Abstract
In order to achieve better outcomes for treatment and in the prophylaxis of malaria, it is imperative to develop a sensitive, specific, and accurate assay for early diagnosis of Plasmodium falciparum infection, which is the major cause of malaria. In this study, we aimed to develop a loop-mediated isothermal amplification (LAMP) assay with P. falciparum unique genes for sensitive, specific, and accurate detection of P. falciparum infection. The unique genes of P. falciparum were randomly selected from PlasmoDB. The LAMP primers of the unique genes were designed using PrimerExplorer V4. LAMP assays with primers from unique genes of P. falciparum and conserved 18S rRNA gene were developed and their sensitivity was assessed. The specificity of the most sensitive LAMP assay was further examined using genomic DNA from Plasmodium vivax, Plasmodium yoelii and Toxoplasma gondii. Finally, the unique gene-based LAMP assay was validated using clinical samples of P. falciparum infection cases. A total of 31 sets of top-scored LAMP primers from nine unique genes were selected from the pools of designed primers. The LAMP assay with PF3D7_1253300-5 was the most sensitive with the detection limit 5 parasites/μl, and it displayed negative LAMP assay with the genomic DNA samples of P. vivax, P. yoelii, and T. gondii. The LAMP assay with PF3D7_0112300 (18S rRNA) was less sensitive with the detection limit 50 parasites/μl, and it displayed negative LAMP assay with the genomic DNA samples of P. yoelii and T. gondii, but displayed positive LAMP detection with P. vivax. The positive detection rate of the LAMP assay with PF3D7_1253300-5 was 90% (27/30), higher than that (80%, 24/30) of the positive rate of PF3D7_0112300 (18S rRNA) in examining clinical samples of P. falciparum infection cases. The LAMP assay with the primer set PF3D7_1253300-5 was more sensitive, specific, and accurate than those with PF3D7_0112300 (18S rRNA) in examining P. falciparum infection, and therefore it is a promising tool for diagnosis of P. falciparum infection.
Keywords: Malaria, Plasmodium falciparum, unique genes, loop-mediated isothermal amplification
Introduction
Malaria is a fatal and infectious disease, prevalent in tropical and subtropical regions, especially in Africa, Asia, and America. The prevalence and death rate of malaria are much higher in sub-Saharan Africa than in other global regions [1]. It can become a major threat to human health and imported malaria has dramatically increased in China, mainly due to the return of Chinese workers from Southeast Asia (SE Asia) and Africa [2]. In 2015, aproximately 212 million cases of malaria were detected worldwide, with an estimated 429,000 deaths [1].
In the early stage after malaria parasite infection, there occurs symptoms which are similar to the common cold, dengue, yellow fever, and typhoid fever, easily leading to confusion and misdiagnosis. If there is no proper treatment in time, even mild and moderate malaria parasitic infections are fatal to children under five and to pregnant women, particularly those infected by Plasmodium falciparum. It has been found that the morbidity and mortality rates of malaria caused by falciparum are higher than those of vivax malaria [3,4]. In Africa, P. falciparum is the most dangerous malaria parasite and responsible for more than 75% of malaria cases [1]. It is imperative to develop a rapid, specific, sensitive, and inexpensive detection method to identify Plasmodium species especially P. falciparum infection for malaria control.
Loop-mediated isothermal amplification (LAMP) was developed as a novel nucleic acid amplification method in 2000 [5]. A 109-fold amplification of a copy of nucleic acid in less than an hour can be achieved using LAMP technology. LAMP reaction is set up under isothermal conditions (60–65 °C) with high specificity. In addition, LAMP assay is simple, convenient, cost-effective, and reliable. Therefore, LAMP technology is being considered for diagnosing infectious diseases including tuberculosis [6], HIV [7], and malaria [8–11], as well as other pathogen infections and diseases such as Q fever and Salmonella Enterica Serotype Enteritidis [12,13]. LAMP also holds promise for use in molecular detection of pathogens in malaria [5,9,14,15]. Numerous LAMP assays have been developed for malaria diagnosis based on PfHRP2 [16], 18S rRNA [8,11,15], Pfs16 and Pfs25 [17], and mitochondrial DNA [18] of malaria parasites. These selected genes for conventional polymerase chain reaction (PCR) or LAMP are conserved, resulting in a high false-positive rate of amplification of target sequences. To solve this problem, genes unique to malaria parasites can be selected. The genome database of the P. falciparum 3D7 strain provided a vital tool for large-scale unique gene screening [19–21].
In this study, we developed a LAMP assay with the unique genes of P. falciparum to diagnose falciparum infection. Our findings support the conclusion that LAMP assay with unique genes was a rapid, specific, sensitive, and cost-effective detection method to identify P. falciparum infection, and therefore it is useful for malaria control.
Materials and methods
Study design
In order to survey malaria caused by P. falciparum on returning Chinese workers from Southeast Asia (SE Asia) and Africa, a LAMP assay based on unique genes was developed. Its sensitivity, specificity, and accuracy in detecting clinically-confirmed P. falciparum infection were assessed.
Collection and preparation of human blood samples
This study was approved by the ethics committees of Wuhan Center for Disease Control and Prevention (CDC). Peripheral blood samples were collected from a patient with falciparum malaria and a patient with P. vivax infection in Wuhan CDC. These two patients were Chinese workers who had returned from Africa. They were diagnosed with One Step Malaria HRP2/pLDH (P.f/Pan) (Wondfo, Guangzhou, China) and microscopic examination of Giemsa-stained thick and thin peripheral blood smear. For quality control, archived malaria positive slides were re-examined and parasitaemia was recorded. The Plasmodium spp. was confirmed by Plasmodium malaria real time PCR diagnostic kit (Shanghai Liferiver Bio-Tech Corp, Shanghai, China). All these samples were delivered to Hubei University of Medicine at low temperature (−20 °C).
Collection and preparation of mice blood samples
All animal experiments were approved by the Animal Care and Use Committee of the Third Military Medical University. Mice were obtained from the Experimental Animal Center of the Third Military Medical University. Female C57BL/6 mice (5–6 weeks) were infected by P. yoelii 17XL 106 infected erythrocytes in 200 μl of sterile PBS via intraperitoneal injection. When the parasitaemia reached 5–15%, blood samples were collected using heparin-pretreated 5-ml tube and stored at −20 °C until use, following the published report [22]. Seroperitoneum sample of T. gondii was kindly provided by Dr Shuang Shen of Jiangsu Institute of Parasitic Diseases. All these samples were delivered to Hubei University of Medicine at low temperature (−20 °C).
DNA extraction
DNA from blood samples of P. falciparum, P. vivax, P. yoelii, and T. gondii were extracted using TIANamp Blood DNA Kit (Tiangen Biotech Co., Ltd., Beijing, China) following the manufacturer’s protocol. All extracted genomic DNA (5 μl) were electrophoretically analysed in a horizontal plate with 2% agarose gels, containing 0.1 μg/ml ethidium bromide, and examined for quality under UV light. The remaining extracted genomic DNA samples were either used immediately or stored at −20 °C.
Selection of unique genes and design of LAMP primers
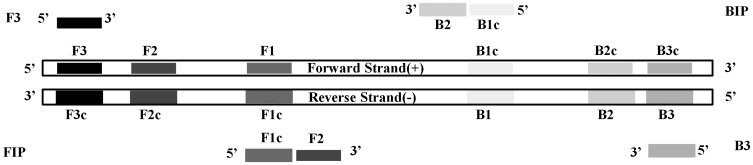
Unique genes were randomly selected from P. falciparum genome database in PlasmoDB [19,23]. Species specificity of target unique genes was verified using OrthoMCL database [24]. 18S rRNA (Gene ID. PF3D7_0112300) was used as a standard reference for unique genes. Nucleotide sequences of target genes were extracted from PlasmoDB (https://plasmodb.org/plasmo/) [23,25]. LAMP primers for target unique genes were designed using the online software PrimerExplorer V4 (https://primerexplorer.jp/elamp4.0.0/index.html) with default parameters. For each target unique gene, 2–5 top-scored sets of LAMP primers were selected and synthesized by Genewiz Company (Soochow, China). The design of LAMP primers was illustrated in Figure 1. The inner primers (FIP/BIP) and outer primers (F3/B3) were dissolved in ultrapure water with concentrations 200 and 100 pmol/μl respectively. A 20 μl working mixture of LAMP primers was made using 4 μl FIP and BIP, 1 μl F3 and B3, and 10 μl of ultrapure water, in which the concentrations of inner primers and outer primers were 40 and 5 μM, respectively.
Figure 1.
Design of LAMP primers for Plasmodium falciparum unique genes.
Note: F3 and B3, outer primer; FIP (F1c, F2) and BIP (B1c, B2), inner primer.
LAMP assay and determination of sensitivity and specificity
LAMP assay was developed according to the published reports [15,23] with modifications. Briefly, the LAMP assay was performed with a DNA amplification kit (Deaou, Guangzhou, China). The reaction mixture in a 0.2-ml PCR tube was set up by adding 12.5 μl 2× reaction buffer, 1.0 μl Bst DNA polymerase, 1.0 μl genomic DNA, 1.0 μl working LAMP primer mixture, and 9.5 μl sterile ultrapure water. Finally, isometric mineral oil (20 μl) was added into the PCR tube, which was then centrifuged for 30 s. The LAMP reaction tubes were incubated at 63 °C for 60 min in a T100™ Thermal Cycler (BIO-RAD, Singapore). SYBR Green was used to indicate the results of LAMP assay. The light green mixture in PCR tubes indicated successful amplification, and the brown mixture indicated negative results of LAMP assay. To assess the sensitivity of LAMP assay, the genomic DNA sample was extracted from parasitaemia 50,000 parasites/μl (1% parasitaemia), which was adjusted from 58,241 P. falciparum parasites/μl (1.16% parasitaemia) of the returned Chinese worker. The DNA sample was then serially diluted in 10-fold (100–10−6) and used as templates. The most sensitive primers were selected for specificity evaluation using the genomic DNA sample of P. vivax, P. yoelii, and T. gondii. For comparison, the 18S rRNA-based LAMP assay was also performed using the same samples. System quality control (positive, negative, and blank) was carried out using the DNA amplification kit.
Validation of the unique gene-based LAMP assay with clinical samples of P. falciparum infection cases
This study was approved by the ethics committees of Malabo Regional Hospital on Bioko Island, Equatorial Guinea. The informed consents were obtained from all participants. Blood samples were collected from Chinese workers with uncomplicated P. falciparum infection at Malabo Regional Hospital on Bioko Island, Equatorial Guinea from March 2014 to September 2015. The initial diagnosis of P. falciparum infection was conducted by microscopic examination of blood smears and a rapid diagnostic assay with the ICT malaria P.f. Cassette Test (ICT Diagnostics, South Africa). Approximately 300 μl of each whole blood sample was aliquoted on 3MM Whatman® filter paper (Whatman International Ltd., Maidstone, England), and air dried. These dried filter papers with blood spots (DBS) were then stored individually in Ziplock bags containing silica desiccant beads and kept at −20 °C. All these DBS were delivered to Hubei University of Medicine at low temperature (−20 °C). Among these DBS, thirty pieces were randomly selected. All these 30 patients were diagnosed with P. falciparum infection with 135,111 ± 216,492 parasites/μl. DNA was extracted from 30 DBS following the Chelex-100 extraction procedure described in our previous study [26]. The DNA samples were subjected to LAMP assay. For comparison, PF3D7_0112300 (18S rRNA) based LAMP assay was also performed.
Results
Selection of P. falciparum unique genes and LAMP primers
To develop unique gene-based LAMP assay, we randomly selected nine unique genes of P. falciparum from genome database of P. falciparum 3D7 strain, including one pseudogene PF3D7_1253300 and eight protein-coding genes (Table 1). PF3D7_0702300, PF3D7_1001900, PF3D7_1253300, and PF3D7_1301700 were only found in P. falciparum but not in other Plasmodium spp. according to OrthoMCL database (Table 1). A total of 31 sets of top-scored LAMP primers for nine unique genes were selected from the pools of primers designed by the online software PrimerExplorer V4 (Table 1).
Table 1.
The LAMP primers of Plasmodium unique genes.
| Gene ID. | Target region | LAMP primer | ||||
|---|---|---|---|---|---|---|
| Name | Start to end | Length (bp) | Name | Oligo (nt) | Sequence (5′-3′) | |
| PF3D7_0202200 | PF3D7_0202200-1 | 921-1121 | 201 | F3 | 18 | GAACAAGTTTCAAAAGCA |
| B3 | 18 | GTCATCTTGTTCCCTAAG | ||||
| FIP | 48 | CATACGTTTTTGCATATCCATATTACCTTTTATATTACCTTTCGTTCC | ||||
| BIP | 39 | TTCATGCTATGTTTAAATGGGC-CTTCATTTCTGCGTTGT | ||||
| PF3D7_0202200-2 | 604-798 | 195 | F3 | 17 | TTGGCGCTATAAGAGAA | |
| B3 | 20 | GTTTATCTTTTCCTTCATCA | ||||
| FIP | 40 | AGTTATACTTGCAGGTAATGGAAATAACGATTCCTTTGGT | ||||
| BIP | 46 | AAGGAGATTCTGAAGCAGAAGTATCATCATTTGAAGAACTTACAGC | ||||
| PF3D7_0202200-3 | 927-1121 | 195 | F3 | 19 | GTTTCAAAAGCATTACCTT | |
| B3 | 18 | GTCATCTTGTTCCCTAAG | ||||
| FIP | 38 | AACATTACATACGTTTTTGCATTACCTTTCGTTCCTCC | ||||
| BIP | 37 | TTCATGCTATGTTTAAATGGCTTCATTTCTGCGTTGT | ||||
| PF3D7_0202200-4 | 597- 798 | 202 | F3 | 17 | AAAAGAATTGGCGCTAT | |
| B3 | 20 | GTTTATCTTTTCCTTCATCA | ||||
| FIP | 45 | TGCAGGTAATGGTAAATGATTTGAGAAATGATAAATAACGATTCC | ||||
| BIP | 41 | ATAACTAAAGGAGATTCTGAAGATTTGAAGAACTTACAGCT | ||||
| PF3D7_0202200-5 | 660-883 | 224 | F3 | 18 | CATTTACCATTACCTGCA | |
| B3 | 20 | TGTAAATTCGATTTACCTGA | ||||
| FIP | 45 | TGATGTATTACGTGAATCATAGTATAACTAAAGGAGATTCTGAAG | ||||
| BIP | 42 | TGAAGGAAAAGATAAACGTCCACATATTTTTGATAATCCCAT | ||||
| PF3D7_0702300 | PF3D7_0702300-1 | 338-543 | 206 | F3 | 20 | ATCAGAATATCAATCGAACT |
| B3 | 19 | TTACTTTTGTAGTGCTTGT | ||||
| FIP | 34 | TGTTCCGGATTTTTTACTATTCTTGGTGGGGGAT | ||||
| BIP | 44 | TATACCAGAAAGTAGTAGTACAGTTGTACTGTTATTTGCTGCTA | ||||
| PF3D7_0702300-2 | 305–544 | 240 | F3 | 20 | ATCAACATACGTTATAAAGC | |
| B3 | 19 | GTTACTTTTGTAGTGCTTG | ||||
| FIP | 43 | GTTCCGGATTTTTTACTATTTACATATCAATCGAACTTTCTTG | ||||
| BIP | 44 | TATACCAGAAAGTAGTAGTACAGTTGTACTGTTATTTGCTGCTA | ||||
| PF3D7_0702300-3 | 347–543 | 197 | F3 | 18 | TCAATCGAACTTTCTTGG | |
| B3 | 19 | TTACTTTTGTAGTGCTTGT | ||||
| FIP | 41 | ACTTTGTATTTACATTTGTTCCGGAGGGATATAGTGCAGCT | ||||
| BIP | 41 | TATACCAGAAAGTAGTAGTACAGTTGTACTGTTATTTGCTG | ||||
| PF3D7_1001900 | PF3D7_1001900-1 | 691–873 | 183 | F3 | 16 | CGGAAGAAGGTGAAGA |
| B3 | 18 | TCAAAAGTATTGCTGTCA | ||||
| FIP | 44 | CCAATTTCTCCTTTATTTGGATATACCTAAACCTTATACAGTTG | ||||
| BIP | 45 | TTCGTAGAAGTGTTATTACTTTGTAATAAGAAAAGTAGGTGACGT | ||||
| PF3D7_1001900-2 | 681–895 | 215 | F3 | 17 | GTTGAAAATGCGGAAGA | |
| B3 | 20 | GCTGTCTTAAAATTGTCATT | ||||
| FIP | 47 | CCAATTTCTCCTTTATTTGGATTAGGTGAAGAAATACCTAAACCTTA | ||||
| BIP | 43 | TTCGTAGAAGTGTTATTACTGTCATTGTAATAAGAAAAGTAGG | ||||
| PF3D7_1002000 | PF3D7_1002000-1 | 707–930 | 224 | F3 | 18 | AATCAAATGTGACAAACG |
| B3 | 19 | GGTCTTTCTCTTTTTCCTT | ||||
| FIP | 48 | GTCTGCTTTTATTTCTTCAATTGAAAAGTGTGAAATTTGAAGAACCAC | ||||
| BIP | 43 | TAAACGTGGATGAAAAAAAGGTGTTTTTTCTTCTTCACTGGAA | ||||
| PF3D7_1002000-2 | 720–930 | 211 | F3 | 17 | AAACGTGAAAAGTGTGA | |
| B3 | 19 | GGTCTTTCTCTTTTTCCTT | ||||
| FIP | 39 | TGTCTGCTTTTATTTCTTCAAATTTGAAGAACCACAAGA | ||||
| BIP | 43 | TAAACGTGGATGAAAAAAAGGTGTTTTTTCTTCTTCACTGGAA | ||||
| PF3D7_1002000-3 | 666–892 | 227 | F3 | 19 | TACCTATTCTAGACGAGAA | |
| B3 | 19 | TTTTTTCTTCTTCACTGGA | ||||
| FIP | 41 | CTCATCTTGTGGTTCTTCTAGTAAATCAAATGTGACAAACG | ||||
| BIP | 39 | AAGCAGACAATAATGATTCAAATCCTTTTTTTCATCCAC | ||||
| PF3D7_1002000-4 | 744–969 | 226 | F3 | 18 | AGAACCACAAGATGAGAA | |
| B3 | 20 | ATAAGATGTGTTACTGTCTT | ||||
| FIP | 43 | TCATCCACGTTTATATTAGTCAATTGAAGAAATAAAAGCAGAC | ||||
| BIP | 42 | AGTGTAATTTCCAGTGAAGAAGATTCTTATGAGACCTTTTGG | ||||
| PF3D7_1148900 | PF3D7_1148900-1 | 48–267 | 220 | F3 | 17 | CGTGAAAACATTGATGA |
| B3 | 18 | CGTTACATAAAATGCTGA | ||||
| FIP | 35 | CTTTGCCAAAAACTTTCAGAAAATTCCTTTCAAGG | ||||
| BIP | 39 | TTCTGCCGTTGTTTTATTTTTTGACTAAAAGTGCTTTGA | ||||
| PF3D7_1148900-2 | 56–267 | 212 | F3 | 19 | CATTGATGAAGAAAATTCC | |
| B3 | 18 | CGTTACATAAAATGCTGA | ||||
| FIP | 28 | GAAGGGCTTTGCCTTTCAAGGGTCCAAG | ||||
| BIP | 39 | TTCTGCCGTTGTTTTATTTTTTGACTAAAAGTGCTTTGA | ||||
| PF3D7_1240100 | PF3D7_1240100-1 | 25–236 | 212 | F3 | 20 | ATTTTATATCTCATTGCTGC |
| B3 | 18 | AGTCCTATACCATATGCA | ||||
| FIP | 48 | CCTTTCTTTTTTTTTTTTCCCTC-TTGGCAATTAATTTAATAGCTCCCA | ||||
| BIP | 42 | TTCAGAAAAAAACATCATAAGGCCAAATAAAAGAGCGATAGC | ||||
| PF3D7_1240100-2 | 41–228 | 188 | F3 | 17 | CTGCCTTATTGGCAATT | |
| B3 | 17 | ACCATATGCAGTACCAA | ||||
| FIP | 42 | GAGGACATCCTTTCTTTTTTTTTATTTAATAGCTCCCAGTGT | ||||
| BIP | 47 | TTCAGAAAAAAACATCATAAGGCTAAAAGAGCGATAGCAGAAACAAC | ||||
| PF3D7_1240100-3 | 41- 228 | 188 | F3 | 17 | CTGCCTTATTGGCAATT | |
| B3 | 17 | ACCATATGCAGTACCAA | ||||
| FIP | 34 | AGAGGACATCCTTTCTTTCCCAGTGTTTGTAACG | ||||
| BIP | 47 | TTCAGAAAAAAACATCATAAGGCTAAAAGAGCGATAGCAGAAACAAC | ||||
| PF3D7_1253300 | PF3D7_1253300-1 | 359–570 | 212 | F3 | 17 | TTGACAAGAGAGGAGTT |
| B3 | 18 | CCATGTGATTTTAGGAGG | ||||
| FIP | 45 | ACCATTTTGTGATTCCATATTTGATTTATTAGTTCAAGTACCACC | ||||
| BIP | 42 | ATGTAGACAAGGAACAAAGGATTTAATTTGGGTTACCCGTAT | ||||
| PF3D7_1253300-2 | 328–532 | 205 | F3 | 18 | AGATAGCAAATTCGAGAG | |
| B3 | 16 | GGTTACCCGTATGTCT | ||||
| FIP | 41 | GTGGTACTTGAACTAATAAATCACGTAACTGAACAGTTGAC | ||||
| BIP | 35 | TATGGAATCACAAAATGGTAGGTGCAATTCCTCTT | ||||
| PF3D7_1253300-3 | 542–738 | 197 | F3 | 18 | ATGGTAAATCACCTCCTA | |
| B3 | 21 | CTTCACATATAAATCGGTATG | ||||
| FIP | 38 | TGTTTCCAAAGTAGAAACCAAATCACATGGCAAGGATG | ||||
| BIP | 45 | ATAACCTACTTAATAAAAAGGCTCCCATCTATACATGAACGAATG | ||||
| PF3D7_1253300-4 | 521–738 | 218 | F3 | 17 | ATACGGGTAACCCAAAT | |
| B3 | 21 | CTTCACATATAAATCGGTATG | ||||
| FIP | 43 | CAAAGTAGAAACCATCATATTACTCACCTCCTAAAATCACATG | ||||
| BIP | 42 | ACCTACTTAATAAAAAGGCTCTATACATGAACGAATGAAACT | ||||
| PF3D7_1253300-5 | 507–703 | 197 | F3 | 20 | ACTTACATATAGACATACGG | |
| B3 | 20 | CATCTATACATGAACGAATG | ||||
| FIP | 45 | AATCATACGAACATCCTTGCACCCAAATCAATATGGTAAATCACC | ||||
| BIP | 47 | ATATGATGGTTTCTACTTTGGATGGAGCCTTTTTATTAAGTAGGTTA | ||||
| PF3D7_1301700 | PF3D7_1301700-1 | 688–913 | 226 | F3 | 21 | TCAATGCACTCAAATCACAAT |
| B3 | 21 | ACATATAACATAGCTGGGACA | ||||
| FIP | 45 | TGCAGCTACTGCTGAAATAGTTAATTAATGTTAGAGCAGCTACCA | ||||
| BIP | 46 | ATATGCTTGCTATTGCAGGAGTTAATAATAATGCCATACCAGGTAA | ||||
| PF3D7_1301700-2 | 722–913 | 192 | F3 | 19 | TGTTAGAGCAGCTACCATT | |
| B3 | 21 | ACATATAACATAGCTGGGACA | ||||
| FIP | 46 | ATGTTGCAGCTACTGCTGAAAATAGCAGGATTCTTATCAATCTTTG | ||||
| BIP | 46 | ATATGCTTGCTATTGCAGGAGTTAATAATAATGCCATACCAGGTAA | ||||
| PF3D7_1334700 | PF3D7_1334700-1 | 351–553 | 203 | F3 | 20 | ACAAGAATGTCAATCAATCA |
| B3 | 17 | TTTGGCTAGTTCCTCTT | ||||
| FIP | 46 | GTAGAGAAAGGAGTATTAACATTATAAAAGAACTTTTGAGTGCTGC | ||||
| BIP | 40 | GCATTAGAAAAAGATGAATTCCTGTATGTCCACATAGTGC | ||||
| PF3D7_1334700-2 | 235–441 | 207 | F3 | 19 | AGTTTATTAGACCAACCAA | |
| B3 | 19 | AGTTGTAGAGAAAGGAGTA | ||||
| FIP | 40 | GGATCATTTGGATTTGTTAAATTTCTACATCAACACAAGG | ||||
| BIP | 42 | CAAGAATGTCAATCAATCAATGTTCATCATTTGTAGCAGCAC | ||||
| PF3D7_1334700-3 | 211–428 | 218 | F3 | 17 | AATTCCCTTGAAGAAGC | |
| B3 | 21 | GGAGTATTAACATGTTCATCA | ||||
| FIP | 48 | GAAGTATTATTTTCACTACCTTGTGACAAAAGAGTTTATTAGACCAAC | ||||
| BIP | 41 | TAACAAATCCAAATGATCCAACACCAGCACTCAAAAGTTCT | ||||
| PF3D7_1334700-4 | 247–441 | 194 | F3 | 23 | CAACCAAATATTTCTACATCAAC | |
| B3 | 19 | AGTTGTAGAGAAAGGAGTA | ||||
| FIP | 45 | GGATCATTTGGATTTGTTAACAAGGTAGTGAAAATAATACTTCAG | ||||
| BIP | 42 | CAAGAATGTCAATCAATCAATGTTCATCATTTGTAGCAGCAC | ||||
| PF3D7_1334700-5 | 278–509 | 232 | F3 | 23 | GTGAAAATAATACTTCAGGTGTT | |
| B3 | 18 | CCACATAGTGCTTCTGTT | ||||
| FIP | 45 | GCAGCACTCAAAAGTTCTTTTATAATAACAAATCCAAATGATCCA | ||||
| BIP | 41 | ATGATGAACATGTTAATACTCCCGTGATACTATCACGGAAT | ||||
| PF3D7_0112300 | 18S rRNA | 506-725 | 220 | F3 | 24 | TGTAATTGGAATGATAGGAATTTA |
| B3 | 23 | GAAAACCTTATTTTGAACAAAGC | ||||
| FIP | 41 | AGCTGGAATTACCGCGGCTGGGTTCCTAGAGAAACAATTGG | ||||
| BIP | 45 | TGTTGCAGTTAAAACGTTCGTAGCCCAAACCAGTTTAAATGAAAC | ||||
LAMP assays with primers from P. falciparum unique genes and their sensitivity and specificity
We first examined whether the primers from P. falciparum unique genes can be used to develop LAMP assay using the DNA of a P. falciparum-infected blood sample (parasitaemia: 50,000 parasites/μl). The results showed that 25 sets of LAMP primers from eight genes displayed positive LAMP assay, and six sets of LAMP primers from five genes displayed negative LAMP assay. Notably, all five sets of LAMP primers from the gene PF3D7_1253300 displayed positive amplification (Table 2). Four sets of LAMP primers from PF3D7_0202200, PF3D7_1002000, or PF3D7_1334700 gene displayed positive LAMP detection. Three and two sets of LAMP primers between PF3D7_1240100 and PF3D7_1001900 displayed positive LAMP assay, respectively. Only one set of LAMP primer from PF3D7_0702300, PF3D7_1148900, and PF3D7_1301700 displayed positive reactions.
Table 2.
LAMP assays with primers from P. falciparum unique genes and their sensitivity.
| LAMP name | Sensitivity (10-fold serial dilutions) specificity | ||||||
|---|---|---|---|---|---|---|---|
| 100 | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | 10−6 | |
| PF3D7_0112300 | P | P | P | P | N | N | N |
| PF3D7_0202200-1 | P | N | N | N | N | N | N |
| PF3D7_0202200-2 | P | P | P | N | N | N | N |
| PF3D7_0202200-3 | P | P | P | N | N | N | N |
| PF3D7_0202200-4 | N | N | N | N | N | N | N |
| PF3D7_0202200-5 | P | P | P | N | N | N | N |
| PF3D7_0702300-1 | N | N | N | N | N | N | N |
| PF3D7_0702300-2 | N | N | N | N | N | N | N |
| PF3D7_0702300-3 | P | P | P | N | N | N | N |
| PF3D7_1001900-1 | P | N | N | N | N | N | N |
| PF3D7_1001900-2 | P | P | P | P | N | N | N |
| PF3D7_1002000-1 | P | P | P | N | N | N | N |
| PF3D7_1002000-2 | P | P | P | N | N | N | N |
| PF3D7_1002000-3 | P | P | N | N | N | N | N |
| PF3D7_1002000-4 | P | P | P | N | N | N | N |
| PF3D7_1148900-1 | N | N | N | N | N | N | N |
| PF3D7_1148900-2 | P | P | N | N | N | N | N |
| PF3D7_1240100-1 | P | P | N | N | N | N | N |
| PF3D7_1240100-2 | P | N | N | N | N | N | N |
| PF3D7_1240100-3 | P | N | N | N | N | N | N |
| PF3D7_1253300-1 | P | P | N | N | N | N | N |
| PF3D7_1253300-2 | P | P | N | N | N | N | N |
| PF3D7_1253300-3 | P | P | N | N | N | N | N |
| PF3D7_1253300-4 | P | P | P | N | N | N | N |
| PF3D7_1253300-5 | P | P | P | P | P | N | N |
| PF3D7_1301700-1 | P | P | N | N | N | N | N |
| PF3D7_1301700-2 | N | N | N | N | N | N | N |
| PF3D7_1334700-1 | P | P | N | N | N | N | N |
| PF3D7_1334700-2 | P | P | P | N | N | N | N |
| PF3D7_1334700-3 | P | P | N | N | N | N | N |
| PF3D7_1334700-4 | P | P | P | N | N | N | N |
| PF3D7_1334700-5 | N | N | N | N | N | N | N |
Note: P and N represented positive and negative, respectively. 100 represented the extracted DNA from blood sample of P. falciparum infection (parasitaemia: 50,000 parasites/μl). The 10−1 represented 10-fold dilution of 100
We subsequently assessed sensitivity of the LAMP assay with primers from unique genes with serial diluted DNA template. The results showed that the LAMP assay with PF3D7_1253300-5 was the most sensitive, as shown by the positive LAMP assay at the maximum dilution (10−4) of DNA template (Table 2), indicating that the P. falciparum detection limit of the LAMP assay with PF3D7_1253300-5 was 5 parasites/μl. The LAMP assay with PF3D7_1001900-2 was the second most sensitive (positive LAMP reaction at the maximum dilution 10−3) among all LAMP primers from unique genes (Table 2), indicating its P. falciparum detection limit was 50 parasites/μl. The sensitivity of PF3D7_0112300 (18S rRNA) was the same as that of PF3D7_1001900-2.
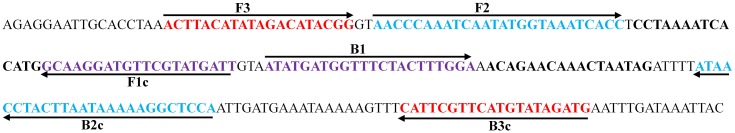
We further examined the specificity of PF3D7_1253300-5 in detecting P. falciparum infection using the genomic DNA samples of P. vivax, P. yoelii, and T. gondii. The results showed that the LAMP assay with PF3D7_1253300-5 displayed negative LAMP assay with the genomic DNA samples of P. vivax, P. yoelii, and T. gondii, and the LAMP assay with PF3D7_0112300 (18S rRNA) displayed negative amplification with the genomic DNA samples of P. yoelii and T. gondii, but displayed positive LAMP assay with P. vivax. These results indicated that LAMP assay with the primer PF3D7_1253300-5 unique to P. falciparum had higher sensitivity and specificity than those with PF3D7_0112300 (18S rRNA). The genomic location and sequence of the LAMP primer set PF3D7_1253300-5 was illustrated in Figure 2.
Figure 2.
The genomic location and sequence of the primer set PF3D7_1253300-5.
Validation of the unique gene-based LAMP assay with clinical samples of P. falciparum infection cases
We further validated the unique gene-based LAMP assay with the primer PF3D7_1253300-5 using clinical samples of P. falciparum infection cases. The results showed that the positive detection rate of PF3D7_1253300-5 was 90% (27/30), higher than that (80%, 24/30) of the positive rate of PF3D7_0112300 (18S rRNA), suggesting that the unique gene-based LAMP assay with the primer PF3D7_1253300-5 was more accurate than PF3D7_0112300 (18S rRNA)-based LAMP assay in examining P. falciparum infection cases.
Discussion
In the current study, we developed LAMP assays using primers of P. falciparum unique genes to diagnose P. falciparum infection. We found that the LAMP assay with the primer set PF3D7_1253300-5 was more sensitive, specific, and accurate than those with PF3D7_0112300 (18S rRNA) in examining P. falciparum infection, and therefore it is a promising tool for diagnosis of P. falciparum infection.
It has been reported that LAMP assay is a highly specific method to detect various pathogens [6–9,11–13]. In the current study, the LAMP assay with the primer set PF3D7_1253300-5 displayed negative LAMP assay with all the genomic DNA samples of P. vivax, P. yoelii, and T. gondii, and the LAMP assay with PF3D7_0112300 (18S rRNA) displayed negative amplification with the genomic DNA samples of P. yoelii and T. gondii, but displayed positive reaction with P. vivax. These results indicate that the LAMP assay with the primer set PF3D7_1253300-5 displayed higher specificity than PF3D7_0112300 (18S rRNA) did, and there is false-positive amplification with 18S rRNA in LAMP assay. It seems that selection of target genes in LAMP is critical for high specificity of LAMP detection.
The conserved gene 18S rRNA has been a conventional target gene in diagnosing malaria [27]. There are both benefits and pitfalls using 18S rRNA as a target to diagnose malaria. On the one hand, 18S rRNA is a conserved gene, and it is easy to identify and design primers for LAMP assay in eukaryotes [27]. On the other hand, there are only seven copies of 18S rRNA gene in P. falciparum while there are many copies of 18S rRNA gene in other eukaryotes [21]. For this reason, the limit of LAMP-based detection is not as good for P. falciparum as that for other eukaryotes. Furthermore, the LAMP detection often generates false-positive results using conserved genes as templates, as indicated in previous studies using PfHRP2 [16], 18S rRNA [8,11,15], Pfs16 and Pfs25 [17], and mitochondrial DNA [18]. In the current study, we found that the detection limit of the LAMP assay with the primer set PF3D7_1253300-5 was as low as 5 parasites/μl, more sensitive than that of the primer set from PF3D7_0112300 (18S rRNA) (50 parasites/μl). The LAMP assay with the primer set PF3D7_1253300-5 displayed higher specificity than PF3D7_0112300 (18S rRNA) did. Further, the unique gene-based LAMP assay with the primer PF3D7_1253300-5 was more accurate than PF3D7_0112300 (18S rRNA)-based LAMP assay in examining P. falciparum infection cases. Therefore, it is likely that the LAMP assay with the primer PF3D7_1253300-5 unique to P. falciparum is superior to the conserved PF3D7_0112300 (18S rRNA).
It is desirable to diagnose P. falciparum infection at an early stage so that better outcomes can be achieved. In the current study, the detection limit of the LAMP assay with the primer set PF3D7_1253300-5 was 5 parasites/μl, lower than that of the primer set from PF3D7_0112300 (18S rRNA) (50 parasites/μl). However, it is not as low as those in some other studies. The detection limit of a LAMP assay with primers from the apicoplast genome achieved 2 parasites/μl [27]. This is probably due to that the apicoplast is semi-autonomous with its own genome and it has 15 copies of genome in P. falciparum [27]. In a recent study, the detection limit of a commercial malaria LAMP assay was reported to be ≤2.0 parasites/μl, reaching the detection limit of a real-time LAMP test [28]. Therefore, the LAMP assay with the primer from unique genes needs to be improved, as also suggested by previous studies [18,29,30]. As far as our LAMP assay system, it can be improved at least in the following aspects. First, genes with a high copy number are selected. Second, loop primers of LAMP (LPF and LPB) are used, because they may improve detection limit by 10-fold [15]. Third, LAMP reaction conditions can be optimized by screening primers, concentrations of Mg2+, reaction buffer, polymerase, and DNA template, and reaction temperature and time. This deserves further investigation.
In conclusion, we have established the LAMP assay with the primer set PF3D7_1253300-5, which is more sensitive, specific, and accurate than that with PF3D7_0112300 (18S rRNA) in examining P. falciparum infection. Since the LAMP assay is rapid, simple, sensitive, specific, and cost-effective, and it can be applied without the aid of sophisticated equipment, the LAMP assay with the primer set PF3D7_1253300-5 is a promising tool widely used to diagnose P. falciparum infection in the regions where malaria is prevalent.
Funding
This work was partially supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars [grant number JYB201448HBMU01] to JL, State Education Ministry; the Natural Science Foundation of Hubei Province of China [grant number 2014CFB648] to JL; the Education Agency Major Project of Hubei Province of China [grant number D20162101] to WXD; The Foundation for Innovative Research Team of Hubei University of Medicine [grant number 2014 CXZ02], [grant number FDFR201603] to JL .The funders had no role in the study design and data analysis, the decision to publish, or reparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
We are grateful to the staff at the Department of Schistosomiasis and Endemic Diseases, Wuhan Center for Disease Prevention and Control, and to all participants who contributed their blood samples.
References
- [1].WHO World malaria report 2016. Geneva (Switzerland): World Health Organization; 2016. [Google Scholar]
- [2].Feng J, Xiao H, Zhang L, et al. The Plasmodium vivax in China: decreased in local cases but increased imported cases from Southeast Asia and Africa. Sci Rep. 2015;5:8847. 10.1038/srep08847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Llinas M, DeRisi JL. Pernicious plans revealed: Plasmodium falciparum genome wide expression analysis. Curr Opin Microbiol. 2004;7(4):382–387. 10.1016/j.mib.2004.06.014 [DOI] [PubMed] [Google Scholar]
- [4].Doolan DL. Plasmodium immunomics. Int J Parasitol. 2011;41(1):3–20. 10.1016/j.ijpara.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63. 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nagai Y, Iwade Y, Nakano M, et al. Rapid and simple identification of Beijing genotype strain of Mycobacterium tuberculosis using loop-mediated isothermal amplification assay. Microbiol Immunol. 2016;60(7):459–467. [DOI] [PubMed] [Google Scholar]
- [7].Odari EO, Maiyo A, Lwembe R, et al. Establishment and evaluation of a loop-mediated isothermal amplification (LAMP) assay for the semi-quantitative detection of HIV-1 group M virus. J Virol Methods. 2015;212:30–38. 10.1016/j.jviromet.2014.10.012 [DOI] [PubMed] [Google Scholar]
- [8].Poschl B, Waneesorn J, Thekisoe O, et al. Comparative diagnosis of malaria infections by microscopy, nested PCR, and LAMP in Northern Thailand. Am J Trop Med Hyg. 2010;83(1):56–60. 10.4269/ajtmh.2010.09-0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abdul-Ghani R, Al-Mekhlafi AM, Karanis P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: would it come to clinical reality as a point-of-care test? Acta Trop. 2012;122(3):233–240. 10.1016/j.actatropica.2012.02.004 [DOI] [PubMed] [Google Scholar]
- [10].Lau YL, Lai MY, Fong MY, et al. Loop-mediated isothermal amplification assay for identification of five human plasmodium species in Malaysia. Am J Trop Med Hyg. 2016;94(2):336–339. 10.4269/ajtmh.15-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ocker R, Prompunjai Y, Chutipongvivate S, et al. Malaria diagnosis by loop-mediated isothermal amplification (Lamp) in Thailand. Rev Inst Med Trop Sao Paulo. 2016;58:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pan L, Zhang L, Fan D, et al. Rapid, simple and sensitive detection of Q fever by loop-mediated isothermal amplification of the htpAB gene. PLoS Neglected Trop Dis. 2013;7(5):e2231. 10.1371/journal.pntd.0002231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Draz MS, Lu X. Development of a loop mediated isothermal amplification (LAMP) – surface enhanced Raman spectroscopy (SERS) assay for the detection of Salmonella enterica serotype enteritidis. Theranostics. 2016;6(4):522–532. 10.7150/thno.14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Poon LL, Wong BW, Ma EH, et al. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem. 2006;52(2):303–306. [DOI] [PubMed] [Google Scholar]
- [15].Han ET, Watanabe R, Sattabongkot J, et al. Detection of four plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J Clin Microbiol. 2007;45(8):2521–2528. 10.1128/JCM.02117-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Paris DH, Imwong M, Faiz AM, et al. Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria. Am J Trop Med Hyg. 2007;77(5):972–976. [PubMed] [Google Scholar]
- [17].Buates S, Bantuchai S, Sattabongkot J, et al. Development of a reverse transcription-loop-mediated isothermal amplification (RT-LAMP) for clinical detection of Plasmodium falciparum gametocytes. Parasitol Int. 2010;59(3):414–420. 10.1016/j.parint.2010.05.008 [DOI] [PubMed] [Google Scholar]
- [18].Polley SD, Mori Y, Watson J, et al. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J Clin Microbiol. 2010;48(8):2866–2871. 10.1128/JCM.00355-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bozdech Z, Llinas M, Pulliam BL, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1(1):E5. 10.1371/journal.pbio.0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vembar SS, Seetin M, Lambert C, et al. Complete telomere-to-telomere de novo assembly of the Plasmodium falciparum genome through long-read (>11 kb), single molecule, real-time sequencing. DNA Res. 2016;23(4):339–351. 10.1093/dnares/dsw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. 10.1038/nature01097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gallego-Delgado J, Baravian C, Edagha I, et al. Angiotensin II moderately decreases plasmodium infection and experimental cerebral malaria in mice. PLoS ONE. 2015;10(9):e0138191. 10.1371/journal.pone.0138191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aurrecoechea C, Brestelli J, Brunk BP, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37(Database issue):D539–543. 10.1093/nar/gkn814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen F, Mackey AJ, Stoeckert CJ Jr, et al. OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic Acids Res. 2006;34(Database issue):D363–368. 10.1093/nar/gkj123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bahl A, Brunk B, Crabtree J, et al. PlasmoDB: the Plasmodium genome resource. A database integrating experimental and computational data. Nucleic Acids Res. 2003;31(1):212–215. 10.1093/nar/gkg081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li J, Chen J, Xie D, et al. High prevalence of pfmdr1 N86Y and Y184F mutations in Plasmodium falciparum isolates from Bioko Island, Equatorial Guinea. Pathogens Global Health. 2014;108(7):339–343. 10.1179/2047773214Y.0000000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Oriero CE, van Geertruyden JP, Jacobs J, et al. Validation of an apicoplast genome target for the detection of Plasmodium species using polymerase chain reaction and loop mediated isothermal amplification. Clin Microbiol Infect. 2015;21(7):e681–e687. [DOI] [PubMed] [Google Scholar]
- [28].Lucchi NW, Gaye M, Diallo MA, et al. Evaluation of the illumigene malaria LAMP: a robust molecular diagnostic tool for malaria parasites. Sci Rep. 2016;6:36808. 10.1038/srep36808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Endeshaw T, Gebre T, Ngondi J, et al. Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malaria J. 2008;7:118. 10.1186/1475-2875-7-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Port JR, Nguetse C, Adukpo S, et al. A reliable and rapid method for molecular detection of malarial parasites using microwave irradiation and loop mediated isothermal amplification. Malaria J. 2014;13:454. 10.1186/1475-2875-13-454 [DOI] [PMC free article] [PubMed] [Google Scholar]