ethanol containing 0.1% crystal violet to quantify plaques.
Quantitative real-time PCR
Flies were collected at 3/condition and homogenized in 1mL of RNAbee (Tel-Test, Inc., Cs-501B). To purify RNA, 200uL chloroform was added to each tube and shaken vigorously for 30 seconds. After a 15 minute incubation on ice, tubes were spun at >13,000g for 15 minutes at 4°C. Aqueous phase was collected and transferred to a fresh tube. An equal volume of isopropanol was added, and tube was shaken. RNA was allowed to precipitate at room temperature for 15 minutes before being spun again at >13,000g for 15 minutes at 4°C. RNA pellet was washed with 70% ethanol and spun in same conditions. Pellet was allowed to dry briefly before being dissolved in DEPC-treated water.
cDNA was generated using the iScript cDNA synthesis kit (Bio-Rad 1708890) per manufacturer instructions. Generated cDNA was used for quantitative PCR. Reactions were prepared as per manufacturer instructions for iQ SYBR Green Supermix (Bio-Rad 1708882). Primers are listed in Key Resources Table. Samples were run alongside a serially diluted standard derived from Dcr2KO flies infected with VSV for 3 days at 29°C. PCR was run on the CFX384 Real-Time System (Bio-Rad).
Dissections and Microscopy
Using two pairs of fine tweezers, brains were dissected from adult flies in PBS. Within a 15 minute span, brains were collected into a wire mesh container soaked in PBS and then were fixed for 20 minutes in PBS containing 4% paraformaldehyde. Sindbis-GFP infected fly brains were washed three times in PBS and then mounted onto slides using Prolong Gold antifade reagent if no other additional staining was necessary (Life Technologies). Otherwise, SINV-GFP dissections were treated the same as VSV-infected brains. Dissected, VSV infected brains were washed three times in PBS. Brains were then blocked in blocking buffer (PBS containing 0.5% bovine serum albumin and 0.2% Triton X-100) for 30 minutes. After three washes in wash buffer (PBS containing 0.2% Triton X-100), brains were stained with primary antibodies overnight at 4°C. After three 10-minute washes, brains were incubated in Alexafluor secondary antibodies (Life Technologies) diluted to 1:600 for 1.5 hours at room temperature. DRAQ5 stain (ThermoFisher 62251) was added for an additional 30-minute incubation. Brains were washed three more times at 10 minute intervals before being mounted to glass slides using Prolong Gold antifade reagent. Imaging was done on a conventional fluorescent microscope or a Zeiss axiovert spinning disk confocal microscope, and ImageJ software was used for image analysis.
Quantification and Statistical Analysis
Statistical details can be found in the results, figures, and figure legends. P-values <0.05 were considered statistically significant. All survival data was tested for statistical significance using the Gehan-Breslow-Wilcoxon test. Acute anesthesia recovery assay results were tested for statistical significance using the log-rank test. For each condition in survival and acute recovery assays, at least 20 flies were used. In acute recovery assay graphs, each data point represents one fly.
For viral titer measurements and qPCR data, the unpaired t-test was used to test for statistical significance. For viral titer data, 3 samples of 5–10 flies were collected for each condition for one experiment. Data from three experiments are shown in aggregate. For qPCR data, one sample for each condition was collected, run in triplicate, and averaged. Averages from three experiments are shown in aggregate.
Behavioral assays (negative geotaxis and FlyWalker analysis) utilized two-way ANOVA to test for statistical significance. For one negative geotaxis experiment, each condition had three cohorts of >15 animals/vial. Flies from each cohort were startled and observed 15 times. The 15 replicates were averaged for an N=1. For FlyWalker studies, N refers to the number of animals recorded for each condition. Mean and standard error are represented on graphs. All error bars represent standard error. Prism software was used for statistical analysis.
Supplementary Material
Related to Figure 2. Example processed FlyWalker movie.
Highlights.
Non-lethal CO2 exposure kills insects that were infected with VSV susceptibility
A single viral protein (VSV G), absent of infection, causes CO2 susceptibility
VSV G only needs to be expressed by glial cells to induce CO2
VSV G fusion activity causes lethal neuro-trauma by inducing syncytia formation
Acknowledgments
We would like to thank members of the Kagan lab for helpful discussions, and Paula I. Watnick for graciously sharing her fly facilities. This work was supported by NIH grants AI093589, AI116550, and P30 DK34854 to J.C.K. J.C.K. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
AUTHOR CONTRIBUTIONS
J.C. designed and performed all Drosophila studies. J.C. and Z.M. set up video recordings for FlyWalker analysis and performed mosquito infections. I.B. created, updated, and customized FlyWalker program. J.C.K. and S.M. helped design and plan research. J.C.K. supervised all research. All authors discussed the results and commented on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardet P, Kolahgar G, Mynett A, Miguel-aliaga I, Briscoe J, Meier P, Vincent J. A fluorescent reporter of caspase activity for live imaging. Proc Natl Acad Sci. 2008;105:13901–13905. doi: 10.1073/pnas.0806983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone MC, Bohmann D. Assessing Neurodegenerative Phenotypes in Drosophila Dopaminergic Neurons by Climbing Assays and Whole Brain Immunostaining. J Vis Exp. 2013;74:e50339. doi: 10.3791/50339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-mcclellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–330. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Ghosh Roy S, Bose P, Saha A. Role of EBNA-3 Family Proteins in EBV Associated B-cell Lymphomagenesis. Front Microbiol. 2016;7:457. doi: 10.3389/fmicb.2016.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster - from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14:797. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussereau F. Etude du symptome de la sensibilite au CO2 Produit par le Virus de la Stomatite Vesiculaire chez Drosophila melanogaster. Ann Microbiol (Paris) 1973;124A:535–554. [PubMed] [Google Scholar]
- Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SPJ. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 2009;5:e1000394. doi: 10.1371/journal.ppat.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio TA, Gutierrez KD, Lagunoff M. Kaposi’s Sarcoma-Associated Herpesvirus Downregulates Transforming Growth Factor β2 To Promote Enhanced Stability of Capillary-Like Tube Formation. J Virol. 2014;88:14301–14309. doi: 10.1128/JVI.01696-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz RZ, Rose JK. A Cell Line Expressing Vesicular Stomatitis Virus Glycoprotein Fuses at Low pH. Science. 1984;225:721–723. doi: 10.1126/science.6087454. [DOI] [PubMed] [Google Scholar]
- Foxman EF, Iwasaki A. Genome–virome interactions: examining the role of common viral infections in complex disease. Nat Rev Microbiol. 2011;9:254–264. doi: 10.1038/nrmicro2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen BL, Whitt MA. Vesicular Stomatitis Virus Glycoprotein Mutations That Affect Membrane Fusion Activity and Abolish Virus Infectivity. J Virol. 1995;69:1435–1443. doi: 10.1128/jvi.69.3.1435-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen BL, Whitt MA. Mutations at Two Conserved Acidic Amino Acids in the Glycoprotein of Vesicular Stomatitis Virus Affect pH-Dependent Conformational Changes and Reduce the pH Threshold for Membrane Fusion. Virology. 1996;217:49–57. doi: 10.1006/viro.1996.0092. [DOI] [PubMed] [Google Scholar]
- Freeman MR. Drosophila Central Nervous System Glia. Col Spring Harb Perspect Biol. 2015 doi: 10.1101/cshperspect.a020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann Ja, Imler J-L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, Johnson EA, Sporn PHS, Sznajder JI, Beitel GJ. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc Natl Acad Sci U S A. 2009;106:18710–18715. doi: 10.1073/pnas.0905925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, Medzhitov R. Role of Tissue Protection in Lethal Respiratory Viral-Bacterial Coinfection. Science. 2013;340:1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci U S A. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Heritier P. Sensitivity to CO2 in Drosophila-- A Review. Heredity (Edinb) 1948 Dec;2:352–348. doi: 10.1038/hdy.1948.20. [DOI] [PubMed] [Google Scholar]
- L’Heritier P, Teissier G. Une anomalie physiologique hereditaire chez la Drosophile. Comptes Rendus Hebd Des Séances L ‘ Académie Des Sci. 1937;205:1099–1101. [Google Scholar]
- L’Heritier P, Teissier G. Un mecanisme hereditaire aberrant chez la Drosophile. Comptes Rendus Hebd Des Séances L ‘ Académie Des Sci. 1938a;206:1193–1195. [Google Scholar]
- L’Heritier P, Teissier G. Transmission hereditaire de la sensibilite au gaz carbonique chez la Drosophile. Comptes Rendus Hebd Des Séances L ‘ Académie Des Sci. 1938b;206:1683–1685. [Google Scholar]
- Lee AS-Y, Burdeinick-Kerr R, Whelan SPJ. A ribosome-specialized translation initiation pathway is required for cap-dependent translation of vesicular stomatitis virus mRNAs. Proc Natl Acad Sci U S A. 2012;110:324–329. doi: 10.1073/pnas.1216454109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuff DA, Reese TA, Kimmey JM, Weiss LA, Song C, Zhang X, Kambal A, Duan E, Carrero JA, Boisson B, et al. Phenotypic complementation of genetic immunodeficiency by chronic herpesvirus infection. Elife. 2015;4:e04494. doi: 10.7554/eLife.04494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E, Edwards J, Brown DT. Polycaryocyte Formation Mediated by Sindbis Virus Glycoproteins. J Virol. 1983;45:1083–1089. doi: 10.1128/jvi.45.3.1083-1089.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Bartos I, Akay T, Márka S, Mann RS. Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. Elife. 2013;2:e00231. doi: 10.7554/eLife.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G, Sillans D. Immediate and Latent Effects of Carbon Dioxide on Insects. Annu Rev Entomol. 1989;34:97–116. [Google Scholar]
- Novo-Veleiro I, Alvela-Suárez L, Chamorro A-J, González-Sarmiento R, Laso F-J, Marcos M. Alcoholic liver disease and hepatitis C virus infection. World J Gastroenterol. 2016;22:1411–1420. doi: 10.3748/wjg.v22.i4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, McCall K, Yang-Zhou D, Flackhart I, Binari R, Shim HS, et al. The Transgenic RNAi Project at Harvard Medical School: Resources and Validation. Genetics. 2015;201:843–852. doi: 10.1534/genetics.115.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TA, Bi K, Kambal A, Filali-Mouhim A, Beura LK, Burger MC, Pulendran B, Sekaly RP, Jameson SC, Masopust D, et al. Sequential Infection with Common Pathogens Promotes Human-like Immune Gene Expression and Altered Vaccine Response. Cell Host Microbe. 2016;19:713–719. doi: 10.1016/j.chom.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H, Kondor-Koch C, Garoff H. Cell surface expression of fusogenic vesicular stomatitis virus G protein from cloned cDNA. EMBO J. 1984;3:1477–1483. doi: 10.1002/j.1460-2075.1984.tb01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rij RP, Saleh M-C, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rørth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Rynda-Apple A, Robinson KM, Alcorn JF. Influenza and bacterial superinfection: Illuminating the immunologic mechanisms of disease. Infect Immun. 2015;83:3764–3770. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, Tassetto M, van Rij RP, Goic B, Gausson V, Berry B, Jacquier C, Antoniewski C, Andino R. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature. 2009;458:346–350. doi: 10.1038/nature07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS, Ayres JS, Brandt SM, Costa A, Dionne MS, Gordon MD, Mabery EM, Moule MG, Pham LN, Shirasu-Hiza MM. Drosophila eiger mutants are sensitive to extracellular pathogens. PLoS Pathog. 2007;3:e41. doi: 10.1371/journal.ppat.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelekati E, Shin H, Doering TA, Dolfi DV, Ziegler CG, Beiting DP, Dawson L, Liboon J, Wolski D, Ali MA, et al. Bystander Chronic Infection Negatively Impacts Development of CD8 + T Cell Memory. Immunity. 2014;40:801–813. doi: 10.1016/j.immuni.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Kanbayashi D, Kurata T, Yoshiyama H, Komano J. Enhanced susceptibility of B lymphoma cells to measles virus by Epstein-Barr virus type III latency that upregulates CD150/signaling lymphocytic activation molecule. Cancer Sci. 2014;105:211–218. doi: 10.1111/cas.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan SPJ, Barr JN, Wertz GW. Identification of a Minimal Size Requirement for Termination of Vesicular Stomatitis Virus mRNA: Implications for the Mechanism of Transcription. J Virol. 2000;74:8268–8276. doi: 10.1128/jvi.74.18.8268-8276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Related to Figure 2. Example processed FlyWalker movie.