Abstract
Tuberculosis (TB), an important issue in the present age, affects millions of people each year. The infectious agent of TB, Mycobacterium tuberculosis (Mtb), interacts with the immune system which prevents the development of this bacterium as much as possible. In fact, the receptors on the surface of immune cells identify the bacteria, one of which is Toll-like receptors (TLRs). Different TLRs including 2, 4, 9 and 8 play critical roles in tuberculosis infection. In this paper, we focused on the role of TLRs which interact with different components of Mtb and, consequently, prevent the entrance and influence of bacteria on the body.
Keywords: Mycobacterium tuberculosis, Toll-like receptors, TLR, immune system, infection
1. Introduction
Mycobacterium tuberculosis (Mtb), the infectious agent of Tuberculosis (TB), causes illness among millions of people each year [1]. Both the emergence of the acquired immune deficiency syndrome and the development of multidrug-resistant (MDR)-TB [2] have increased such estimation to 10.4 million with 1.8 million deaths in 2015 among which 2,50,000 were MDR/rifampicin resistant (RR)-TB [1]. Moreover, about one-third of the world’s population has latent TB, but are not yet capable to transmit the disease [3]. Different environmental, genetic, and pathogenic factors influence the progression of active TB [4,5] and also interplay some crucial roles with the immune system during both the early and late phases of infection [6]. Both adaptive [7] and innate [8] immune mechanisms modulate host susceptibility to TB [9]. Innate immune system as early warning part of the system recognizes bacteria through its own receptors such as Toll-like receptors (TLRs). This review summarizes some new aspects of TLR roles in Mtb infection.
2. The role of TLRs against TB
TLRs, a family of single membrane-spanning receptors of which 1 to 10 have been nominated in human beings, are expressed in both immune and non-immune cells [10,11]. TLRs generally play a critical role in both innate immune responses and the initiation of adaptive immunity to Mtb. Actually, polymorphisms of TLRs have been associated with mutated susceptibility to tuberculosis among different populations [12–20]. Innate immune cells initiate subsequent adaptive immune responses after recognizing Mtb by Lucine Rich Repeats of the extracellular domains of the their TLRs [21]. Such interaction among Mtb’s ligand and TLRs activates Myeloid Differentiation Primary Response 88 (MyD88) which, as a central role player [22], is used by all TLRs except TLR3 [23]. MyD88, links initial complex to subsequent molecules including Interleukin-1 receptor-associated kinase (IRAK), TNF receptor associated factor (TRAF) 6, transforming growth factor beta-activated kinase 1 (TAK1) and mitogen-activated protein kinases (MAPK). Such signaling pathway mediates the translocation of NF-κ B into the nucleus [24] to induce transcription of inflammatory mediators, expression of adhesion molecules, and further recruitment and activation/apoptosis of macrophages, dendritic cells (DCs) and polymorphonuclear cells (PMNs) in the Mtb infected area [2]. From one side, MyD88-deficient mice are highly susceptible to Mtb infection [25,26] and from the other side, Mtb is able to meddle such signaling as its cell wall proline-proline-glutamic acid (PPE) family protein Rv1808 manipulates the host cytokine profile via MAPK and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways [27]. To clarify mechanisms underlying such effects, one recent study revealed the critical role of MyD88 and TIR-domain-containing adapter-inducing interferon-β (TRIF) in the activation and maturation of DCs in response to a potent adjuvant activating antigen presenting cells (APCs), named heat shock protein (Hsp) 70 derived from Mtb [28]. Another study showed the apoptotic effect of 38-kDa antigen of Mtb on macrophages through TLRs 2 and 4 [29]. Among the TLRs been identified, TLR2, TLR4, TLR9 and possibly TLR8 are the key receptors that are involved in the recognition of Mtb [30–36].
3. TLR2
3.1. TLR2 and innate immune cells
TLR2 expression on macrophages is important in determining the fate of innate immune responses to Mtb [37,38]. From one side, initial high TLR2 expression on macrophages may worsen the outcomes of infection via different mechanisms such as secretion of anti-inflammatory cytokines [39] as well as conferring to signaling pathways [40,41]. From the other side, it may maintain the dormant state of the Mtb and survive the bacilli in a latent form to avoid its activation [42]. In this regard, different components of Mtb have been shown to elicit the production of a broad range of components by macrophages in a TLR2-dependent manner [43]. For example, a cell-associated lipoglycoprotein of Mtb, called MPT83, acts as a TLR2 agonist which mediates the induction of matrix metalloproteinase 9 (MMP-9) by human THP-1 cells [44] (Figure 1). The other example is a heat-killed Mtb H37Ra called MTBRa which upregulates TNF-α expression through activation of TLR2/ERK signaling, and increases MMP-1 and MMP-9 production in human pleural mesothelial cells [45]. The Rv2660c protein, preferentially expressed during latent infection of Mtb for adaptation to lack of nutrition and hypoxia, stimulates human macrophages by interacting with TLR2 to secrete pro inflammatory cytokines which might maintain latency of Mtb [46]. Early secreted antigen 6 (ESAT-6) of Mtb promotes apoptosis of macrophages via TLR2/NF-κB activation [47], mycolic acid [48] as well as lipoprotein components [31,49,50] of Mtb activate macrophages via TLR2 (Figure 1) to bypass two strategies allowing Mtb to evade host immunity: down regulation of major histocompatibility complex (MHC) class II molecules (which restricts its antigen presentation) [51] and restriction of pro inflammatory responses (which delay the onset of adaptive immune responses) [52]. There is evidence emphasizing the effects of TLR2 on other innate immune cells, some of which are as follows: TLR2/dectin-1 cooperation induces Reactive Oxygen Species (ROS) production to induce the activation and apoptosis of neutrophils [53]; peptidoglycan components of mycobacterial cell wall are able to interact with TLR-2 which promote the activation of resting natural killer cells and Interferon gamma (IFN-γ) production [54]; TLR2-induced epithelial-derived C-X-C motif chemokine ligand 5 (CXCL5) is critical for PMN-driven destructive inflammation in pulmonary tuberculosis [55]; TLR2-induced pro-inflammatory cytokines produced by DCs or monocytes may contribute to the pathogenesis of Mtb -associated immune restoration disease [56]. From the point of vitamin D, the effect of TLR2 in macrophages should also be noted. TLR2 enhances the expression of genes of vitamin D receptor and vitamin-D-1-hydroxylase and in this way promotes the production of the antimicrobial peptide cathelicidin by human macrophages [57,58]. Such links between TLR2 and vitamin D-mediated innate immunity suggests the contribution of TLR2 in resistance to Mtb infection.
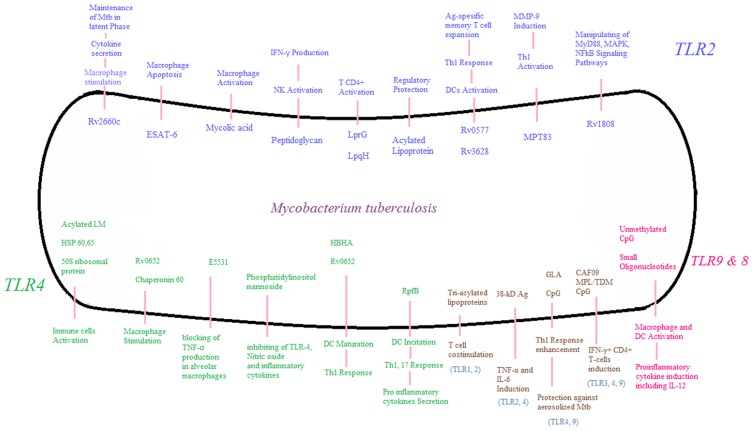
Figure 1.
Different Mtb components stimulate the immune system through Toll-like receptors (TLRs). The role of each TLR is depicted in this figure separately. TLRs interact with Mtb components and cause the activation of macrophages, NK cells, dendritic and T cells and also induce cytokine secretion. Such roles of TLRs is crucial in primary identification of Mtb and development of appropriate immune responses to overcome the Mtb infection. LM: Lipomannan, Hsp: Heat Shock Protein, HBHA: Heparin-binding hemagglutinin, Rpf: Resuscitation-promoting factor, CAF: Cationic adjuvant formulation, MPL: Monophosphoryl lipid-A, TDM: Trehalose dimycolate, ESAT: Early secreted antigen, Nk cell: Natural killer cell, IFN-γ: Interferon-Gamma, DC: Dendritic cell, Th: T helper, MMP: Matrix metalloproteinase, MAPK: Mitogen-activated protein kinase, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, TNF: Tumor necrosis factor, IL: Interleukin.
3.2. TLR2 and T cells
According to some studies [59], TLR2 signaling may not influence the memory as well as the induction of T cell immunity to Mtb. Its engagement on T cells may affect T-cell trafficking [60] in some ways such as inducing the production of C-C motif chemokine ligand 8 (CCL8) chemokines to recruit CD4+ cells to pleural effusion of Mtb infected patients [61] or mediating the recruitment of forkhead box P3 (Foxp3⁺) T regulatory cells (Tregs) to the lungs to control inflammation [62]. Some other studies reveal that TLR2 engagement on CD4+ T cells may increase Mtb Ag-specific responses and contribute to protection against Mtb infection [63]. The role of such an engagement in the contribution of CD4+ cells against Mtb is supported by the study in which pretreatment with TLR2-antagonistic antibody significantly inhibits the cytokine production caused by a major membrane protein II of Mtb [64]. Furthermore, Mtb lipoproteins LprG and LpqH induce the activation of memory CD4+ T lymphocytes via the existence of TLR2 on their surface [65]. (Figure 1) From one side, TLR2 serves as a co-stimulatory receptor for mycobacterium-specific T cell development and participates in the maintenance of T cell memory [66]. From the other side, it plays some protective regulatory roles [22,67–69] especially when it is engaged on CD4+ T cells [63] by related ligands such as acylated lipoproteins of Mtb [65]. An evidence emphasizing the stimulatory function of TLR2 is about Rv0577 [70] and Rv3628 [71] proteins, 2 TLR2 agonists, which have critical roles in the activation of DCs in a TLR2-dependent manner and the initiation of the adaptive immune response by polarizing the development of T cells to a type 1 T helper (Th1) response and the expansion of Ag-specific memory CD4+ T cells. (Figure 1) Also, an evidence emphasizing the regulatory activity of TLR2 is related to a recent study which has shown that TLR2 activates extracellular-signal-regulated Kinase (ERK) signaling in macrophages to promote anti-inflammatory macrophage responses and blunts Th1 responses against the Mtb [72]. Furthermore, Wnt-β-catenin, a critical regulator of pathogen-specific TLR2 responses, accompanied by Notch1, controls the expression of genes that could foster the generation of Treg cells [73].
In the case of CD4+ subtypes, type 17 T helper (Th17) cells play a critical role in conferring optimal protection against Mtb. In this regard, TLR2 may be an important upstream molecule in mediating Th17 responses to Mtb via mediating the induction of p19 (a subunit of IL-23), Interleukin 1 (IL-1) β [74], Interleukin 6 (IL-6), and transforming the beta growth factor (TGF-β) in DCs [75]. In this way the Th17 cells show their protective effect by speeding up the Th1 cells to populate in the site of infection [76]. This condition results in the sustainability of Th1 responses mediated by TLR2 and now could be an attractive target for effective vaccination [74]. Actually, deficiencies in TLRs may fail some responses to Mtb. For example, such deficiency inhibits Th17 differentiation (following complete Freund’s adjuvant immunization) [77]. Protection induced by novel vaccines may be achieved by TLR2 engagement. For example, S-[2,3-bis(palmitoyloxy)propyl]cysteine (Pam2Cys) [78], PPE57 [79], Rv3628 [71] and Rv3203 Mtb proteins [80], as TLR agonists, are potential candidate antigens to be used in future prophylactic vaccines against Mtb strains. Although a TLR2 agonist such as recombinant MPT83 (rMPT83) may induce the macrophage function [81], the inclusion of such agonists into new vaccines may not be fully effective in some situations such as when they are used in the elderly population [82].
Findings about TLR2-Mtb interaction may yield some clinical applications regarding treatment considerations. For example, TLR2 rescues Th1 cells from exhaustion and therefore can be considered as an important target in the treatment of patients with chronic infections [83]. Also, Mtb promotes arthritis development through TLR2, and TLR2 could represent a therapeutic target for this form of arthritis [84]. However, some limitation should be considered in the case of such clinical applications. For example, CD36-TLR2 cooperation may lead to a decreased macrophage response [85], and some mycobacterium antigens expressed inside infected macrophage may suppress protective immune responses such as TLR2-induced IL-12 production [86]. TLR2 gene polymorphisms may increase the risk of susceptibility to Mtb [87–89].
4. TLR4
Binding TLR4 to different components of Mtb such as 3- and 4-acylated lipomannan (LM), 60- and 65-HSPs, and 50S ribosomal protein [24] activates immune cells in different ways. In this regard, macrophages from TLR4−/− mice do not respond to Mtb HSP65 [33] and show less, yet not completely abolished, tumor necrosis factor alpha (TNF-α) production [90,91]. TLR4 agonists substantially increase the pool of effector memory CD4 and CD8 T cells and reduce the dose and Mtb burden in the lungs [92]. For example, both Mtb protein of Rv0652, a potent TLR4 agonist [93], and Mtb heparin-binding hemagglutinin (HBHA), a Novel TLR4 agonist [94], enhance the polarization of T effector cells toward a Th1 phenotype through dendritic cell maturation. However, TLR4 antagonist E5531 blocks Mtb induced TNF-α production in primary human alveolar macrophages [35] (Figure 1).
The immune-stimulatory impact of TLR4 may be controversial concerning some studies stating that TLR4-deficient mice do not show high susceptibility to Mtb infection; otherwise, non-functional TLR4 and TLR4-deficient mice develop a chronic lung infection when exposed to aerosolized Mtb [90,91,95,96]. Actually, similar to TLR2, TLR4 plays some dual beneficial and pathologic effects on the host immune responses against Mtb. For instance, lipopolysaccharide (LPS), a major mediator of TLR4-mediated inflammatory responses, might negatively be down-regulated by Phosphatidylinositol mannosides of Mtb in such a way that it may inhibit TLR4 and MyD88-dependent production of nitric oxide as well as inflammatory cytokines [97]. Such a strategy, developed by Mtb, may repress host immune responses. Mutually, Mtb proteins such as Rv0652 [98] and chaperonin 60 [99] stimulate macrophages and Resuscitation-promoting factor (Rpf)B [100] and (Rpf)E [101] proteins incite DCs toward Th1/Th17 cell expansion in a TLR4-dependent pathway to secrete pro inflammatory cytokines and hereon have the potential to be effective Mtb vaccines. (Figure 1) If vaccines can succeed in inducing Th1 memory cells for a long time, they can ensure the high efficacy of tuberculosis vaccines [100]. Unexpectedly, the deficiency of Toll-interacting protein (TOLLIP), as a negative regulator of TLR signaling, which has some anti-inflammatory responses in humans by suppressing pro inflammatory cytokines via TLR2 and TLR4 and also by inducing IL-10 through a TLR4-specific mechanism, is associated with a risk of Mtb pathogenesis [102]. In the case of CD4+ subtypes, Th17 cells may secrete IL-17A by the engagement of TLR4 as the main receptor mediating responses to Mtb via the induction of IL-1 [103]. Like TLR2, TLR4 genetic polymorphisms may influence the risk of developing Mtb infection [104].
5. TLR9 and TLR8
TLR9 interacts with mycobacterial DNA and activates macrophages to induce pro inflammatory cytokines [105]. Such activation is ascribed to unmethylated CpG motif [34,106] as well as small oligonucleotides that mimic bacterial CpG motifs [107] which interact with both TLR9 and TLR8. DCs are activated in such a way that their Mtb-induced IL-12 release is TLR9-dependent [108]. (Figure 1) One subtype of DCs, called plasmacytoid DCs, play an important role in the initiation of innate responses and inflammation after the induction of TLR9 stimulation with mycobacterial infection [109]. Such pivotal roles of TLR9 make those findings reasonable stating that TLR9-deficient mice are susceptible to Mtb infection rather than wild-type animals [38,110]. The other confirming idea is that Single Nucleotide Polymorphisms (SNPs) in the TLR9 gene region are associated with susceptibility to pulmonary and meningeal Mtb [111]. A tuberculosis (TB) vaccine consisting of a recombinant fusion protein (H4) and a novel TLR9 adjuvant (IC31) is in clinical development [112].
TLR8 expression can be up-regulated in macrophages after exposure to Bacillus Calmette–Guérin (BCG). Such a finding reveals a role for TLR8 in susceptibility to pulmonary tuberculosis in different populations [36]. The association of TLR8 to such susceptibility depends on its polymorphism [113].
6. Cooperation of TLRs
By cooperating with other TLRs, TLR2 forms heterodimers with TLR1 [114], TLR6 [115] and TLR4 [116] to activate the macrophages in response to tri-acylated lipoproteins, soluble tuberculosis factor, and mycobacterial 38-kDa glycolipoprotein antigen of Mtb, respectively. The primary CD4+ T cells use TLR2/TLR1 heterodimers to interact with Mtb lipoproteins, and this interaction results in direct T cell costimulation [65]. (Figure 1) The gene expression of TLR1 and 2 increases in intestinal Mtb infection through the induction of innate immune activation and Th1 polarization [117] from the first months of life and afterwards even after vaccination [118].
In line with this idea, a decrease in TLR1 and TLR6 genes modulate adaptive immunity from the point of the production of BCG-induced cytokines by T cells [119].
The different aspects of the cooperation of both TLR2 and TLR4 have been introduced. A study reported that the mycobacterial 38-kDa glycolipid antigen uses both TLR2 and TLR4 to induce pro inflammatory cytokines such as TNF-α and IL-6 in monocytes during Mtb infection [116]. Another study revealed that the invasion of Mtb to DCs might enhance their maturation [120] and antigen-presenting function [121] through activation of TLR2/4 signaling pathway. In regards to emphasizing the paradoxical effects of TLRs, two studies showed that TLR2 and TLR4 expression causes the Mtb infected cells more susceptible to death and drug resistance [122,123], whereas, two others associated the anti-Mtb activity of macrophages to the expression of such TLRs [124,125].
It seems that TLR2 also has some cooperation to TLR9. Double knockout TLR2/TLR9 mice display greater defects of IL-12 and IFN-γ production in comparison with both single TLR knockout mice [34]. Moreover, mice lacking TLR2/TLR4/TLR9 show a milder phenotype MyD88 deficient mice [126]. However, one recent study stated that signaling through TLR2 and through TLR2 and TLR9 is not required to generate immunity against Mtb growth [127].
Recently, the cooperation of other TLRs has been introduced. For example, Rv2034, a protein that is expressed during pulmonary infection which is strongly recognized by human T-cells, can be used as a new vaccine if introduced in the presence of TLR3, 4 and 9-adjuvants including cationic adjuvant formulation (CAF) 09, monophosphoryl lipid-A (MPL)/trehalose dimycolate (TDM), and CpG, respectively (Figure 1). Such combinations would be able to induce IFN-γ+CD4+T-cells [128]. Combining glucopyranosyl lipid adjuvant (GLA) and CpG, as TLR4 and TLR9 agonists, in order to enhance the Th1 response against ID93 antigen which is a fusion of four Mtb proteins and leads to an increased protection against aerosolized Mtb challenge is another example in this field [129] (Figure 1).
7. Conclusion
TLRs play a significant role against the invasion of TB in the body. Actually, each one alone can activate different components of the immune system and reinforce anti-TB responses. Therefore, defects and polymorphisms in TLRs may increase the risk of infection and vulnerability to TB. Moreover, TLR agonists may be used in the development of vaccines against Mtb. In the attempt to properly understand the interactions between the host and pathogen receptors, including the TLRs, we greatly hope to achieve an optimal combination for targeting various pathogen components to vaccinate the infection.
Likewise; TLRs can be used as important targets in the treatment of chronic mycobacterial infections. Although various studies have been conducted in the past decade to develop new findings in mechanism of TLRs function, more serious efforts would be needed to prevent the increasing risk of the tuberculosis infection. Such efforts should better clarify signal transduction pathways employed by the immune system to overwhelm Mtb and escape mechanisms employed by Mtb to resist the immune system.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].World Health Organization [Internet] Geneva: Global tuberculosis report 2016, Available from: http://www.who.int/entity/tb/publications/global_report/gtbr2016_main_text.pdf?ua=1 [Google Scholar]
- [2].Ahmad S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:814943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization [Internet] Geneva: Tuberculosis Fact sheet N°104. 2016, [Reviewed March 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/ [Google Scholar]
- [4].Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microb Infect. 2008;10(9):995–1004. 10.1016/j.micinf.2008.07.039 [DOI] [PubMed] [Google Scholar]
- [5].Bhatt K, Salgame P. Host innate immune response to Mycobacterium tuberculosis. J Clin Immunol. 2007;27(4):347–362. 10.1007/s10875-007-9084-0 [DOI] [PubMed] [Google Scholar]
- [6].García-Vallejo JJ, van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev. 2009;230(1):22–37. 10.1111/imr.2009.230.issue-1 [DOI] [PubMed] [Google Scholar]
- [7].Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21(6):455–462. 10.1016/j.cytogfr.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- [9].Schröder NW, Schumann RR. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis. 2005;5(3):156–164. 10.1016/S1473-3099(05)01308-3 [DOI] [PubMed] [Google Scholar]
- [10].Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- [11].Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- [12].Wu L, Hu Y, Li D, et al. Screening toll-like receptor markers to predict latent tuberculosis infection and subsequent tuberculosis disease in a Chinese population. BMC Med Genet. 2015;16(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Graustein A, Horne DJ, Arentz M, et al. . TLR9 gene region polymorphisms and susceptibility to tuberculosis in Vietnam. Tuberculosis. 2015;95(2):190–196. 10.1016/j.tube.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bukhari M, Aslam M, Khan A, et al. . TLR8 gene polymorphism and association in bacterial load in southern Punjab of Pakistan: an association study with pulmonary tuberculosis. Int J Immunogenet. 2015;42(1):46–51. 10.1111/iji.12170 [DOI] [PubMed] [Google Scholar]
- [15].Arji N, Busson M, Iraqi G, et al. . Genetic diversity of TLR2, TLR4, and VDR loci and pulmonary tuberculosis in Moroccan patients. J Infect Dev Ctries. 2014;8(4):430–440. [DOI] [PubMed] [Google Scholar]
- [16].Torres-García D, Cruz-Lagunas A, Figueroa MCG-S, et al. . Variants in toll-like receptor 9 gene influence susceptibility to tuberculosis in a Mexican population. J Trans Med. 2013;11(1):1–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zaki H, Leung K, Yiu W, et al. Common polymorphisms in TLR4 gene associated with susceptibility to pulmonary tuberculosis in the Sudanese. Int J Tuberc Lung Dis. 2012;16(7):934–940. 10.5588/ijtld.11.0517 [DOI] [PubMed] [Google Scholar]
- [18].Kobayashi K, Yuliwulandari R, Yanai H, et al. . Association of TLR polymorphisms with development of tuberculosis in Indonesian females. Tissue Antigens. 2012;79(3):190–197. 10.1111/j.1399-0039.2011.01821.x [DOI] [PubMed] [Google Scholar]
- [19].Dalgic N, Tekin D, Kayaalti Z, et al. Relationship between toll-like receptor 8 gene polymorphisms and pediatric pulmonary tuberculosis. Dis Markers. 2011;31(1):33–38. 10.1155/2011/545972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Velez DR, Wejse C, Stryjewski ME, et al. . Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet. 2010;127(1):65–73. 10.1007/s00439-009-0741-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu G, Zhang L, Zhao Y. Modulation of immune responses through direct activation of Toll-like receptors to T cells. Clin Exp Immunol. 2010;160(2):168–175. 10.1111/j.1365-2249.2010.04091.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jo E-K. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21(3):279–286. 10.1097/QCO.0b013e3282f88b5d [DOI] [PubMed] [Google Scholar]
- [23].Koets A, Santema W, Mertens H, et al. . Susceptibility to paratuberculosis infection in cattle is associated with single nucleotide polymorphisms in Toll-like receptor 2 which modulate immune responses against Mycobacterium avium subspecies paratuberculosis. Prev Vet Med. 2010;93(4):305–315. 10.1016/j.prevetmed.2009.11.008 [DOI] [PubMed] [Google Scholar]
- [24].Hossain MM, Norazmi M-N, Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection – the double-edged sword? Bio Med Res Int. 2013;2013:179174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fremond CM, Yeremeev V, Nicolle DM, et al. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114(12):1790–1799. 10.1172/JCI200421027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Scanga CA, Bafica A, Feng CG, et al. . MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72(4):2400–2404. 10.1128/IAI.72.4.2400-2404.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deng W, Li W, Zeng J, et al. . Mycobacterium tuberculosis PPE family protein Rv1808 manipulates cytokines profile via co-activation of MAPK and NFκB signaling pathways. Cell Physiol Biochem. 2014;33(2):273–288. 10.1159/000356668 [DOI] [PubMed] [Google Scholar]
- [28].Kim T-H, Shin SJ, Park Y-M, et al. . Critical role of TRIF and MyD88 in Mycobacterium tuberculosis Hsp70-mediated activation of dendritic cells. Cytokine. 2015;71(2):139–144. 10.1016/j.cyto.2014.09.010 [DOI] [PubMed] [Google Scholar]
- [29].Lim YJ, Choi JA, Lee JH, et al. Mycobacterium tuberculosis 38-kDa antigen induces endoplasmic reticulum stress-mediated apoptosis via toll-like receptor 2/4. Apoptosis. 2015;20(3):358–370. 10.1007/s10495-014-1080-2 [DOI] [PubMed] [Google Scholar]
- [30].Means TK, Wang S, Lien E, et al. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163(7):3920–3927. [PubMed] [Google Scholar]
- [31].Brightbill HD, Libraty DH, Krutzik SR, et al. . Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285(5428):732–736. 10.1126/science.285.5428.732 [DOI] [PubMed] [Google Scholar]
- [32].Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. 2003;9(4):264–268. 10.1177/09680519030090040801 [DOI] [PubMed] [Google Scholar]
- [33].Bulut Y, Michelsen KS, Hayrapetian L, et al. . Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biological Chem. 2005;280(22):20961–20967. 10.1074/jbc.M411379200 [DOI] [PubMed] [Google Scholar]
- [34].Bafica A, Scanga CA, Feng CG, et al. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202(12):1715–1724. 10.1084/jem.20051782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Means TK, Jones BW, Schromm AB, et al. . Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166(6):4074–4082. 10.4049/jimmunol.166.6.4074 [DOI] [PubMed] [Google Scholar]
- [36].Davila S, Hibberd ML, Dass RH, et al. . Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet. 2008;4(10):e1000218. 10.1371/journal.pgen.1000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Drage MG, Pecora ND, Hise AG, et al. . TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258(1):29–37. 10.1016/j.cellimm.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kleinnijenhuis J, Joosten LA, van de Veerdonk FL, et al. . Transcriptional and inflammasome-mediated pathways for the induction of IL-1β production by Mycobacterium tuberculosis. Eur J Immunol. 2009;39(7):1914–1922. 10.1002/eji.200839115 [DOI] [PubMed] [Google Scholar]
- [39].Wang JY, Chang HC, Liu JL, et al. . Expression of toll-like receptor 2 and plasma level of interleukin-10 are associated with outcome in tuberculosis. Eur J Clin Microbiol Infect Dis. 2012;31(9):2327–2333. 10.1007/s10096-012-1572-3 [DOI] [PubMed] [Google Scholar]
- [40].Das S, Bhattacharjee O, Goswami A, et al. Arabinosylated lipoarabinomannan (Ara-LAM) mediated intracellular mechanisms against tuberculosis infection: Involvement of protein kinase C (PKC) mediated signaling. Tuberculosis. 2015;95(2):208–216. 10.1016/j.tube.2014.11.007 [DOI] [PubMed] [Google Scholar]
- [41].Liu Y, Li J-Y, Chen S-T, et al. The rLrp of Mycobacterium tuberculosis inhibits proinflammatory cytokine production and downregulates APC function in mouse macrophages via a TLR2-mediated PI3K/Akt pathway activation-dependent mechanism. Cell Mol Immunol. 2016;13(6):729–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kumar A, Lewin A, Rani PS, et al. . Dormancy Associated Translation Inhibitor (DATIN/Rv0079) of Mycobacterium tuberculosis interacts with TLR2 and induces proinflammatory cytokine expression. Cytokine. 2013;64(1):258–264. 10.1016/j.cyto.2013.06.310 [DOI] [PubMed] [Google Scholar]
- [43].Prados-Rosales R, Baena A, Martinez LR, et al. . Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest. 2011;121(4):1471–1483. 10.1172/JCI44261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chambers MA, Whelan AO, Spallek R, et al. . Non acylated Mycobacterium bovis glycoprotein MPB83 binds to TLR1/2 and stimulates production of matrix metalloproteinase 9. Biochem Biophys Res Commun. 2010;400(3):403–408. 10.1016/j.bbrc.2010.08.085 [DOI] [PubMed] [Google Scholar]
- [45].Chen W-L, Sheu J-R, Chen R-J, et al. . Mycobacterium tuberculosis upregulates TNF-α expression via TLR2/ERK signaling and induces MMP-1 and MMP-9 production in human pleural mesothelial cells. PLOS ONE. 2015;10(9):e0137979. 10.1371/journal.pone.0137979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yihao D, Hongyun H, Maodan T. Latency-associated protein Rv2660c of Mycobacterium tuberculosis augments expression of proinflammatory cytokines in human macrophages by interacting with TLR2. Infect Dis. 2015;47(3):168–177. 10.3109/00365548.2014.982167 [DOI] [PubMed] [Google Scholar]
- [47].Yang S, Li F, Jia S, et al. . Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes apoptosis of macrophages via targeting the MicroRNA155-SOCS1 interaction. Cell Physiol Biochem. 2015;35(4):1276–1288. 10.1159/000373950 [DOI] [PubMed] [Google Scholar]
- [48].Sequeira PC, Senaratne RH, Riley LW. Inhibition of toll-like receptor 2 (TLR-2)-mediated response in human alveolar epithelial cells by mycolic acids and Mycobacterium tuberculosis mce1 operon mutant. Pathog Dis. 2014;70(2):132–140. 10.1111/fim.2014.70.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Aliprantis AO, Yang R-B, Mark MR, et al. . Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285(5428):736–739. 10.1126/science.285.5428.736 [DOI] [PubMed] [Google Scholar]
- [50].Drage MG, Tsai HC, Pecora ND, et al. . Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat Struct Mol Biol. 2010;17(9):1088–1095. 10.1038/nsmb.1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bakhru P, Sirisaengtaksin N, Soudani E, et al. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell Immunol. 2014;287(1):53–61. 10.1016/j.cellimm.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Madan-Lala R, Peixoto kV, Re F, et al. Mycobacterium tuberculosis Hip1 dampens macrophage proinflammatory responses by limiting toll-like receptor 2 activation. Infect Immun. 2011;79(12):4828–4838. 10.1128/IAI.05574-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Alemán M. Neutrophil apoptosis in the context of tuberculosis infection. Tuberculosis. 2015;95(4):359–363. 10.1016/j.tube.2015.03.010 [DOI] [PubMed] [Google Scholar]
- [54].Esin S, Counoupas C, Aulicino A, et al. . Interaction of Mycobacterium tuberculosis cell wall components with the human natural killer cell receptors NKp44 and Toll-like receptor 2. Scand J Immunol. 2013;77(6):460–469. 10.1111/sji.2013.77.issue-6 [DOI] [PubMed] [Google Scholar]
- [55].Nouailles G, Dorhoi A, Koch M, et al. . CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest. 2014;124(3):1268–1282. 10.1172/JCI72030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tan DB, Lim A, Yong YK, et al. . TLR2 induced cytokine responses may characterize HIV-infected patients experiencing mycobacterial immune restoration disease. AIDS. 2011;25(12):1455–1460. 10.1097/QAD.0b013e328348fb18 [DOI] [PubMed] [Google Scholar]
- [57].Liu PT, Stenger S, Li H, et al. . Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- [58].Liu PT, Stenger S, Tang DH, et al. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. 10.4049/jimmunol.179.4.2060 [DOI] [PubMed] [Google Scholar]
- [59].McBride A, Bhatt K, Salgame P. Development of a secondary immune response to Mycobacterium tuberculosis is independent of Toll-like receptor 2. Infect Immun. 2011;79(3):1118–1123. 10.1128/IAI.01076-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nicolò C, Di Sante G, Procoli A, et al. . M tuberculosis in the adjuvant modulates time of appearance of CNS-specific effector T cells in the spleen through a polymorphic site of TLR2. PLoS ONE. 2013;8(2):e55819. 10.1371/journal.pone.0055819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu H, Liu Z, Chen J, et al. . Induction of CCL8/MCP-2 by mycobacteria through the activation of TLR2/PI3K/Akt signaling pathway. PLoS ONE. 2013;8(2):e56815. 10.1371/journal.pone.0056815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].McBride A, Konowich J, Salgame P. Host defense and recruitment of Foxp3(+) T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires toll-like receptor 2. PLoS Pathog. 2013;9(6):e1003397. 10.1371/journal.ppat.1003397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Reba SM, Li Q, Onwuzulike S, et al. . TLR2 engagement on CD4+ T cells enhances effector functions and protective responses to Mycobacterium tuberculosis. Eur J Immunol. 2014;44(5):1410–1421. 10.1002/eji.201344100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tsukamoto Y, Endoh M, Mukai T, et al. . Immunostimulatory activity of major membrane protein II from Mycobacterium tuberculosis. Clin Vaccine Immunol. 2011;18(2):235–242. 10.1128/CVI.00459-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lancioni CL, Li Q, Thomas JJ, et al. . Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4+ T cell activation via Toll-like receptors 1 and 2. Infect Immun. 2011;79(2):663–673. 10.1128/IAI.00806-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rahman MJ, Chuquimia OD, Petursdottir DH, et al. Impact of Toll-like receptor 2 deficiency on immune responses to mycobacterial antigens. Infect Immun. 2011;79(11):4649–4656. 10.1128/IAI.05724-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Drennan MB, Nicolle D, Quesniaux VJ, et al. . Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164(1):49–57. 10.1016/S0002-9440(10)63095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sutmuller RP, Morgan ME, Netea MG, et al. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27(8):387–393. 10.1016/j.it.2006.06.005 [DOI] [PubMed] [Google Scholar]
- [69].Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8(4):296–307. 10.1038/nrmicro2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Byun E-H, Kim WS, Kim J-S, et al. . Mycobacterium tuberculosis Rv0577, a novel TLR2 agonist, induces maturation of dendritic cells and drives Th1 immune response. The FASEB J. 2012;26(6):2695–2711. 10.1096/fj.11-199588 [DOI] [PubMed] [Google Scholar]
- [71].Kim WS, Jong-Seok K, Cha SB, et al. . Mycobacterium tuberculosis Rv3628 drives Th1-type T cell immunity via TLR2-mediated activation of dendritic cells and displays vaccine potential against the hyper-virulent Beijing K strain. Oncotarget. 2016;7(18):24962–24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Richardson ET, Shukla S, Sweet DR, et al. . TLR2- dependent ERK signaling in Mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits Th1 polarization of responding T cells. Infect Immun. 2015;83(6):2242–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bansal K, Trinath J, Chakravortty D, et al. Pathogen-specific TLR2 protein activation programs macrophages to induce Wnt-β-catenin signaling. J Biol Chem. 2011;286(42):37032–37044. 10.1074/jbc.M111.260414 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [74].Teixeira-Coelho M, Cruz A, Carmona J, et al. . TLR2 deficiency by compromising p19 (IL-23) expression limits Th 17 cell responses to Mycobacterium tuberculosis. Int Immunol. 2011;23(2):89–96. 10.1093/intimm/dxq459 [DOI] [PubMed] [Google Scholar]
- [75].Chatterjee S, Dwivedi VP, Singh Y, et al. . Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Path. 2011;7(11):e1002378. 10.1371/journal.ppat.1002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Khader SA, Bell GK, Pearl JE, et al. . IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
- [77].Shenderov K, Barber DL, Mayer-Barber KD, et al. . Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol. 2013;190(11):5722–5730. 10.4049/jimmunol.1203343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gowthaman U, Rai PK, Khan N, et al. Lipidated promiscuous peptides vaccine for tuberculosis-endemic regions. Trends Mol Med. 2012;18(10):607–614. 10.1016/j.molmed.2012.07.008 [DOI] [PubMed] [Google Scholar]
- [79].Xu Y, Yang E, Huang Q, et al. . PPE57 induces activation of macrophages and drives Th1-type immune responses through TLR2. J Mol Med. 2015;93(6):645–662. 10.1007/s00109-014-1243-1 [DOI] [PubMed] [Google Scholar]
- [80].Mohammad O, Kaur J, Singh G, et al. . TLR agonist augments prophylactic potential of acid inducible antigen Rv3203 against Mycobacterium tuberculosis H37Rv in experimental animals. PLOS ONE. 2016;11(3):e0152240. 10.1371/journal.pone.0152240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen ST, Li JY, Zhang Y, et al. Recombinant MPT83 derived from Mycobacterium tuberculosis induces cytokine production and upregulates the function of mouse macrophages through TLR2. J Immunol. 2012;188(2):668–677. 10.4049/jimmunol.1102177 [DOI] [PubMed] [Google Scholar]
- [82].Rottinghaus EK, Vesosky B, Turner J. TLR-2 independent recognition of Mycobacterium tuberculosis by CD11c+ pulmonary cells from old mice. Mech Ageing Dev. 2010;131(6):405–414. 10.1016/j.mad.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chodisetti SB, Gowthaman U, Rai PK. Triggering through toll-like receptor 2 limits chronically stimulated T-helper type 1 cells from undergoing exhaustion. J Infect Dis. 2014;211(3):486–496. [DOI] [PubMed] [Google Scholar]
- [84].Kanagawa H, Niki Y, Kobayashi T, et al. . Mycobacterium tuberculosis promotes arthritis development through toll-like receptor 2. J Bone Miner Metab. 2015;33(2):135–141. 10.1007/s00774-014-0575-9 [DOI] [PubMed] [Google Scholar]
- [85].Almeida PE, Roque NR, Magalhães KG, et al. . Differential TLR2 downstream signaling regulates lipid metabolism and cytokine production triggered by Mycobacterium bovis BCG infection. Biochim Biophys Acta Mol Cell Biol Lipids. 2014;1841(1):97–107. 10.1016/j.bbalip.2013.10.008 [DOI] [PubMed] [Google Scholar]
- [86].Gupta D, Sharma S, Singhal J, et al. Suppression of TLR2-induced IL-12, reactive oxygen species, and inducible nitric oxide synthase expression by Mycobacterium tuberculosis antigens expressed inside macrophages during the course of infection. J Immunol. 2010;184(10):5444–5455. 10.4049/jimmunol.0903283 [DOI] [PubMed] [Google Scholar]
- [87].Zhao Y, Bu H, Hong K, et al. . Genetic polymorphisms of CCL1 rs2072069 G/A and TLR2 rs3804099 T/C in pulmonary or meningeal tuberculosis patients. Int J clin Exp Pathol. 2015;8(10):12608–12620. [PMC free article] [PubMed] [Google Scholar]
- [88].Yu G, Cui Z, Sun X, et al. . Gene expression analysis of two extensively drug-resistant tuberculosis isolates show that two-component response systems enhance drug resistance. Tuberculosis. 2015;95(3):303–314. 10.1016/j.tube.2015.03.008 [DOI] [PubMed] [Google Scholar]
- [89].Pöyhönen L, Nuolivirta K, Vuononvirta J, et al. . Toll-like receptor 2 subfamily gene polymorphisms are associated with Bacillus Calmette-Guérin osteitis following newborn vaccination. Acta Paediatr. 2015;104(5):485–490. 10.1111/apa.2015.104.issue-5 [DOI] [PubMed] [Google Scholar]
- [90].Reiling N, Hölscher C, Fehrenbach A, et al. . Cutting edge: toll-like receptor (TLR) 2-and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169(7):3480–3484. 10.4049/jimmunol.169.7.3480 [DOI] [PubMed] [Google Scholar]
- [91].Branger J, Leemans JC, Florquin S, et al. Toll-like receptor 4 plays a protective role in pulmonary tuberculosis in mice. Int Immunol. 2004;16(3):509–516. 10.1093/intimm/dxh052 [DOI] [PubMed] [Google Scholar]
- [92].Khan N, Pahari S, Vidyarthi A, et al. . NOD-2 and TLR-4 signaling reinforces the efficacy of dendritic cells and reduces the dose of TB drugs against Mycobacterium tuberculosis. J Innate Immun. 2016;8(3):228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lee SJ, Shin SJ, Lee MH, et al. . A potential protein adjuvant derived from Mycobacterium tuberculosis Rv0652 enhances dendritic cells-based tumor immunotherapy. PLoS ONE. 2014;9(8):e104351. 10.1371/journal.pone.0104351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jung ID, Jeong SK, Lee C-M, et al. . Enhanced efficacy of therapeutic cancer vaccines produced by co-treatment with Mycobacterium tuberculosis heparin-binding hemagglutinin, a novel TLR4 agonist. Cancer Res. 2011;71(8):2858–2870. 10.1158/0008-5472.CAN-10-3487 [DOI] [PubMed] [Google Scholar]
- [95].Heldwein KA, Liang MD, Andresen TK, et al. . TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J Leukocyte Biol. 2003;74(2):277–286. 10.1189/jlb.0103026 [DOI] [PubMed] [Google Scholar]
- [96].Abel B, Thieblemont N, Quesniaux VJ, et al. . Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169(6):3155–3162. 10.4049/jimmunol.169.6.3155 [DOI] [PubMed] [Google Scholar]
- [97].Doz E, Rose S, Vasseur V, et al. . Mycobacterial phosphatidylinositol mannosides negatively regulate host Toll-like receptor 4, MyD88 dependent proinflammatory cytokines, and TRIF-dependent co-stimulatory molecule expression. J Biol Chem. 2009;284(35):23187–23196. 10.1074/jbc.M109.037846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kim K, Sohn H, Kim JS, et al. . Mycobacterium tuberculosis Rv0652 stimulates production of tumour necrosis factor and monocytes chemoattractant protein-1 in macrophages through the Toll-like receptor 4 pathway. Immunology. 2012;136(2):231–240. 10.1111/imm.2012.136.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cehovin A, Coates AR, Hu Y, et al. . Comparison of the moonlighting actions of the two highly homologous chaperonin 60 proteins of Mycobacterium tuberculosis. Infect Immun. 2010;78(7):3196–3206. 10.1128/IAI.01379-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kim J-S, Kim WS, Choi H-G, et al. . Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J Leukocyte Biol. 2013;94(4):733–749. 10.1189/jlb.0912435 [DOI] [PubMed] [Google Scholar]
- [101].Choi HG, Kim WS, Back YW, et al. . Mycobacterium tuberculosis RpfE promotes simultaneous Th1-and Th17-type T-cell immunity via TLR4-dependent maturation of dendritic cells. Eur J Immunol. 2015;45(7):1957–1971. 10.1002/eji.v45.7 [DOI] [PubMed] [Google Scholar]
- [102].Shah JA, Vary JC, Chau TT, et al. . Human TOLLIP regulates TLR2 and TLR4 signaling and its polymorphisms are associated with susceptibility to tuberculosis. J Immunol. 2012;189(4):1737–1746. 10.4049/jimmunol.1103541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, et al. . Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukocyte Biol. 2010;88(2):227–232. 10.1189/jlb.0809550 [DOI] [PubMed] [Google Scholar]
- [104].Jafari M, Nasiri MR, Sanaei R, et al. . The NRAMP1, VDR, TNF-α, ICAM1, TLR2 and TLR4 gene polymorphisms in Iranian patients with pulmonary tuberculosis: A case–control study. Infect Genet Evol. 2016;39:92–98. [DOI] [PubMed] [Google Scholar]
- [105].Hemmi H, Takeuchi O, Kawai T, et al. . A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. [DOI] [PubMed] [Google Scholar]
- [106].Griebel PJ, Brownlie R, Manuja A, et al. . Bovine toll-like receptor 9: a comparative analysis of molecular structure, function and expression. Vet Immunol Immunopathol. 2005;108(1–2):11–16. 10.1016/j.vetimm.2005.07.012 [DOI] [PubMed] [Google Scholar]
- [107].Choi S-S, Chung E, Jung Y-J. Newly identified CpG ODNs, M5-30 and M6-395, stimulate mouse immune cells to secrete TNF-α and enhance Th1-mediated immunity. J Microbiol. 2010;48(4):512–517. 10.1007/s12275-010-0053-6 [DOI] [PubMed] [Google Scholar]
- [108].Pompei L, Jang S, Zamlynny B, et al. . Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol. 2007;178(8):5192–5199. 10.4049/jimmunol.178.8.5192 [DOI] [PubMed] [Google Scholar]
- [109].Guillerey C, Mouriès J, Polo G, et al. . Pivotal role of plasmacytoid dendritic cells in inflammation and NK-cell responses after TLR9 triggering in mice. Blood. 2012;120(1):90–99. 10.1182/blood-2012-02-410936 [DOI] [PubMed] [Google Scholar]
- [110].Kleinnijenhuis J, Oosting M, Joosten LA, et al. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011:405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Graustein A, Horne D, Arentz M, et al. . TLR9 gene region polymorphisms and susceptibility to tuberculosis in Vietnam. Tuberculosis. 2015;95(2):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Aboutorabian S, Hakimi J, Boudet F, et al. . A high ratio of IC31® adjuvant to antigen is necessary for H4 TB vaccine immunomodulation. Hum Vaccin Immunother. 2015;11(6):1449–1455. 10.1080/21645515.2015.1023970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Salie M, Daya M, Lucas LA, et al. . Association of toll-like receptors with susceptibility to tuberculosis suggests sex-specific effects of TLR8 polymorphisms. Infect Genet Evol. 2015;34:221–229. 10.1016/j.meegid.2015.07.004 [DOI] [PubMed] [Google Scholar]
- [114].Jin MS, Kim SE, Heo JY, et al. . Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130(6):1071–1082. 10.1016/j.cell.2007.09.008 [DOI] [PubMed] [Google Scholar]
- [115].Bulut Y, Faure E, Thomas L, et al. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001;167(2):987–994. 10.4049/jimmunol.167.2.987 [DOI] [PubMed] [Google Scholar]
- [116].Jung S-B, Yang C-S, Lee J-S, et al. . The mycobacterial 38 kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74(5):2686–2696. 10.1128/IAI.74.5.2686-2696.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pugazhendhi S, Jayakanthan K, Pulimood A, et al. Cytokine gene expression in intestinal tuberculosis and Crohn’s disease. Int J Tuberc Lung Dis. 2013;17(5):662–668. 10.5588/ijtld.12.0600 [DOI] [PubMed] [Google Scholar]
- [118].Shey MS, Nemes E, Whatney W, et al. . Maturation of innate responses to mycobacteria over the first nine months of life. J Immunol. 2014;192(10):4833–4843. 10.4049/jimmunol.1400062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Randhawa AK, Shey MS, Keyser A, et al. . Association of human TLR1 and TLR6 deficiency with altered immune responses to BCG vaccination in South African infants. PLoS path. 2011;7(8):e1002174. 10.1371/journal.ppat.1002174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Uehori J, Fukase K, Akazawa T, et al. . Dendritic cell maturation induced by muramyl dipeptide (MDP) derivatives: monoacylated MDP confers TLR2/TLR4 activation. J Immunol. 2005;174(11):7096–7103. 10.4049/jimmunol.174.11.7096 [DOI] [PubMed] [Google Scholar]
- [121].Xu Q, Jin MM, Zheng WW, et al. Role of Toll-like receptor 2/4-nuclear factor-kappaB signaling pathway in invasion of Mycobacterium tuberculosis to mouse dendritic cells. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2014;43(2):200–206. [DOI] [PubMed] [Google Scholar]
- [122].Sun Z, Ren W, Xu Y, et al. Preliminary study on the virulence of XDR-TB: low virulence owing to less cytokine expression through the TLR 2 and TLR4 pathways in BLAB/C mice. Bio-med Mater Eng. 2014;24(6):3873–3882. [DOI] [PubMed] [Google Scholar]
- [123].Chavez-Galan L, Sada-Ovalle I, Baez-Saldana R, et al. Monocytes from tuberculosis patients that exhibit cleaved caspase 9 and denaturalized cytochrome c are more susceptible to death mediated by Toll-like receptor 2. Immunology. 2012;135(4):299–311. 10.1111/imm.2012.135.issue-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ranjbar S, Haridas V, Jasenosky LD, et al. A role for IFITM proteins in restriction of Mycobacterium tuberculosis Infection. Cell Rep. 2015;13(5):874–883. 10.1016/j.celrep.2015.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Li N, Liu P, Wang L, et al. . Effect of Ipr1 on expression levels of immune genes related to macrophage anti-infection of mycobacterium tuberculosis. Int J Clin Exp Med. 2015;8(3):3411–3419. [PMC free article] [PubMed] [Google Scholar]
- [126].Holscher C, Reiling N, Schaible UE, et al. . Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. Eur J Immunol. 2008;38(3):680–694. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- [127].Gopalakrishnan A, Dietzold J, Salgame P. Vaccine-mediated immunity to experimental Mycobacterium tuberculosis is not impaired in the absence of Toll-like receptor 9. Cell Immunol. 2016;(302):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Commandeur S, van den Eeden SJ, Dijkman K, et al. . The in vivo expressed Mycobacterium tuberculosis (IVE-TB) antigen Rv2034 induces CD4+ T-cells that protect against pulmonary infection in HLA-DR transgenic mice and guinea pigs. Vaccine. 2014;32(29):3580–3588. 10.1016/j.vaccine.2014.05.005 [DOI] [PubMed] [Google Scholar]
- [129].Orr MT, Beebe EA, Hudson TE, et al. . A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93. PLoS ONE. 2014;9(1):e83884. 10.1371/journal.pone.0083884 [DOI] [PMC free article] [PubMed] [Google Scholar]