Introduction
Leptospirosis, caused by serovars of the pathogenic spirochetes belong to the genus Leptospira. It is an emerging and most neglected zoonosis in a wide range of animal hosts, has a worldwide distribution [1,2]. Globally, the disease is estimated to cause 1.03 million cases and 58,900 deaths each year. These estimates make it a leading zoonotic cause of morbidity and mortality [3,4].
Leptospira are antigenically diverse with more than 250 serovars, belonging to 25 serogroups and about 21 species [5], with multiple serovars being endemic in a given area. Protective immunity against Leptospira infection is mediated predominantly through humoral, by antibodies directed against lipopolysaccharide (LPS) and is generally serovar specific (but not exclusively so) [6,7].
Immunity acquired after natural infection is mostly limited to the infecting serovar. Heterologous immunity does not usually follow a natural infection (at least in humans), though some degree of cross protection between serovars has been observed [8,9]. The nature of immune response after natural infection has implications in the pathogenicity, clinical course of the disease, sero-diagnosis and in the effectiveness of vaccines [10–12].
The stimulation of cross-protective immunity still remains an important focus in leptospirosis vaccine research. There are very few studies that investigated the immune cross-protection among Leptospira serovars, and long-term antibody response in endemic human population. The present study assessed the long-term clinical course and also the level and duration of antibody persistence against autologous, homologous and heterologous strains of Leptospira, among those infected naturally with diverse serovars.
Material and methods
The archipelago of Andaman and Nicobar (92-94°E; 6-14°N), a chain of more than 500 Islets situated about 1200 km south-east of Indian peninsula in the Bay of Bengal, is a Union Territory (UT) of India. The present study was carried out at three primary health centres (PHCs) in the district of South Andaman (SA), which are the local source of healthcare services.
Probable case of leptospirosis is considered as those patients who report to PHC with symptoms, viz, fever or headache of sudden onset along with severe calf muscle tenderness, conjunctival suffusion, symptoms or signs of lung or liver dysfunction or any haemorrhagic tendency. Any probable case with a positive blood culture for Leptospira or a four-fold rise in seroconversion by microscopic agglutination test (MAT), was considered as a confirmed case.
Patients fulfilling the criteria for probable case were included in the study after obtaining written consent. Patient enrolment was carried out for a period of 15 months. Follow-up blood samples were collected from a subsample of culture positive patients at intervals of 1, 3, 6, 9, 12, 24, 36 and 48 months, in addition to the sample obtained at the time of reporting, and the convalescent sample obtained 2–3 weeks thereafter.
Blood culture: Acute samples collected from all the patients were inoculated into Ellinghausen–McCullough–Johnson–Harris (EMJH) semisolid medium and incubated at 30 °C. Inoculated media were examined under darkground microscope (DGM) at weekly intervals. Cultures were declared negative if no growth of Leptospira was detected within six weeks.
Microscopic agglutination test (MAT): MAT was performed following standard procedure [13] on all serum samples using a panel of 33 strains of Leptospira as antigens. The panel included 19 reference strains of 18 serovars, belonging to 17 serogroups of Leptospira and 14 strains, belonging to 6 serovars of 5 serogroups, recovered from the patients enrolled in the study. The details of the strains used in MAT as antigens are listed in Table S1 (supplementary material). MAT was performed in doubling dilutions, starting from a dilution of 1 in 20. Positive samples were titrated up to end titres [14].
The distribution of culture positive patients obtained by MAT titre against the infecting strain, reference strain of homologous serogroup and the highest titre obtained against reference strains of heterologous serogroups, were analyzed by dot plots with medians. The proportion of the patients seropositive to each of the above three antigens was plotted against different time interval from infection. The incidence density of sero-reversion from positive to negative against the three antigens was studied by Kaplan-Meier survival analysis, and the mean and median duration of sero-reversion with 95% confidence limits were estimated.
Results and discussion
In the present study (for a period of 15 months), a total of 334 patients with a clinical diagnosis of probable leptospirosis were included. Among them, leptospirosis was confirmed in 103 patients. Of these, 99 recovered with therapy and four patients died due to pulmonary haemorrhage and respiratory distress (case fatality ratio = 3.9%). Assuming that the PHC could detect all the clinical cases of leptospirosis among the population of 65,751 (Directorate of Economics and Statistics, Andaman and Nicobar Administration) of rural South Andaman, the annual incidence of leptospirosis in the area would be about 125 cases per 100,000 population.
The common infecting serovars were Australis, Canicola, Copenhageni, Ratnapura, Valbuzzi, Hebdomadis, Hardjo, Icterohaemorrhagiae, Pomona and Saxkoebing. Previous reports showed post-monsoon epidemics, as well as inter-epidemic sporadic cases occurring every year. Serovars, viz, Australis, Canicola, Copenhageni, Ratnapura, Valbuzzi [15], Hebdomadis, Hardjo, Icterohaemorrhagiae, Lai like [16], Pomona and Saxkoebing have been found to be responsible for the infection [17]. Regular occurrence of leptospirosis in a relatively small and stable community provided an opportunity to constitute a cohort of individuals with high risk of infection, with a wide spectrum of Leptospira serovars. There are very few studies on the immune cross-protection among Leptospira serovars and long-term antibody response in culture-confirmed patients of leptospirosis, which could be probably due to the difficulty in obtaining a suitable cohort [1,18].
Among the 99 confirmed leptospirosis patients, who recovered from the disease, 48 were followed up clinically for a period of four years (out of 48, there were 14 culture positives). Among them, 12 developed acute febrile illness, fulfilling the case definition for probable case of leptospirosis and a diagnosis of leptospirosis was confirmed in one patient. Re-infection occurred at 10 months, post initial infection. The cumulative incidence of re-infection during the four year follow up period was 2.08%, and the incidence density of re-infection was 0.52/100 person-years. The individuals who were initially infected with serovar Pomona, of the serogroup Pomona (confirmation was based on isolation and identification) was re-infected by serovar Ratnapura, of the serogroup Grippotyphosa (confirmation was based on paired MAT) (Supplementary data). However, the incidence density of Leptospira re-infection was only 0.52/100 person-years, compared to infection among non-confirmed cases, where incidence density was 1.32/100 person-years (data set). Similar observations were reported by other workers, viz. culture confirmed infection with one serovar was re-infected later by a different serovar in less than 3 months [1,18]. The initial infection may have acted as a natural live vaccine, conferring cross-protection among unrelated Leptospira serovars in an endemic setting.
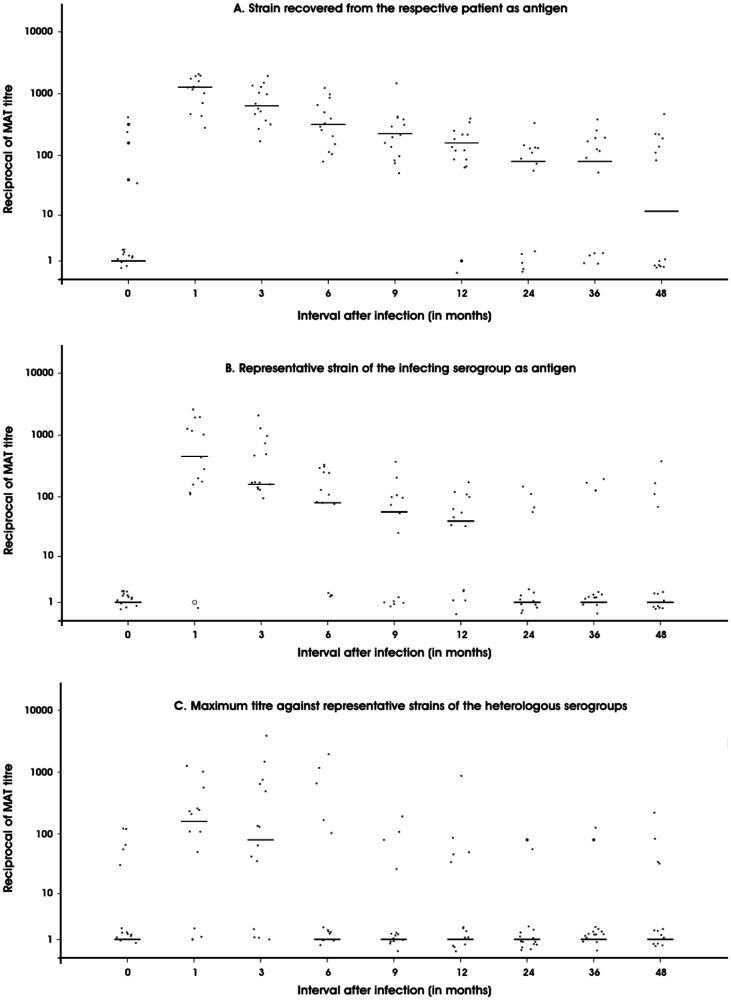
Figure 1 shows the trend in antibody titre in culture positive patients. All the patients remained seropositive to the infecting strain between the 1st and 9th month. Although it dropped later, (even after four years), half of the patients remained seropositive to the strain. Seropositivity to the reference strain of the infecting serogroup followed a similar pattern, but with lower proportion of seropositivity at all intervals, except at three months, when all the patients were seropositive to the reference strain of homologous serogroup. At the end of four years, only a third of the patients retained detectable levels of antibodies against the homologous serogroup’s reference strain. Seropositivity to heterologous serogroups peaked to about 70% during 1–3 months and then dropped rapidly. Only a third of the patients had detectable antibodies against heterologous serogroups at 9 months and at 24 months, only one patient had heterologous antibodies.
Figure 1.
Distribution of reciprocal of the titre in MAT done with culture positive patients’ sera against the strain recovered from the respective patient (A), representative strain of homologous serogroup (B) and representative strains of heterologous serogroups (C) (horizontal scores indicate the median titres)
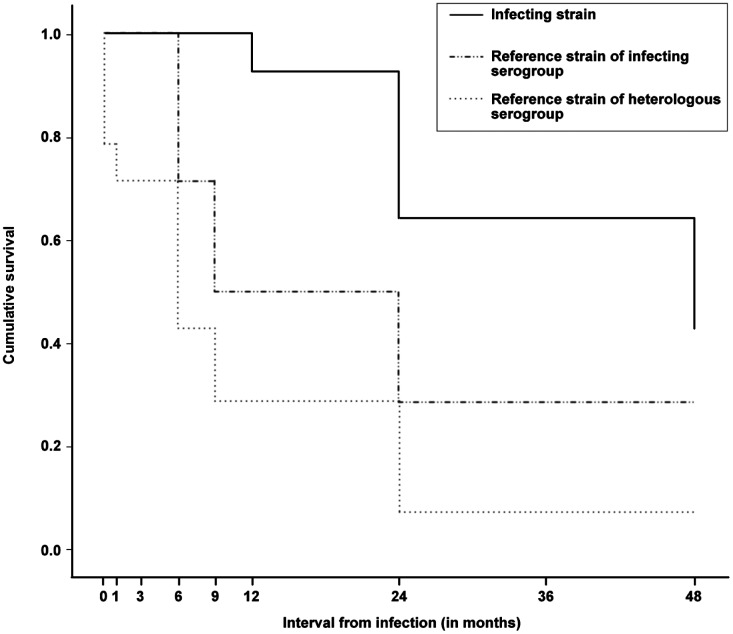
The survival curves of antibody levels, ≥1 in 40 against the three antigens, viz. infecting strain, homologous serogroup reference strain and heterologous serogroups reference strain, created by Kaplan-Meier analysis is shown in Figure 2. The cumulative survival (presence of antibodies ≥1 in 40) was substantially higher for antibodies against the infecting strain as compared to homologous or heterologous reference strains. At the end of four year follow-up period, the cumulative persistence of antibodies was 0.43 against the infecting strain, 0.29 against the homologous reference strain and 0.07 against heterologous reference strains. The mean and median duration of persistence along with 95% confidence intervals for antibody levels ≥1 in 40 against the infecting strain, homologous and heterologous reference strains is summarized in Table 1. The median survival of antibodies against the infecting strain was four years, while it was 9 and 6 months for antibodies against homologous and heterologous serogroups.
Figure 2.
Survival curves of antibody levels ≥1 in 40 against infecting strain reference strain of the serogroup of infecting strain and reference strain of any of the other serogroups constructed by Kaplan-Meier analysis.
Table 1.
Mean and median period (in months)of seropositivity to the infecting strain, reference strain of infecting serogroup and reference strains of heterologous serogroups after Leptospira infection.
| Antigen | Mean | 95% confidence interval | Median | 95% confidence interval |
|---|---|---|---|---|
| Strain | 38.6 | 31.3, 45.8 | 48.0 | 19.0, 77.0 |
| Homologous serogroup | 22.5 | 13.4, 31.6 | 9.0 | 0.0, 20.0 |
| Heterologous serogroups | 11.6 | 4.7, 18.6 | 6.0 | 1.5, 10.5 |
The present study demonstrated the persistence of antibodies after a Leptospiral natural infection, which may have implications on various aspects of the disease. The antibody titre against autologous and homologous antigen had long duration of persistence up to 4 years. The persistence of antibodies may influence the diagnostic accuracy of screening tests [19]. Pre-existing antibodies may lead to complications, such as severe pulmonary haemorrhage (SPH) during subsequent infections with the same or a different serovar. The immune response also has a role in the pathogenesis of leptospirosis through the formation of immunological complex, by the liberation of cytokines and the generation of autoimmune vasculitis [10–12]. Signs and symptoms of the lung, renal and hepatic involvement thus appear in the immune phase when specific antibodies begin to be detected [12]. Previous subclinical exposure to one serovar may possibly worsen the disease caused by a different serovar, if the immune system is deregulated, analogous to the situation observed for Dengue [20]. Rise in antibody titres due to past infections has been linked to the increased severity in leptospirosis [21].
The antibodies against the infecting strain rose to a higher peak and lasted longer than the homologous titre. This observation indicates that microscopic agglutinating antibodies are mainly serovar and strain specific, and may not neutralize the diverse serovars and strains even within a serogroup. Experimental bacterins, LPS and antigenic purified protein protected against homologous and heterologous strains, thus demonstrating the existence of cross-protection [22]. However, it seems apparent that heterologous immunity does not usually follow natural infection, at least in humans.
There are very few prospective studies that evaluated the long term immune cross-protection among Leptospira serovars in humans. After a natural infection with Leptospira, antibodies persist for many years. The specificity of the agglutinating antibodies are restricted to the level of the infecting strain. Consequently, the spectrum of strains, which the whole-cell vaccines can protect against, will be very limited. The duration of persistence of antibodies might be an indicator of the effective period of protection offered by vaccines. Although several attempts have been made to develop an effective vaccine, none that confers protection across heterogeneous large number of serovars of Leptospira has yet been developed [1]. However, these studies are often lacking for antibodies which are generated against protein antigens which may not show up in MAT. Further, immune-protection should also involve such protein antigens in a long term follow up study in such endemic setting.
Ethical clearance
This study was approved by Institutional Ethics Committee.
Supplemental data
The supplemental material for this paper is available online at https://doi.org/10.1080/20477724.2017.1333782.
Funding
This work was supported by Indian Council of Medical Research [Intramural Grant].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Acknowledgement
The authors are thankful to the Directorate of Health Services (DHS), Andaman & Nicobar Administration, Andaman & Nicobar Islands for providing support in carrying out the present study. The authors are also thankful to clinician and staff at Manglutan PHC for their support. The authors would like to thank Indian Council of Medical Research, New Delhi for providing grant support for this study.
References
- [1].Adler B. Vaccines against leptospirosis. Curr Top Microbiol Immunol. 2015;387:251–272. [DOI] [PubMed] [Google Scholar]
- [2].Vijayachari P, Sugunan A, Shriram A. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(4):557–569. 10.1007/s12038-008-0074-z [DOI] [PubMed] [Google Scholar]
- [3].Costa F, Hagan JE, Calcagno J, et al. . Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898 10.1371/journal.pntd.0003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Torgerson PR, Hagan JE, Costa F, et al. . Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Negl Trop Dis. 2015;9(10):e0004122 10.1371/journal.pntd.0004122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Levett PN. Systematics of leptospiraceae. Curr Top Microbiol Immunol. 2015;387:11–20. [DOI] [PubMed] [Google Scholar]
- [6].Sonrier C, Branger C, Michel V, et al. . Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine. 2000;19(1):86–94. 10.1016/S0264-410X(00)00129-8 [DOI] [PubMed] [Google Scholar]
- [7].Adler B, de la Pena MA. Leptospira and leptospirosis. Vet Microbiol. 2010;140(3–4):287–296. 10.1016/j.vetmic.2009.03.012 [DOI] [PubMed] [Google Scholar]
- [8].Myers DM, Coltorti EA. Broadly reacting precipitating and agglutinating antigen of leptospirae. J Clin Microbiol. 1978;8(5):580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rosario LA, Arencibia DF, Suarez YE, et al. . Cross-protection among unrelated leptospira pathogens serovars: an unfinished story. Adv Clin Exp Med. 2012;21(5):581–589. [PubMed] [Google Scholar]
- [10].Zuerner RL. Host response to leptospira infection. Curr Top Microbiol Immunol. 2015;387:223–250. [DOI] [PubMed] [Google Scholar]
- [11].Levett P. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cinco M, Domenis R, Perticarari S, et al. . Interaction of leptospires with murine microglial cells. New Microbiol. 2006;29(3):193–199. [PubMed] [Google Scholar]
- [13].Wolff JW. The laboratory diagnosis of leptospirosis In: Charles CT, editor. Springfield (IL) Blackwell Scientific Publications, Ltd. 1954. p. 99. [Google Scholar]
- [14].Faine S. Guidelines for control of leptospirosis. Geneva: WHO; offset publication no. 67; 1982. [Google Scholar]
- [15].Vijayachari P, Sehgal S, Goris M, et al. . Leptospira interrogans serovar Valbuzzi: a cause of severe pulmonary haemorrhages in the Andaman Islands. J Med Microbiol. 2003;52(10):913–918. 10.1099/jmm.0.05094-0 [DOI] [PubMed] [Google Scholar]
- [16].Sehgal SC, Vijayachari P, Smythe LD, et al. . Lai-like leptospira from the Andaman Islands. Indian J Med Res. 2000;112:135–139. [PubMed] [Google Scholar]
- [17].Vijayachari P, Ahmed N, Sugunan A, et al. . Use of fluorescent amplified fragment length polymorphism for molecular epidemiology of leptospirosis in India. J Clin Microbiol. 2004;42(8):3575–3580. 10.1128/JCM.42.8.3575-3580.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Esteves LM, Bulhões SM, Branco CC, et al. . Human leptospirosis: seroreactivity and genetic susceptibility in the population of Sao Miguel Island (Azores, Portugal). PLoS One. 2014;9(9):e108534 10.1371/journal.pone.0108534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vijayachari P, Sugunan AP, Sehgal SC. Evaluation of microscopic agglutination test as a diagnostic tool during acute stage of leptospirosis in high & low endemic areas. Indian J Med Res. 2001;114:99–106. [PubMed] [Google Scholar]
- [20].Truong KN, Coburn J. The emergence of severe pulmonary hemorrhagic leptospirosis: questions to consider. Front Cell Infect Microbiol. 2011;1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abdulkader RC, Daher EF, Camargo ED, et al. . Leptospirosis severity may be associated with the intensity of humoral immune response. Rev Inst Med Trop Sao Paulo. 2002;44(2):79–83. 10.1590/S0036-46652002000200005 [DOI] [PubMed] [Google Scholar]
- [22].Dib CC, Gonçales AP, Morais ZM, et al. . Cross-protection between experimental anti-leptospirosis bacterins. Braz J Microbiol. 2014;45:1083–1088. 10.1590/S1517-83822014000300042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.