Abstract
Earlier, low-temperature-active polygalacturonase isoforms from Saccharomyces cerevisiae PVK4 were isolated and purified. Substrate specificity of polygalacturonase isoforms indicated high affinity for pectins and very low enzyme activity towards non-pectic polysaccharides. To characterize the polygalacturonase isoforms, biochemical, spectral, and in silico approaches were used. The apparent K m and V max values for hydrolysis of pectin and galacturonic acid were 0.31 mg/ml and 3.15 mmol min/mg, respectively. Interestingly, the polygalacturonase isoforms were found to be more stable at optimal pH and temperature of 4.5 and 40 °C, respectively. These isoforms were reacted with different metal ions; Cd2+ and Ni2+ severely inhibited the enzyme activity, while Mg2+, Zn2+, Cd2+, Fe2+ Cu2+, and Ni2+ inhibited to a lesser extent, which clearly demonstrated that variations in enzyme activity were due to their differential binding affinity of metal ions. Furthermore, decrease in the viscosity of polygalacturonic acid and citrus pectin by these isoforms was approximately four and six times higher than the rate of release of reducing sugars. This indicates that polygalacturonase isoforms have an endo-mode of action. In addition to the above, thermostability of purified polygalacturonase isoforms was studied by circular dichroism and fluorescence spectroscopy. Circular dichroism showed 18% alpha helix and 57% beta sheets at pH 5, while at pH 7, 8, and 9 there was an increase of random coil. Fluorescence studies revealed small conformational changes, which were observed at 30–50 °C, while unfolding transition region was noticed between 60 and 70 °C. The purified enzyme fractions were analyzed by MALDI-TOF MS. Finally, 3D model structures for isoenzymes of polygalacturonase of S. cerevisiae were generated and validated as good quality models, which are also suitable for molecular interaction studies.
Keywords: Saccharomyces cerevisiae, Polygalacturonase isoforms, Circular dichroism, Fluorescence spectroscopy, MALDI-TOF MS, Homology modeling
Introduction
Pectinolytic enzymes are generally produced by higher plants, fungi, bacteria, and yeasts and have widespread biotechnological as well as industrial applications (Lang and Dornenburg 2000). They act on plant tissues, especially on pectins, causing cell lysis. Pectins are complex structural polysaccharides that occur mainly in the middle lamella and primary cell wall of higher plants. They consist of a backbone of partially methyl-esterified galacturonic acid subunits linked by α-1,4 glycosidic linkages, whereas demethylated compound is known as pectic acid or polygalacturonic acid (Rombouts and Pilnik 1989). Pectinolytic enzymes are classified into two types based on their mechanism of action on the galacturonan backbone, namely pectin esterases (PE) and depolymerases. Depolymerizing enzymes are sub-divided into polygalacturonases that catalyze hydrolytic cleavage of glycosidic bonds and pectin lyases (PL) that break down the glycosidic bonds by β-elimination (Fogarty and Kelly 1983).
Polygalacturonases (PG) are one of the most studied and widely used commercial pectinases. These enzymes are used in various commercial sectors including fruit juice, textile, papermaking industries, fermentation of coffee and tea, oil extractions, and treatment of pectic waste water (Kashyap et al. 2001; Gummadi and Panda 2003; Olsson et al. 2003). These are also used for liquefaction of fruit juices and wines, retting plant fibers, and de-clogging pulps. Endo-polygalacturonases have an important role in fruit softening and plant infection processes (Bateman and Basham 1976), which are also widely used in the industrial processing of fruits and vegetables specifically in beverage clarification (Whitaker 1984).
The studies on production, purification, and characterization of pectinolytic enzymes from fungi and bacteria have been widely reported (Kashyap et al. 2001; Gummadi and Panda 2003; Esquivel and Vogel 2004). However, very few studies addressed the yeast pectinases including pectin methyl esterase (PE) and polygalacturonase (PG) activities in Rhodotorula sp. (Vaughn et al. 1969). Some have demonstrated PG activity in Saccharomyces fragilis (Wimborne and Rickard 1978), PG, PE and PL activities in S. cerevisiae (SCPP 2180) (Gainvors et al. 1994), PG activity in Cryptococcus aquaticus, C. macerans, Cystofilobasidium capitatum and C. lari-marini (Birgisson et al. 2003), and PG activity in Kluyveromyces wickerhamii, K. marxianus and Debaryomyces hansenii (Da Silva et al. 2005).
Considering the fact that pectinases differ in molecular sizes, pH, temperature optima, and other characteristics from different sources of microorganisms as well as their prospective applications in various fields, it is important to understand nature and properties of these enzymes for efficient and effective usage. We have previously reported the production, isolation, and purification of low-temperature-active polygalacturonase isoforms by Saccharomyces cerevisiae strain PVK4 using citrus pectin as substrate (Vijayakumar et al. 2015, 2016). In the present work, the characterizations of purified polygalacturonase isoforms from S. cerevisiae and substrate specificities of the enzymes with pectic and non-pectic polysaccharides were studied. The purified enzyme fractions were analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) along with homology studies.
Materials and methods
Culture conditions, production, and purification of enzyme
The yeast Saccharomyces cerevisiae strain PVK4 was previously isolated from fruit waste and low-temperature-active polygalacturonase isoforms were characterized (Vijayakumar et al. 2015, 2016). In the present study, the same isoforms were used for further characterization.
Polygalacturonase (PG) assay
It was assayed by incubating a mixture of 0.5 ml of 1% PGA (dissolved in 0.05 M acetate buffer, pH 5.0), 9 ml of sodium acetate buffer, and 0.5 ml of culture filtrate at 45 °C for 1 h in a shaker incubator (Baracat et al. 1989). Enzyme activity was measured by quantifying the amount of reducing sugar groups that were liberated after incubation with 1% polygalacturonic acid at 45 °C, by DNS method (Miller 1959) using standard galacturonic acid. One unit of PG activity (U) was defined as the amount of enzyme that liberates 1 μmol of galacturonic acid per min under standard assay conditions.
Characterization of polygalacturonase isoenzymes from S. cerevisiae
Substrate specificity
Polygalacturonase activity was tested with different pectins and was compared with standard polygalacturonic acid (PGA) (Sigma), which was regarded to have 100% activity. The tested substrates were citrus pectin, apple pectin, xylan, glycogen, corn starch, potato starch, dextran, and CM-cellulose. The reaction was performed in 50 mM sodium acetate buffer (pH 4.8) at 50 °C for 30 min and the total amount of reducing sugars liberated in the reaction mixture was determined by the dinitrosalicylic acid (DNS) method using galacturonic acid (GalA) as standard (Gao et al. 2012).
Determination of enzyme kinetics
For the determination of enzyme kinetics, the reaction mixture contains 1 ml of 0.1% polygalacturonic acid in 50 mM acetate buffer (pH 5.0) along with the appropriate amount of enzyme and the kinetic parameters were determined by incubating this mixture at 45 °C for 1 h. For kinetic experiments, 1 unit of enzyme activity was defined as the amount of enzyme which liberates 1 µmol of GalA per minute per ml under standard assay conditions. The Michaelis–Menten constants, K m and V max, for substrate hydrolysis were calculated by double reciprocal Lineweaver–Burk plot (Graph Pad Prism 4.00 for Windows, San Diego, CA, U.S.A (www.graphpad.com).
Effect of pH on enzyme stability
The effect of pH on enzyme stability was evaluated at various pH values by incubating enzyme in 50 mM sodium acetate (pH 3.0–5.0), 50 mM sodium citrate (pH 5.0–5.5), and 50 mM sodium phosphate buffer (pH 6.0–9.0) with 2.5 mg/ml of citrus pectin at 40 °C for 120 min. After incubation, the residual activity of the enzyme was determined.
Effect of temperature on enzyme stability
To evaluate the effect of temperature on the stability of enzyme, purified enzyme was incubated with 100 mM acetate buffer (optimum pH) for 15 min at different temperatures in the range of 4–90 °C and residual activity was assayed soon after the incubation period.
Effect of metal ions on enzyme activity
The effect of metal ions (1 mM) on the enzyme activity of polygalacturonase was determined by evaluating the residual activity. Enzyme was incubated in 100 mM acetate buffer (optimum pH) containing the respective metal ions for 20 min at 4 °C. Tested metal ions were Ca2+, Mg2+, Mn2+, Fe2+, Zn2+, Cu2+, Cd2+, and Ni2+. After incubation, the residual activity was measured.
Determination of change in specific viscosity (η)
Viscometric assays were carried out in a Brookfield DV2TLV Viscometer wherein the reaction mixture consisted of 7 ml of 0.1% PGA and citrus pectin in 0.05 M sodium acetate buffer (pH 5.0) and 1 unit of purified enzyme. Viscosity measurements were conducted at room temperature in a glass tube viscometer and the loss in viscosity measurements was determined at 30-min intervals for 180 min. The hydrolysis of pectins was also determined by following the above-mentioned PG enzyme assay.
Circular dichroism (CD) studies
CD spectroscopy is one of the best methods for examining the secondary structure of protein. The CD spectra of polygalacturonase exhibit two negative bands in the far-ultraviolet region at 208 and 220 nm; these peaks attributed to n → π* transition for the peptide bond of α-helix. The isoenzymes of polygalacturonase were incubated with 0.1 M acetate buffer at different pH (5, 7, 8, and 9) for 24 h at 30 °C. CD spectra were recorded using Jasco J-810 spectropolarimeter using 1-cm path length cell from 190 to 250 nm at 25 °C. Secondary structural elements were calculated using the CDNN 2.2 software program. According to the method of Sreerama and Woody (1993) the CD results were analyzed for individual buffer spectra.
Fluorescence spectroscopy measurements
The steady-state fluorescence emission spectra were analyzed in Perkin Elmer LS-55 fluorimeter (PerkinElmer Corporate, USA) with 1-cm path length cuvette. Here the protein excited at 295 nm and the spectra were recorded and maintained at 30–70 °C temperature range at 300–400 nm. A bandwidth of 5 nm is set for both excitation and emission of light. The collected data were analyzed and the changes in emission fluorescence intensities at 330 nm were evaluated. Evaluations were carried out before and after the incubation period.
MALDI-TOF MS and validation
Protein bands obtained from SDS-PAGE were sliced from the gel and subjected to trypsin digestion. The gel slice was diced into small pieces and placed in new Eppendorf tubes and the gel pieces were destained using destaining solution at 10-min intervals (3–4 times) until the gel pieces turned translucent white. The gels were dehydrated using acetonitrile and SpeedVac till complete dryness. The gel pieces were rehydrated with DTT (dithiothreitol) and incubated for 1 h. After incubation, the DTT solution was removed. Again the gel pieces were incubated with iodoacetamide for 45 min. Supernatant was removed and the gel pieces were incubated with ammonium bicarbonate solution for 10 min. The supernatant was removed and the gel pieces were dehydrated again with acetonitrile for 10 min and SpeedVac till complete dryness. To the dried gel slices, trypsin solution was added and incubated overnight at 37 °C. The trypsin-digested solution was transferred to fresh Eppendorf tubes and the gel pieces were extracted thrice with extraction buffer and the supernatant was collected each time into the above-mentioned Eppendorf tubes and then SpeedVac was applied till complete dryness. The dried pepmix was suspended in TA (trifluoroacetic acid) buffer. The peptides obtained were mixed with HCCA (α-cyano-4-hydroxycinnamic acid) matrix in 1:1 ratio and 2 µl of the resulting solution was spotted onto the MALDI plate. After air drying the sample, it was analyzed on the MALDI-TOF/TOF ULTRAFLEX III MS instrument and further analysis was done with FLEX ANALYSIS SOFTWARE for obtaining the peptide mass fingerprint. The masses obtained in the peptide mass fingerprint were submitted for Mascot search in CONCERNED database for identification of the protein. These peptides were used in in silico studies for the elucidation of its 3D structure.
Modeling study
The amino acid sequences (FASTA sequence) of PG1 and PG2 of S. cerevisiae were obtained from MALDI-TOF and UniProt, respectively, and have been used to search for homologous sequences against PDB using BLASTp at www.ncbi.nlm.nih.gov/blast.html (Altschul et al. 1990). Two templates were downloaded from PDB with PDB ID: 2V5P and PDB ID: 1CZF and these were selected based on high bit score and low E value for the target sequences. The derived homologous sequences of PG1 and PG2 of S. cerevisiae and templates were aligned using command line interface ClustalW (Chenna et al. 2003) to identify the similarities between those sequences. Model was built using MODELER 9v11 (Fiser et al. 2002), this program is an automated approach to comparative modeling by satisfaction of spatial restraints (Browne et al. 1996; Sanchez and Sali 2000). Hundred models were generated and inspected. Later RMS values of all models were inspected using SWISS-PDB Viewer (spdbv) (Peitsch 1995; Guex and Peitsch 1997). Pymol software was used for representation of 3D modeled structures.
Validation of the homology model
Therefore, the generated model was assessed by the geometric quality of the backbone conformation residue interaction and the energy profile of the structure using different methods, including PROCHECK (Laskwoski et al. 1993). ProSA (Sippl 1993) tool is used for internal consistency and reliability. The stereochemical quality checking of the developed structure was carried out by PROCHECK assessing the residues in the favorable regions of Ramachandran plot. Online server PDBSum was used for the prediction of secondary structure of target and template sequences (Baker and Sali 2001). Active site of template structure was obtained by submitting PDB ID 2V5P and 1CZF to PDBSum. Z-score of developed PG1 and PG2 of S. cerevisiae was obtained by submitting its PDB structure (target) to ProSA (Protein Structure Analysis).
Results and discussion
Substrate specificity
The specificity of the purified polygalacturonase isoforms was evaluated with various substrates. The enzyme had no detectable activity against xylan, glycogen, corn starch, potato starch, dextran, and CM-cellulose. Among the tested substrates, the PG isoforms had remarkable specificity with citrus pectin (72%) followed by apple pectin (25%). For the period of increasing esterification, the rate of hydrolysis of pectins was decreased (Table 1). Similarly, polygalacturonase produced by Trichoderma harzianum showed substrate specificity for citrus pectins (64%) (Mohamed et al. 2003), very low enzyme activity was observed towards non-pectic polysaccharides. These results revealed that S. cerevisiae polygalacturonase isoforms had very high affinity and specificity towards PGA and pectins.
Table 1.
Substrate specificity of S. cerevisiae PG isoforms toward different substrates
| Substrates | % Relative activity | |
|---|---|---|
| PG1 | PG2 | |
| PGA | 100 | 100 |
| Citrus pectin | 72 | 69 |
| Apple pectin | 25 | 23 |
| Xylan | 5.2 | 4.3 |
| Glycogen | 2 | 1 |
| Corn starch | 3.1 | 2.5 |
| Potato starch | 3.5 | 2.4 |
| Dextran | 2.9 | 1.8 |
| CM-cellulose | 1.8 | 1.2 |
Determination of enzyme kinetics
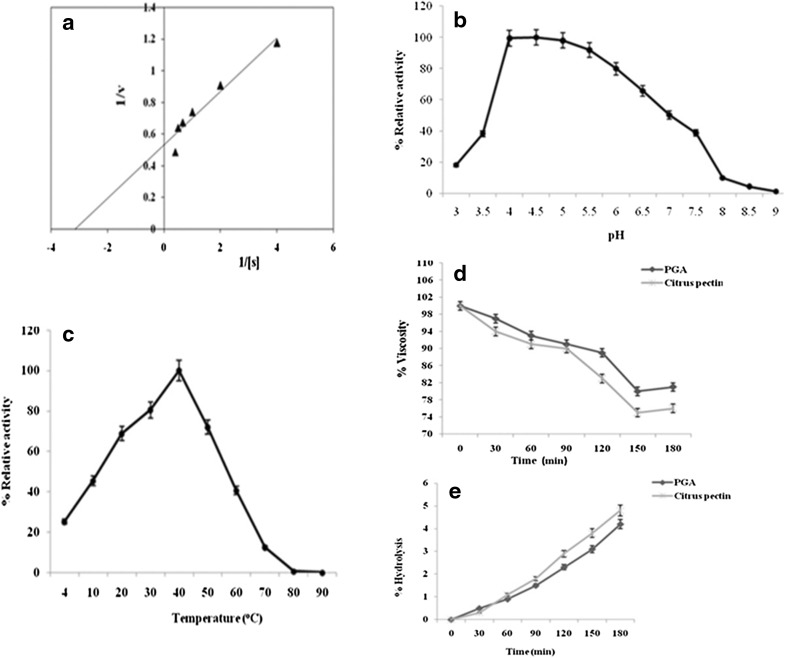
Michaelis–Menten equation was found to fit the reaction of polygalacturonase isoform PG1 from S. cerevisiae (Fig. 1a). V max was found to be 3.15 µmol per unit time GalA equivalent released h−1 and K m was found to be 0.31 mg/ml for PG1. The low K m values indicate that the isolated PG1 has high affinity towards PGA. Similar results were reported in T. harzianum PGII for the kinetic parameter hydrolysis towards PGA and different pectins at pH 5.0 and 40 °C (Mohamed et al. 2003). From this the marked K m and V max values for this enzyme were 0.25, 0.23, and 0.22 mg/ml and 5.7, 3.6, and 4.4 µmol GalA equivalent released h−1, respectively. These results are concurrent with the previous reports on K m values ranging from 2.5 to 14.08 mg/ml of PGs from Aspergillus niger (Parenicova et al. 1998), Neurospora crassa (Polizeli et al. 1991), and Saccharomyces cerevisiae (Blanco et al. 1997). Behere et al. (1993) obtained K m values of 0.24 and 0.12 for two exopolygalacturonases produced by Aspergillus niger. From these results, it can be concluded that the polygalacturonase isoenzyme PG1 had high affinity with low K m and V max towards citrus pectin. Similar results were observed for PG2 (data not included).
Fig. 1.
a Lineweaver–Burk plot of purified enzyme (PG1), b effect of pH on the polygalacturonase (PG1) activity, c effect of temperatures on the polygalacturonase (PG1) activity, d and e changes in viscosity and reducing sugars of PGA and citrus pectin by S. cerevisiae PG1
Effect of pH and temperature on enzyme stability
The stability of PG1 was affected by physical parameters, i.e., pH and temperature (Fig. 1b, c). This enzyme exhibited a maximal activity at 40 °C and pH 4.5 with a gradual decline after pH 6 and at temperature 60 °C. However, the enzyme was found stable over a pH range of 4–6 and temperature range of 30–60 °C. Relative activity of enzyme at 4 °C was recorded as 25.2% which indicates the enzyme is active at low temperature. Few studies on cold active nature of polygalacturonases were previously reported in the literature (Birgisson et al. 2003; Tomoyuki et al. 2002; Nakagawa et al. 2004). The enzyme lost about 70–90% of its activity at pH 3.0 and pH range from 7 to 9 which is supported by previous findings on pH stability of PG from Mucor flavus, where the enzyme was completely stable between pH 2.5 and 6.0 but at pH 7.0, the stability decreased to 60% (Grade et al. 2003). Similar results were observed for PG2 (data not included).
Effect of metal cations
The metal ions tested in this study showed different and partial inhibitory effects on the activity of enzyme except Cd2+ and Ni2+ which severely inhibited the enzyme activity. The presence of metals up to 1 mM Ca2+, Mn2+, and Zn2+ stimulated enzyme activity by 86.7, 96.4, and 81.5%. These results were in agreement with previous findings by Vijayakumar et al. (2016), whereas less inhibition was observed with Mg2+, Zn2+, Cd2+, Fe2+ Cu2+, and Ni2+ on the relative activity of PG isoforms from S. cerevisiae (Table 2). The pectinase of Kluyveromyces wickerhamii was moderately thermostable and retained over 80% activity for 45–50 min at 60 and 70 °C in the presence of 3 mM Ca2+ ions (Moyo et al. 2003). Kapoor et al. (2000) reported that Bacillus MG-CP-2 PG was stimulated by Ca2+ ions.
Table 2.
Effect of different metals on the activity of purified PG isoforms
| Metal ions (1 mM) | % Relative activity | |
|---|---|---|
| PG1 | PG2 | |
| Control | 100 | 100 |
| Ca2+ | 86.7 | 85.5 |
| Mg2+ | 45.2 | 44.5 |
| Mn2+ | 96.4 | 95.3 |
| Fe2+ | 95 | 94 |
| Zn2+ | 81.5 | 80.6 |
| Cu2+ | 39.3 | 38.6 |
| Cd2+ | 24 | 23 |
| Ni2+ | 12 | 10 |
Determination of change in specific viscosity (η)
The mode of action of the enzyme was determined using viscometric analysis. When the PG1 enzyme caused 19 and 24% decrease in the viscosity of PGA and citrus pectin solutions, the extent of hydrolysis was 4.2 and 4.8%, respectively (Fig. 1d, e). These results reveal that the rate of enzyme-catalyzed reactions and decrease in viscosity of solutions of PGA and citrus pectin was approximately four and six times higher than the rate of release of reducing sugars. This showed an endo-mode of action of PG (Gillespie et al. 1990). According to Guebitz et al. (1996), a rapid decrease in viscosity corresponding to the release of reducing groups specify that internal glycosidic bonds of the polymer were divided by the enzyme preferentially (endo-enzymes), while a relatively slow change in viscosity specifies the process of hydrolysis more at the end of the chains (exo-enzymes). Similar results were observed for PG2 (data not included).
Circular dichroism (CD) studies
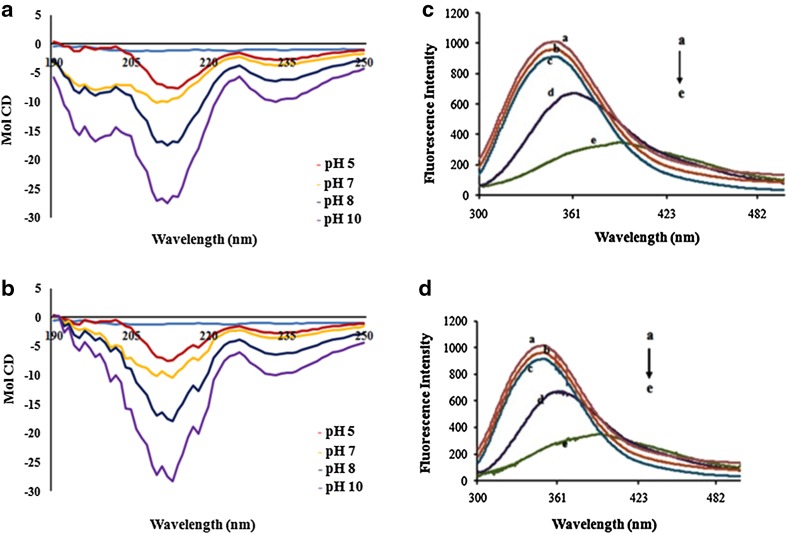
The CD structure of polygalacturonases mainly shows a significant decrease in secondary structure at different pH (5, 7, 8, and 9) and at room temperature over 30 °C, in accordance with the differences in enzyme activity and transition temperatures (Tm). The polygalacturonases showed the secondary structural change at different pH in different buffers, approximately 18% of alpha helix and 57% of beta sheets were observed at pH 5 (Fig. 2a, b) which is in accordance with the findings of Niture et al. (2008). Whereas at pH 7, 8, and 9 there was an increase in random coil, while decrease in the alpha helix and beta sheets which explains the distortion of secondary structure. Thus, at pH 5 in acetate buffer, the polygalacturonases have shown good secondary structural elements. These results confirm that these polygalacturonases are stable at pH 5 and at temperature 30 °C. The secondary structural changes of enzymes at different pH are shown in Table 3. The unfolding transition curves of polygalacturonases were analyzed using a two-state model at pH 7, 8, and 9 (Pace 1990).
Fig. 2.
a and b Far-UV CD spectra of PG1 and PG2 at different pH (5, 7, 8, and 9) and room temperature (around 30 °C). Secondary structure contents were estimated from these spectra and are presented as α-helix and β-sheet. c and d Temperature-induced unfolding profile of PG1 and PG2 at pH 7.0 monitored by changes in tryptophan fluorescence intensity at 300–400 nm. The temperature dependence transition curve was observed with significant fluorescence quenching at 360 nm, corresponding to a complete unfold of protein and a → e represents 30–70 °C
Table 3.
Effect of pH on PG1 and PG2 structural changes in S. cerevisiae
| pH | Activity (U/ml) | Helix (%) | Anti-parallel (%) | Parallel (%) | Beta-turn (%) | Random coil (%) | Total sum (%) |
|---|---|---|---|---|---|---|---|
| PG1 | |||||||
| 5 | 0.40 | 17.70 | 21.70 | 12.30 | 0.90 | 47.40 | 100 |
| 7 | 0.28 | 18.40 | 42.00 | 15.00 | 0.80 | 48.00 | 100 |
| 8 | 0.22 | 16.50 | 20.30 | 11.90 | 0.10 | 52.20 | 100 |
| 9 | ND | 12.40 | 18.30 | 10.30 | 0.70 | 58.30 | 100 |
| PG2 | |||||||
| 5 | 0.36 | 16.20 | 18.00 | 11.00 | 0.60 | 49.00 | 100 |
| 7 | 0.25 | 17.50 | 44.20 | 13.50 | 0.60 | 46.10 | 100 |
| 8 | 0.18 | 15.20 | 16.20 | 9.40 | 0.40 | 56.30 | 100 |
| 9 | ND | 14.50 | 19.20 | 12.20 | 0.40 | 59.20 | 100 |
Fluorescence studies
The thermostability of PG1 and PG2 was also evaluated by fluorescence spectroscopy at different temperature intervals of 30–70 °C. The temperature induced unfolding profile of pectinase at pH 7.0, which is confirmed by changes in tryptophan fluorescence intensity at 300–400 nm. Transition curve was temperature dependent with significant fluorescence quenching at 360 nm, corresponding to a complete unfold of protein from 60 to 70 °C (Fig. 2c, d). The emission spectra indicated small conformational changes at 30–50 °C. The structural unfolding of protein was more prominent at 60 and 70 °C, which indicates that the quenching or the transition in fluorescence band between 360 and 380 nm corresponds to tryptophan side chain exposure. Similar results were reported by Celestino et al. (2006), for an extracellular pectinase (PECI) isolated from Acrophialophora nainiana. These observations support the low enzymatic activity and less stability of pectinase at 60 and 70 °C.
MALDI-TOF MS protein identification
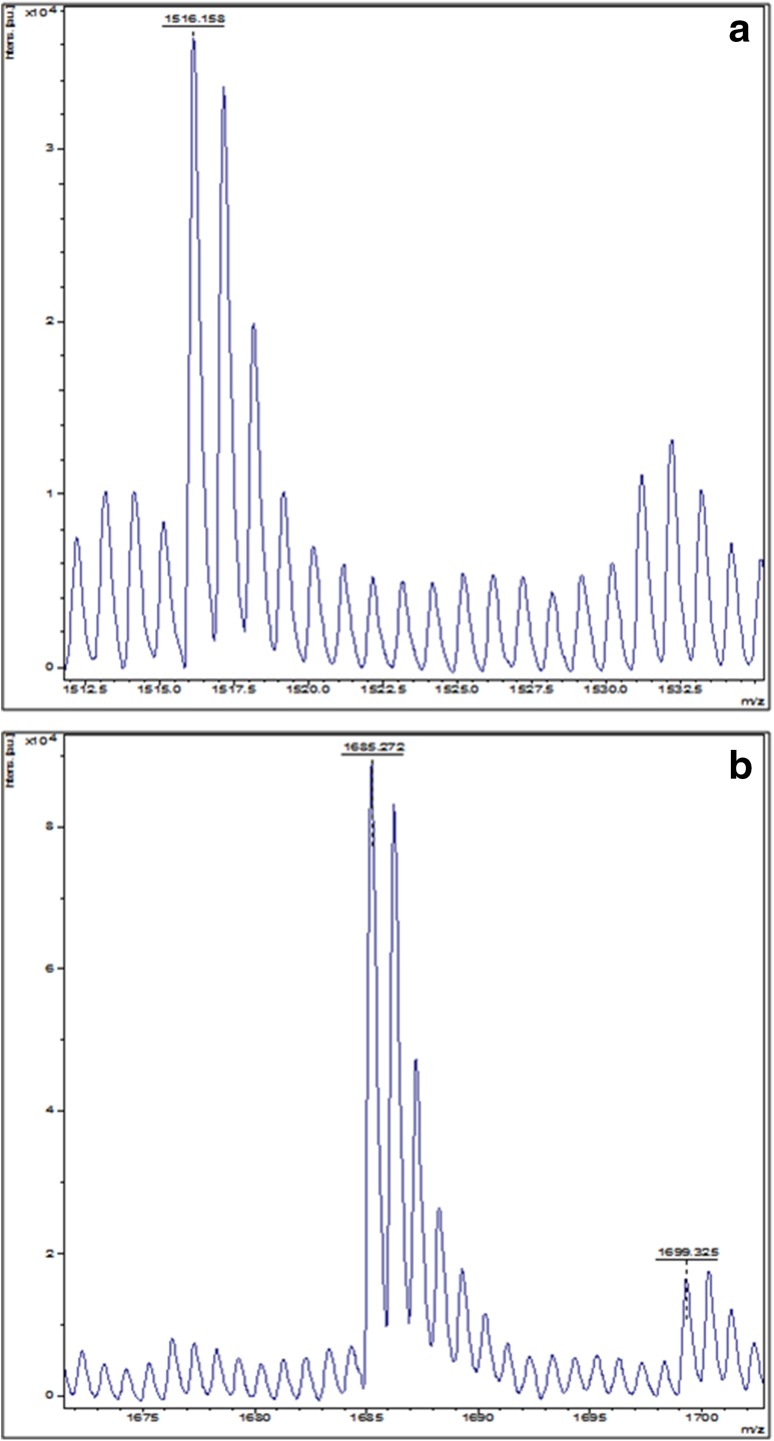
The two purified protein fractions from SDS-PAGE were subjected to MALDI-TOF MS analysis and identified as isoforms of polygalacturonases. From this analysis, 17 and 16 amino acids of similar peptide sequences of isolated enzyme fractions were obtained (Fig. 3a, b). These peptide sequences were matched with pectinase gene in the RCSB Protein Data Bank. The masses obtained in the peptide mass fingerprint were submitted for Mascot search in “CONCERNED” database for identification of the protein. The isolated proteins were identified as polygalacturonase isoenzymes of S. cerevisiae strain PVK4 isolate (Vijayakumar et al. 2015, 2016).
Fig. 3.
a and b MALDI-TOF MS spectra of PG1 and PG2 from S. cerevisiae
Homology modeling of pectinases
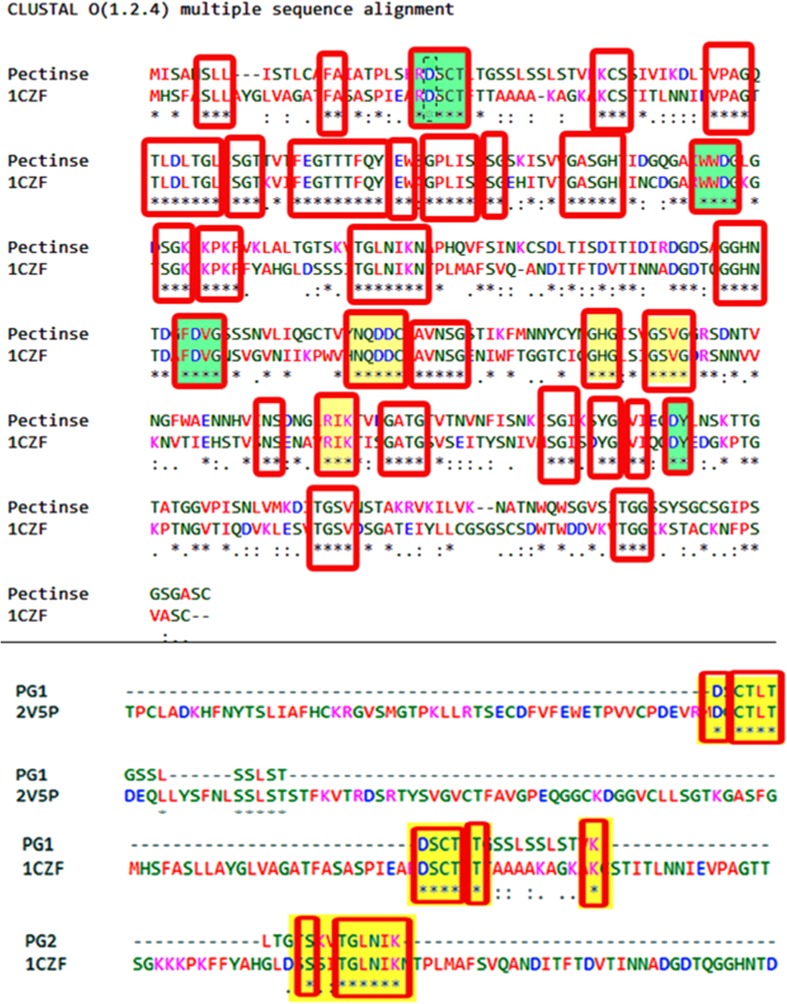
The search using the BLASTp alignment algorithm within the PDB database showed that a variety of potential templates for molecular modeling of PG1 and PG2 of S. cerevisiae was conserved with templates PDB ID: 2V5P (Brown et al. 2008) and 1CZF (van Santen et al. 1999) and the sequence pairwise alignment of pectinases, PG1 and PG2, are shown in red-colored boxes in Fig. 4a–c. The 3D model structures of PG1 and PG2 pectinases of S. cerevisiae were generated using these templates.
Fig. 4.
a Sequence alignment of the target sequences PG1 with template 2V5P, b PG2 with template 1CZF, c multiple sequence alignment of the target sequences PG1 and PG2 with template pectinase, d and e developed 3D structures of PG1 and PG2, f developed secondary structure of polygalacturonase of S. cerevisiae, the structure representing helix and β sheets
For each protein 100 models were developed through Linux-based offline server of Modeler 9v11, among them the one which is having a high percentage of amino acids is the most favorable region in the Ramachandran plot, and lowest RMS values were chosen for further analysis. The 3D structure of protein is a significant source of information to know the function of a protein, its interactions with other compounds (ligands, proteins, DNA), and also to understand the phenotypical effects of mutations (Tramontano 1998). Structural refinement of the developed model was performed using Swiss Pdb Viewer (spdbv) (Peitsch 1995).
Evaluation of quality of PG1 and PG2 of S. cerevisiae models
The total quality of G-factor was 0.3, which indicates a good quality model (acceptable values of the G-factor in PROCHECK are between 0 and 0.5, with the best models displaying values close to zero) (Laskowski et al. 1993). Thus, the approach that we followed to model proteins of PG1 and PG2 of S. cerevisiae resulted in reliable structures. The energy profile of the PG1 and PG2 of S. cerevisiae homology models is consistent with reliable conformation based on its similarity to that of the templates PDB ID: 2V5P and 1CZF (Fig. 4a, b).
The generated 3D model validation was carried out using Ramachandran plot computed with PROCHECK program to study the stereochemical quality of a protein structure by checking the detailed residue by residue level (Laskowski et al. 1996). The Ramachandran plot analysis showed that residues of proposed peptides of PG1 and PG2 of S. cerevisiae were 82.3 and 85.7% in the most favorable region and 15 and 12% in the allowed region, respectively. The root mean square deviation (RMSD) between the C-alpha atom of the template and the model was 0.93 Å, indicating close homology. Thus, the selected structures are proposed proteins of 3D models of PG1 and PG2 of S. cerevisiae, as the proteins have a reasonable and consistent conformation and can be used for further docking study. Thus, both PROCHECK and RMSD confirmed that both protein structures were reliable and can be used for further studies. The selected 3D structures are depicted in Fig. 4d, e. Superposition of the pectinases of S. cerevisiae with template structure PDB ID: 1CZF is presented as cartoon diagram. The secondary structure of pectinases of S. cerevisiae are colored in yellow, while the template is shown in red (Fig. 4f).
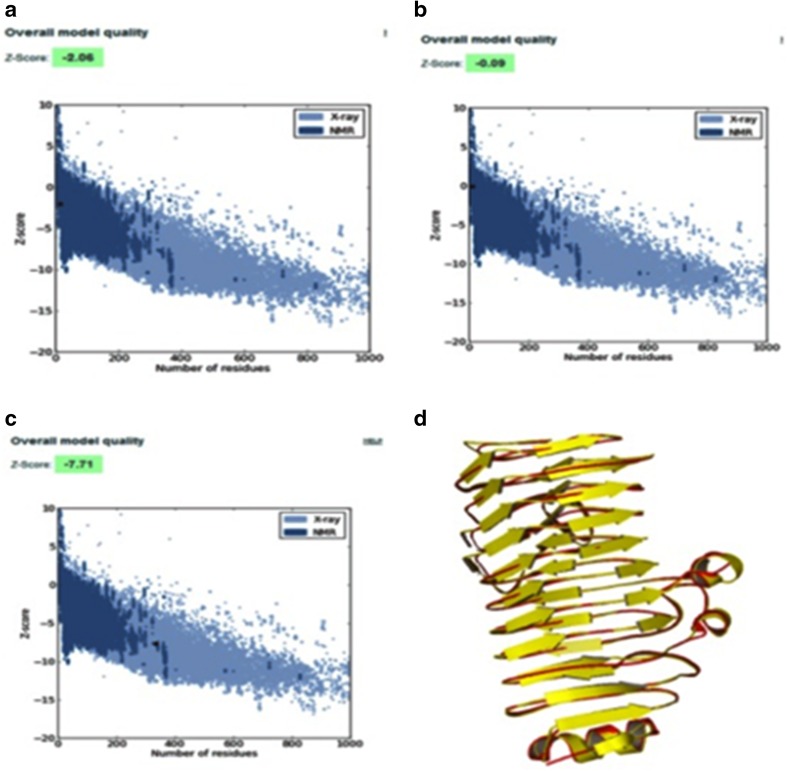
Evaluation of the energy-minimized model of PG1 and PG2 of S. cerevisiae with ProSA web reveals that the Z-score value is −2.06, −0.09, and −7.71, respectively (Fig. 5a–c) which are in the range of native conformations of the crystal structures. ProSA web analysis had shown that overall the residue energy of PG1 and PG2 of S. cerevisiae models was largely negative except for some peaks in the middle region. Figure 5d does not show major structural changes in comparison with the preliminary model, which is consistent with the relatively low RMSD values.
Fig. 5.
a, b and c ProSA web Z-scores of all protein chains in PDB determined by X-ray crystallography (light blue) and NMR spectroscopy (dark blue) with respect to their length, d superposition of the polygalacturonases of S. cerevisiae with template structure 1CZF. The structures are presented as cartoon diagram. The polygalacturonase of S. cerevisiae structure is colored in yellow; the 1CZF structure is colored in red
The CAZy database (www.cazy.org) has offered for more than 15 years online and continuously updated the classification of proteins based on sequence similarity to functionally characterized CAZymes (Lombard et al. 2013). O-Glycosyl hydrolases (EC: 3.2.1.) are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycosyl hydrolases based on sequence similarity has led to the definition of 85 different families (Ruttkowski et al. 1990; Huang and Schell 1990). This classification is available on the CAZy (CArbohydrate-Active EnZymes) web site. Glycoside hydrolase family 28 (GH28) comprises enzymes with several known activities: polygalacturonase (EC:3.2.1.15), exo-polygalacturonase (EC:3.2.1.67), exo-polygalacturonase (EC:3.2.1.82), and rhamnogalacturonase (EC not defined). Both PG1 and PG2 sequences were compared with PDB ID: 2V5P and 1CZF separately, and the aligned sequences are highlighted in yellow (Fig. 6). PDB ID: 1CZF has shown best single pairwise align with both PG1 and PG2. The single pairwise alignment comparison of pectinases and PDB ID: 1CZF shows NTD, DD, GHG, and RIK regions and confirmed that the pectinase belongs to GH28 family (Fig. 6) (Palanivelu 2006). In Fig. 4c is shown the single pairwise analysis of binding regions of pectinases; PG1 and PG2 have arginine, isoleucine, and lysine of RIK region and are highlighted with yellow color blocks and the isoform enzymes were considered under family GH28.
Fig. 6.
Analysis of substrate-binding regions: the blocks indicate the important sequence–structural features/conserved motifs and green colored blocks indicate active site Asp (proton donor regions) in the enzyme, yellow color blocks are related to arginine, isoleucine and lysine of RIK region
Figure 7a, b represents active catalytic residues of PG1 and PG2 in ball and stick models. In PG1, the catalytic residues are aspartic acid-1, serine-2 & 14, cystine-3, valine-15, and lysine-17. In PG2, the catalytic residues are leucine-1 & 3, valine-9, asparagine-13, isoleucine-14, and lysine-15. The above residues were aligned with template residues in sequence alignment. For this, our FASTA sequences of PG1 and PG2 of S. cerevisiae were submitted to PDBSum and ProFunc programs to find out the active catalytic residues. We have also submitted our FASTA sequence of PG1 and PG2 of S. cerevisiae to the servers of CAZy database, EMBL EBI Pfam (Protein Family http://pfam.xfam.org/ncbiseq/398365647) and NCBI (Conserved Protein Domain Family). They classified our PG1and PG2 protein sequences as belonging to glycosyl hydrolases family 28 based on sequence similarity (Lombard et al. 2013; Marchler-Bauer et al. 2008). Figure 7c shows the substrate binding with enzyme targets that are represented in white color sticks. This may play a key role in developing ligands or inhibitors for studying the enzyme mechanism.
Fig. 7.
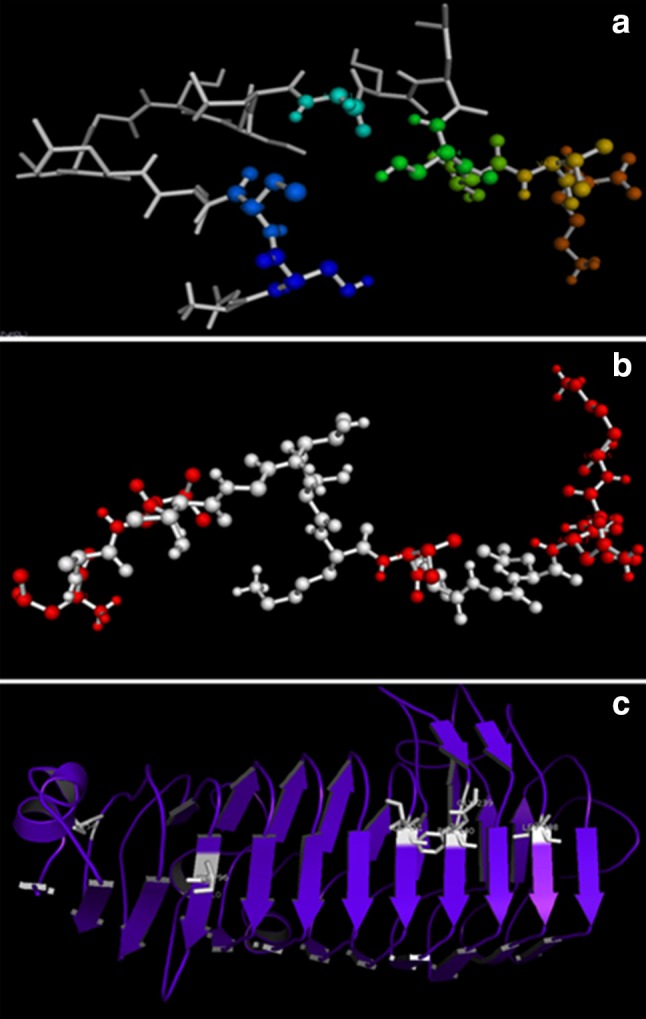
a and b Ball and stick models representing the active catalytic residues of PG1 and PG2; c substrate binding with enzyme target represented in white color sticks
Conclusions
This study presents data on the analysis of various factors that influence the activity of polygalacturonase isoforms as well as their characterization using MALDI-TOF, fluorescence, and CD spectral analyses. These polygalacturonase isoforms are active at low temperature and moderately thermostable exhibiting maximum stability over acidic and alkaline pH, prompting their use in degumming and retting of fiber crops and pretreatment of pectic wastewater from fruit juice industries. The results from substrate specificity and amino acid sequence suggest that they are endo-polygalacturonases. This study also provides valuable insight into the homology of the polygalacturonases for further research and rational designs of new generation of wide-spectrum activators.
Acknowledgements
This work was financially supported by the DST-SERB (SB/SO/PS/11/2013). We are also thankful to CDFD, Hyderabad, for providing the MALDI-TOF instrumentation facility. Special thanks to Dr. S.C. Basappa, former Deputy Director and Scientist, CFTRI, Mysore for his encouragement and critical comments on the manuscript.
Compliance with ethical standard
Conflict of interest
The authors do not have any conflict of interest for this research work.
References
- Altschul SF, Gish W, Miller W (1990) Basic local alignment search tool. J Mol Bio 403–410. doi:10.1016/S0022-2836(05)80360-2 [DOI] [PubMed]
- Baker D, Sali A. Protein structure prediction and structural genomics. Science. 2001;294:93–96. doi: 10.1126/science.1065659. [DOI] [PubMed] [Google Scholar]
- Baracat MC, Valentim C, Muchovej JJ, Silvia DO. Selection of pectinolytic fungi for degumming natural fibres. Biotechnol Lett. 1989;11(12):809–902. doi: 10.1007/BF01026849. [DOI] [Google Scholar]
- Bateman D, Basham H (1976) Degradation of plant cell walls and membranes by microbial enzymes. In: Heitefuss R, Williams P (eds), Physiological plant pathology, pp 316–355. Springer, Berlin. doi:10.1007/978-3-642-66279-9_13
- Behere A, Satyanarayan V, Padwal-Desai SR. Separation and limited characterization of three polygalacturonases of Aspergillus niger. Enzyme Microb. 1993;15:158–161. doi: 10.1016/0141-0229(93)90042-Z. [DOI] [Google Scholar]
- Birgisson H, Delgado O, Garcia AL, Hatti-kaul R, Mattiasson B. Cold adapted yeasts as producers of cold-active polygalacturonases. Extremophiles. 2003;7:185–193. doi: 10.1007/s00792-002-0310-7. [DOI] [PubMed] [Google Scholar]
- Blanco P, Sieiro C, Reboredo NM, Villa TG. Genetic determination of polygalacturonase production in wild-type and laboratory strains of Saccharomyces cerevisiae. Arch Microbiol. 1997;167:284–288. doi: 10.1007/s002030050445. [DOI] [PubMed] [Google Scholar]
- Brown J, Delaine C, Zaccheo OJ, Siebold C, Gilbert RJ, Van Boxel G, Denley A, Wallace JC, Hassan AB, Forbes BE, Jones EY. Structure and functional analysis of the Igf-II/Igf2R interaction. EMBO J. 2008;27:265–276. doi: 10.1038/sj.emboj.7601938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne WJ, North AC, Philips DC. A possible three dimensional structure of bovine alpha-lactalbumin based on that of hen’s egg-white lysozyme. J Mol Bio. 1996;42:65–86. doi: 10.1016/0022-2836(69)90487-2. [DOI] [PubMed] [Google Scholar]
- Celestino SMC, de Freitas SM, Medrano FJ, de Sousa MV, Ferreira Filho EX. Purification and characterization of a novel pectinase from Acrophialophora nainiana with emphasis on its physicochemical properties. J Biotechnol. 2006;123(1):33–42. doi: 10.1016/j.jbiotec.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31(13):3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva EG, Borges MF, Medina C, Piccoli RH, Schwan RF. Pectinolytic enzymes secreted by yeasts from tropical fruits. FEMS Yeast Res. 2005;5:859–865. doi: 10.1016/j.femsyr.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Esquivel JC, Vogel CE. Purification and partial characterization of an acidic polygalacturonase from Aspergillus kawachii. J Biotechnol. 2004;110:21–28. doi: 10.1016/j.jbiotec.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Fiser A, Feig M, Brooks CL, III, Sali A. Evolution and physics in comparative protein structure modeling. Acc Chem Res. 2002;35:413–421. doi: 10.1021/ar010061h. [DOI] [PubMed] [Google Scholar]
- Fogarty WM, Kelly CT (1983) Pectic enzymes. In: Fogarty WM (ed) Microbial enzymes and biotechnology. Applied Science Publishers, London, pp 131–182. ISBN: 0853341850
- Gainvors A, Karam N, Lequart C, Belarbi A. Use of Saccharomyces cerevisiae for the clarification of fruit juices. Biotechnol Lett. 1994;16:1329–1334. [Google Scholar]
- Gao L, Gao F, Wang LS, Geng CL, Zhao J, Qu YB. N-glycoform diversity of CBHIs and the new features of a non-enzymatic glycoform from Penicillium decumbens. J Biol Chem. 2012;287:15906–15915. doi: 10.1074/jbc.M111.332890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie AM, Cook K, Coughlan MP. Characterization of an endo polygalacturonase produced by solid-state cultures of the aerobic fungus Penicillium capsulatum. J Biotechnol. 1990;13:279–292. doi: 10.1016/0168-1656(90)90076-N. [DOI] [Google Scholar]
- Grade RV, Van Driessche G, Van Beeumen J, Bhat MK. Purification, characterization and mode of action of an endopolygalacturonase from the psychrophilic fungus Mucor flavus. Enzyme Microb Technol. 2003;32:321–330. doi: 10.1016/S0141-0229(02)00291-0. [DOI] [Google Scholar]
- Guebitz GM, Schnitzhofer W, Balakrishnan H, Steiner W. Two mannanases from Sclerotium rolfsii in total chlorine-free bleaching of softwood kraft pulp. J Biotechnol. 1996;50:181–188. doi: 10.1016/0168-1656(96)01563-5. [DOI] [Google Scholar]
- Guex N, Peitsch MC. SWISS MODEL and the Swiss Pdb Viewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Gummadi SN, Panda T. Purification and biochemical properties of microbial pectinases—a review. Process Biochem. 2003;38:987–996. doi: 10.1016/S0032-9592(02)00203-0. [DOI] [Google Scholar]
- Huang JH, Schell MA. DNA sequence analysis of pglA and mechanism of export of its polygalacturonase product from Pseudomonas solanacearum. J Bact. 1990;172(7):3879–3887. doi: 10.1128/jb.172.7.3879-3887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Beg QK, Bhushan B, Dadhich KS, Hoondal GS. Production and partial purification of a thermo-alkali stable polygalacturonase from Bacillus sp. MG-cp-2. Process Biochem. 2000;36:467–473. doi: 10.1016/S0032-9592(00)00238-7. [DOI] [Google Scholar]
- Kashyap DR, Vohra PK, Chopra S, Tewari R. Applications of pectinases in the commercial sector: a review. Bioresour Technol. 2001;77:215–227. doi: 10.1016/S0960-8524(00)00118-8. [DOI] [PubMed] [Google Scholar]
- Lang C, Dornenburg H. Perspectives in the biological function and the technological application of polygalacturonases. Appl Microbiol Biotechnol. 2000;53:366–375. doi: 10.1007/s002530051628. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26(2):283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- Laskowski RA, Rullmann JAC, MacArthur MW, Kaptein R, Thornton JM (1996) AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR 8(4):477–486. doi:10.1007/BF00228148 [DOI] [PubMed]
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2013;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, He S. CDD: specific functional annotation with the conserved domain database. Nucleic Acids Res. 2008;37:D205–D210. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mohamed SA, Christensen TMIE, Mikkelsen JD. New polygalacturonases from Trichoderma reesei: characterization and their specificities to partially methylated and acetylated pectins. Carbohydr Res. 2003;338:515–524. doi: 10.1016/S0008-6215(02)00398-1. [DOI] [PubMed] [Google Scholar]
- Moyo S, Gashe BA, Collison EK, Mpuchane S. Optimising growth conditions for the pectinolytic activity of Kluyveromyces wickerhamii by using response surface methodology. Int J Food Microbiol. 2003;85(1):87–100. doi: 10.1016/S0168-1605(02)00503-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nagaoka T, Taniguchi S, Miyaji T, Tomizuka N. Isolation and characterization of psychrophilic yeasts producing cold adapted pectinolytic enzymes. Lett Appl Microbiol. 2004;38(5):383–387. doi: 10.1111/j.1472-765X.2004.01503.x. [DOI] [PubMed] [Google Scholar]
- Niture SK, Kumar AR, Parab PB, Pant A. Inactivation of polygalacturonase and pectate lyase produced by pH tolerant fungus Fusarium moniliforme NCIM 1276 in a liquid medium and in the host tissue. Microbiol Res. 2008;163(1):51–62. doi: 10.1016/j.micres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Olsson L, Christensen TMIE, Hansen KP, Palmqvist EA. Influence of the carbon source on production of cellulases hemicellulases and pectinases by Trichoderma reesei Rut C-30. Enzyme Microbial Technol. 2003;33:612–619. doi: 10.1016/S0141-0229(03)00181-9. [DOI] [Google Scholar]
- Pace CN. Conformational stability of globular proteins. Trends Biochem Sci. 1990;15:14–17. doi: 10.1016/0968-0004(90)90124-T. [DOI] [PubMed] [Google Scholar]
- Palanivelu P. Polygalacturonases: active site analyses and mechanism of action. Indian J Biotechnol. 2006;5:148–162. [Google Scholar]
- Parenicova L, Benen JAE, Kester HCM, Visser J. pga E encodes a fourth member of the endo polygalacturonase gene family from Aspergillus niger. J Biochem. 1998;251:72–80. doi: 10.1046/j.1432-1327.1998.2510072.x. [DOI] [PubMed] [Google Scholar]
- Peitsch MC (1995) Protein modelling by E-mail. BioTechnology 13(7):658–660
- Polizeli MT, Jorge JA, Terenzi HF. Pectinase production by Neurospora crassa: purification and biochemical characterization of extracellular polygalacturonase activity. J Gen Microbiol. 1991;137:1815–1823. doi: 10.1099/00221287-137-8-1815. [DOI] [PubMed] [Google Scholar]
- Rombouts FM, Pilnik WL. Pectic enzymes. In: Rose AH, editor. Economic microbiology. London: Academic Press; 1989. pp. 227–282. [Google Scholar]
- Ruttkowski E, Labitzke R, Khanh NQ, Löffler F, Gottschalk M, Jany KD. Cloning and DNA sequence analysis of a polygalacturonase cDNA from Aspergillus niger RH5344. BBA-Gene Struct Expr. 1990;1087(1):104–106. doi: 10.1016/0167-4781(90)90130-T. [DOI] [PubMed] [Google Scholar]
- Sanchez R, Sali A. Comparative protein structure modeling. Introduction and practical examples with modeller. Method Mol Biol. 2000;143:97–129. doi: 10.1385/1-59259-368-2:97. [DOI] [PubMed] [Google Scholar]
- Sippl MJ. Recognition of errors in three dimensional structures of proteins. Proteins Struct Funct Bioinf. 1993;17(4):355–362. doi: 10.1002/prot.340170404. [DOI] [PubMed] [Google Scholar]
- Sreerama N, Woody RW. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal Biochem. 1993;209:32–44. doi: 10.1006/abio.1993.1079. [DOI] [PubMed] [Google Scholar]
- Tomoyuki N, Kaichiro Y, Tatsuro M, Noboru T. Cold-active pectinolytic activity of psychrophilic-basidiomycetous yeast Cystofilobasidium capitatum strain PPY-1. J Biosci Bio Eng. 2002;94(2):175–177. doi: 10.1016/S1389-1723(02)80140-2. [DOI] [PubMed] [Google Scholar]
- Tramontano A. Homology modeling with low sequence identity. Methods. 1998;14(3):293–300. doi: 10.1006/meth.1998.0585. [DOI] [PubMed] [Google Scholar]
- van Santen Y, Benen JA, Schroter KH, Kalk KH, Armand S, Visser J, Dijkstra BW. 68-A crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis. J Biol Chem. 1999;274:30474–30480. doi: 10.1074/jbc.274.43.30474. [DOI] [PubMed] [Google Scholar]
- Vaughn ILH, Jakubczyl T, McMillan JD, Higgins TE, Dave BA, Crampton VM. Some pink yeast associated with softening of olives. Appl Microbiol. 1969;18:771–775. doi: 10.1128/am.18.5.771-775.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar P, Ramesh B, Rajagopal S, Reddy OVS. Low temperature active pectinases production by Saccharomyces cerevisiae isolate and their characterization. Biocatal Agric Biotechnol. 2015;4:70–76. [Google Scholar]
- Vijayakumar P, Sudheer Kumar Y, Sathyanarayana NG, Rajagopal S, Reddy OVS. Enhanced production of pectinase by Saccharomyces cerevisiae isolate using fruit and agro-industrial wastes: its application in fruit and fiber processing. Biocatal Agric Biotechnol. 2016;6:40–50. [Google Scholar]
- Whitaker JR. Pectic substances, pectic enzymes and haze formation in fruit juices. Enzyme Microb Technol. 1984;6:341–349. doi: 10.1016/0141-0229(84)90046-2. [DOI] [Google Scholar]
- Wimborne MP, Rickard PAD. Pectinolytic activity of Saccharomyces fragilis cultured in controlled environments. Biotechnol Bioeng. 1978;20:231–242. doi: 10.1002/bit.260200206. [DOI] [PubMed] [Google Scholar]