Abstract
We report a method for programming complexity into the glycocalyx of live cells. Via a combination of glycomaterial synthesis and membrane remodeling, we have engineered cells to display native-like, mixed sialoglycan populations, while confining the activity of each glycan into a specific nanoscale presentation.
Graphical abstract
A cell surface engineering method allows for building glycan complexity with control over nanoscale presentation.
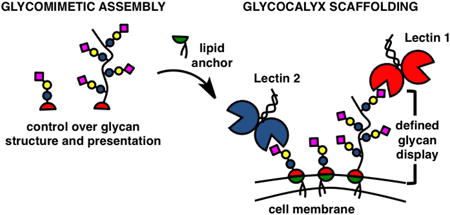
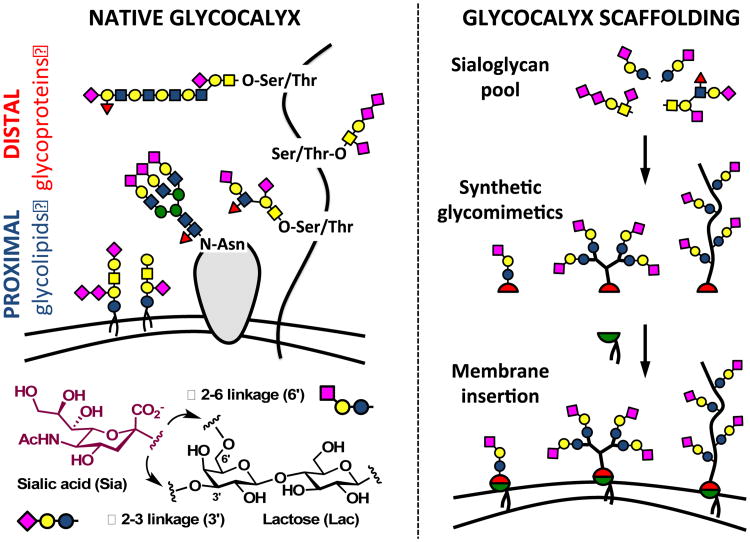
The glycocalyx is an intricate network of carbohydrate-rich biomolecules delineating the boundary between cells and the extracellular environment (Fig 1, left). Composed primarily of glycolipids, glycoproteins and proteoglycans, and reaching hundreds of nanometers from the cell surface, this biological interface contributes key functions to cellular and tissue physiology by providing a protective microenvironment, mediating nutrient exchange while keeping pathogens at bay, and facilitating intercellular communication.1
Figure 1.
Building glycocalyx complexity. The hierarchical organization of sialoglycans in native glycocalyx (left) can be recapitulated in a controlled fashion via precision glycan engineering (right). Specific glycans are assembled into synthetic glycomimetic structures and primed with a lipid anchor for insertion into cell membranes. Delivery of unique glycans in pre-defined nanoscale arrangements to the cell surface allows for spatial organization of glycan activity in specific regions of the glycocalyx.
Among the structurally diverse pool of glycans presented within the glycocalyx, sialic acids stand out as a particularly prominent class of carbohydrates (Fig 1). Introduced as a terminal modification on glycans during their biosynthesis, these negatively charged, nine-carbon monosaccharides are often recognized by protein receptors.2 Over evolution, sialoglycans have acquired immunomodulatory functions and emerged as molecular markers that delineate “self” from “non-self”.3 For instance, sialic acids are prominently displayed in the glycocalyx of B lymphocytes. At concentrations close to 100 mM, as established by Paulson and co-workers,4 they help calibrate signaling responses to antigenic challenge by masking the CD22 receptor, a sialic acid-binding negative regulator of B cell activation. Due to their high abundance on epithelial and endothelial tissues, sialic acids are also often subverted by opportunistic pathogens trying to gain entry into host cells.5
The sialoglycome of a cell can harbor a large degree of complexity, stemming not only from the type of sialic acid (N-acetylated or N-glycolylated) and its glycosidic linkage to underlying glycans (α2-3 or α2-6, Fig 2), but also from their spatial distribution within the glycocalyx (Fig 1).6 Sialoglycans can be presented in the vicinity of the plasma membrane as glycolipids, or project tens to hundreds of nanometers away from the cell surface displayed on glycoprotein scaffolds. The three-dimensional organization of the glycocalyx likely contributes to its biological functions7 and must be considered when devising methods for engineering of the cell-matrix interface for biomedical applications. Here, we report a hierarchical de novo assembly of a synthetic glycocalyx with spatially targeted sialic acid activity using a combination of nanoscale glycomaterial design and non-covalent cell surface engineering (Fig 1, right).
Figure 2.
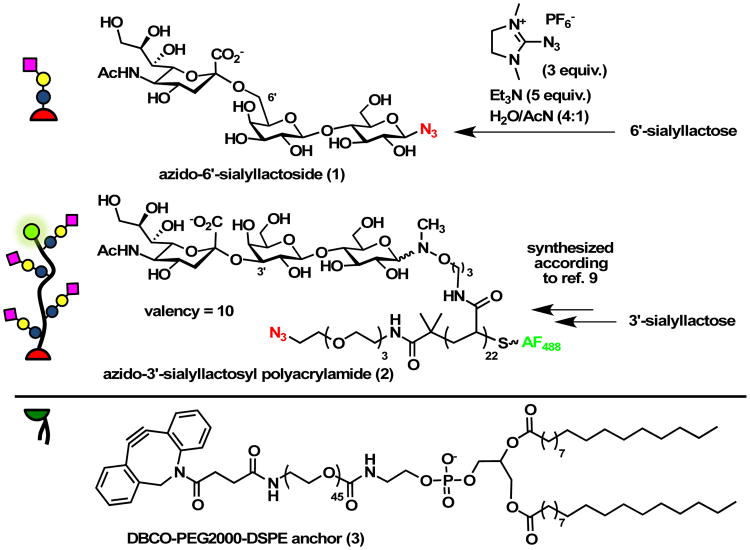
Glycocalyx building blocks. Monovalent glycolipid and multivalent glycopolymer precursors 1 and 2 were prepared as azide conjugates for the attachment of dibenzocyclooctyne-lipid 3 via the SPAAC (or “copper-free click”) reaction.
Synthetic glycomaterials, such as polymers or dendrimers, have long been explored as ligands for glycan-binding proteins. These materials can approximate the shape and scale of various types of glycoproteins and provide tunable multivalent glycan presentation for high-avidity receptor binding.8 However, the diversity of glycan structures introduced into these materials is typically limited to simple mono- and disaccharides, which is mostly due to difficulties associated with the synthesis and functionalization of more complex carbohydrates.8 We have recently reported a general method for directly introducing reducing glycans, including an array of increasingly complex sialoglycans, into polymeric scaffolds armed with N-methylaminooxy side chains.9,10 When furnished with hydrophobic phospholipid anchors, such materials can be targeted to the plasma membranes of live cells to incorporate new glycan structures into their glycocalyx.11,12,13
To test whether this approach can be used to systematically build glycan complexity at the cell surface, we set to install both α2-3 and α2-6 linked sialoglycans simultaneously, while maintaining control over their presentation within the glycocalyx. Whereas the latter would be introduced as a monovalent 6′-sialyllactose glycolipid and form the underbrush of the glycocalyx, the former would be presented as 3′-sialyllactose glycopolymer with predefined nanoscale glycan organization (Fig 1).
We synthesized the glycocalyx building block precursors, 6′-sialyllactoside (1) and a 3′-sialyllactose glycopolymer (2), as azide-conjugates for the attachment of dibenzocyclooctyne-phospholipids via the strain promoted azide-alkyne cycloaddition (SPAAC) reaction (Fig 2).14 We chose, the post-synthetic introduction of lipid anchors for its increased generality, because it obviates the need for the de novo synthesis of individual lipid conjugates, and for the improved solution properties and long-term stability of the azide precursors. Whereas the monovalent β-azido glycoside 1 was prepared in a single step from 6′-sialyllactose,15,16 (Fig. S1-S3) polymer 2 was assembled by ligation of 3′-sialyllactose to a RAFT-derived polyacrylamide precursor (DP = 22, Mn = 6,800, Đ = 1.13) with pendant N-methylaminooxy groups and end-labelled with AlexaFluor 488 for visualization.9 (Fig. S1-S8†) The efficiency of the glycan ligation step, as determined by 1H NMR spectroscopy, was 45%, giving glycopolymer 2 with a sialoglycan valency of ∼ 10. (Fig. S9†) The resulting glycoconjugates 1 and 2 were then primed for membrane insertion via an overnight reaction with the dibenzocyclooctyne-functionalized PEG-lipid conjugate, DBCO-PEG2000-DSPE (3, 1.1 equiv). (Fig. S10-S11†)
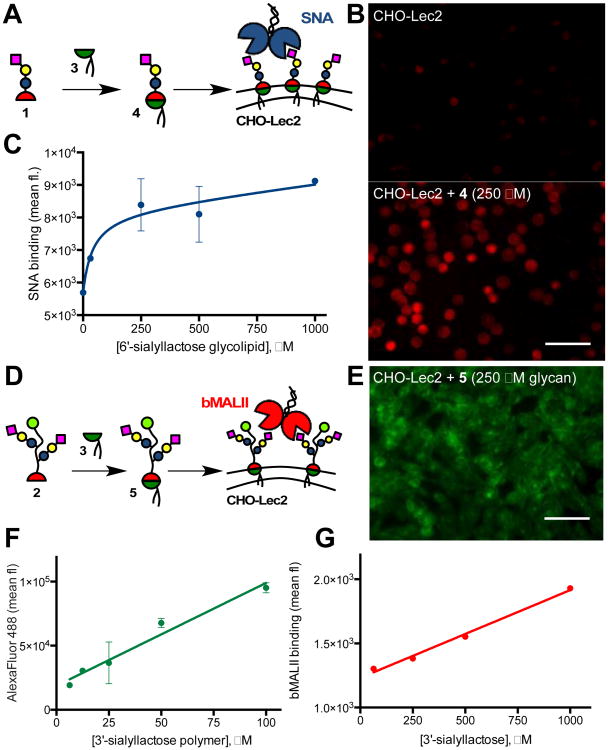
With the desired lipidated monovalent (4) and polymeric (5) glycoconjugates in hand, we set to test their incorporation into the glycocalyx of living cells (Fig 3). For our model cell system, we chose the Chinese hamster ovary Lec 2 (CHO-Lec2, ATCC Cat # CRL-1736) cells.17 The CHO-Lec2 cells are mutants with defective transport of the activated nucleotide sugar precursor, CMP-sialic acid, into the Golgi apparatus. As a consequence, these cells show low levels of sialic acid modification on their glycoproteins and glycolipids.17 (Fig. S12†) First, we evaluated cell membrane insertion of the monovalent 6′-sialyllactose glycolipid 4 (Fig 3A; Fig. S13†). The CHO-Lec2 cells were incubated in the basal MEMα medium containing increasing amounts of 4 for 1 hr at 37 °C. The sialoglycan introduced to the cell surface was detected by staining with Dylight649 conjugated Sambucus nigra agglutinin (SNA), which has known specificity for sialic acids with α2-6 linkages (Fig 3B).18 Flow cytometry analysis revealed that cell surface saturation is reached at concentrations of glycolipid 4 above ∼ 500 μM (Fig 3C). Next, we assessed the incorporation of the lipidated 3′-sialyllactose glycopolymer 5 by incubating the CHO-Lec2 cells with 5 at concentrations ranging between 2.5-100 μM (or 25 μM - 1 mM with respect to 3′-sialyllactose, Fig 3D). The presence of the AlexaFluor488 label in polymer 5 allowed for a direct observation of membrane remodeling. (Fig. 3E; Figs. S14 and S15†) Interestingly, the amount of polymer delivered to the cell surface increased linearly with concentration of 5 in the incubation media without reaching a saturation point (Fig 3F). Staining of the remodeled cells with biotinylated Maackia amurensis lectin II (bMALII) with specificity for α2-3 sialosides18 followed by visualization with Cy5-streptavidin, also showed a linear increase in lectin binding as a function of polymer concentration (Fig 3G). Both glycoconjugates can be used to tune the sialoglycan composition of the cellular glycocalyx, with monovalent glycolipid 4 reaching a saturation of lectin binding sites at lower concentrations compared to the multivalent glycopolymer 5.
Figure 3.
Glycocalyx remodeling with glycomimetics 4 and 5. (A) Remodeling of CHO-Lec2 cells with glycolipid 4. (B) Fluorescence micrograph of SNA-stained CHO-Lec2 cells before and after remodeling with 4. (C) Levels of incorporation of 4 were determined by Dylight648-SNA staining and flow cytometry. (D) Remodeling of CHO-Lec2 cells with glycopolymer 5. (E) Fluorescence micrograph of cells after introduction of AlexaFluor488-labeled 5. Flow cytometry analysis of polymer incorporation based on polymer fluorescence (F) and biotin-MALII/Cy5-streptavidin staining (G).
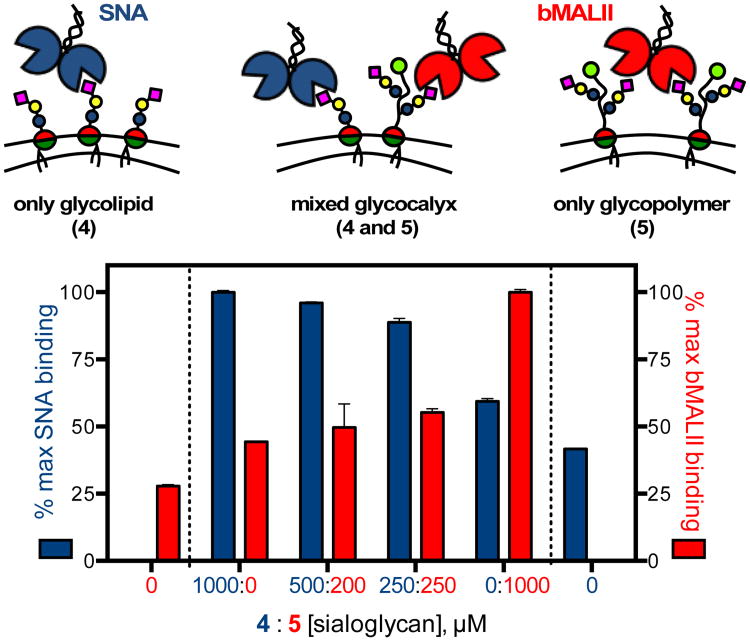
In cells, different sialoglycan structures are targeted to distinct regions of the glycocalyx to perform specific biological functions. We set out to test whether our cell surface engineering approach can artificially recapitulate this behavior, by presenting sialic acid ligands for SNA and bMALII either directly on the plasma membrane via the α2-6 sialoglycolipid 4 or in the form of pre-organized multivalent ensembles on α2-3 sialoglycopolymer scaffolds 5 (Fig 4). To test this concept, CHO-Lec2 cells were incubated for 1 hr in media containing glycoconjugates 4 and 5 premixed at specific concentrations (4:5 = 1000:0, 500:200, 250:250, and 0:1000 μM sialoglycan). The cells were stained with SNA and bMALII and analyzed by flow cytometry. We observed reduction in SNA staining commensurate with the decreasing fraction of 4 in the incubation media, and in agreement with lectin-binding activities previously observed for cells remodeled with the glycolipid only (Fig 3). The gradual decrease in SNA binding is mirrored by enhanced bMALII signal corresponding to the growing contributions from the sialoglycan polymers 5 introduced into the cellular glycocalyx (Fig. S16†).
Figure 4.
Glycocalyx scaffolding. The glycocalyx of CHO-Lec2 cells were remodeled with glycolipid 4 and glycopolymer 5 at varying ratios to modulate the levels of SNA and MALII binding. The nanoscale architecture of 4 and 5 confines the lectin-binding activity into the membrane proximal and distal regions of the glycocalyx, respectively.
Conclusions
Collectively, our data demonstrate that more than one glycoconjugate can be simultaneously introduced into the glycocalyx of live cells to modulate their responses toward lectins. At the same time, the nanoscale organization of the newly acquired biological activity can be defined through molecular design of the synthetic glycomaterial. Whereas our study validates the concept of hierarchical assembly of glycocalyx complexity using simple glycolipids and linear glycopolymers, this method can be extended to any type of glycoconjugate, such as a glycocluster, glycodendrimer, branched glycopolymer and others, as long as they can be functionalized with a membrane-targeting lipid anchor. The ability to tailor complex glycan interactions at the cell-matrix interface with nanoscale precision is poised to provide new insights into the biological roles of glycans and open new opportunities for controlling cellular functions in artificial tissue scaffolds and cell-based therapeutics.
Supplementary Material
Acknowledgments
This work was supported by the NIH Director's New Innovator Award (1DP2HS087954-01) to K.G. and the Glycoscience Common Fund (1R21AI129894-01). M. L. H. was supported in part by the Program of Excellence in Glycosciences (PEG, NHLBI: 4P01HL107150-06). We thank Dr. Lisa Adamiak for assistance with the purification and analysis of 1 and Prof. Jeffrey Esko, UCSD, for providing CHO-K1 (WT) cell line to us.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details and 1H and 13C NMR structure determination. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Varki A, Lowe JB. In: Essentials of Glycobiology. 2nd. Varki A, editor. Chapter 2. Cold Spring Harbor Press; Cold Spring Harbor, NY: 2009. [PubMed] [Google Scholar]
- 2.Varki A. Trends Mol Med. 2008;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer SA, Gagneux P. J Biol Chem. 2013;288:6904–6911. doi: 10.1074/jbc.R112.424523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Proc Natl Acad Sci. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A, Gagneux P. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dam TK, Brewer CF. Biochemistry. 2008;47:8470–8476. doi: 10.1021/bi801208b. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M, Varki A. OMICS. 2010;14:455. doi: 10.1089/omi.2009.0148. [DOI] [PubMed] [Google Scholar]
- 8.Miura Y, Hoshino Y, Seto H. Chem Rev. 2016;116:1673–1692. doi: 10.1021/acs.chemrev.5b00247. [DOI] [PubMed] [Google Scholar]
- 9.Huang ML, Cohen M, Fisher CJ, Schooley RT, Gagneux P, Godula K. Chem Commun. 2015;31:5326–5329. doi: 10.1039/c4cc08613a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, Fisher CJ, Huang ML, Lindsay LL, Plancarte M, Boyce WM, Godula K, Gagneux P. Virology. 2016;493:128–135. doi: 10.1016/j.virol.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. J Am Chem Soc. 2008;130:5947–5953. doi: 10.1021/ja710644g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ML, Smith RAA, Trieger GW, Godula K. J Am Chem Soc. 2014;136:10565–10568. doi: 10.1021/ja505012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulsipher A, Griffin ME, Stone SE, Brown JM. J Am Chem Soc. 2014;136:6794–6797. doi: 10.1021/ja5005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jewett JC, Sletten EM, Bertozzi CR. J Am Chem Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim D, Brimble MA, Kowalczyk R, Watson AJ, Fairbanks AJ. Angew Chem Int Ed Engl. 2014;53:11907–11911. doi: 10.1002/anie.201406694. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Nagai H, Noguchi M, Kobayashi A, Shoda SI. Chem Commun. 2009;23:3378–3379. doi: 10.1039/b905761g. [DOI] [PubMed] [Google Scholar]
- 17.Stanley P. Meth Enzymol. 1983;96:157–184. doi: 10.1016/s0076-6879(83)96015-9. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein IJ, Poretz RD. In: The Lectins-Properties, Functions, and Applications in Biology and Medicine. Liener IE, Sharon N, Goldstein IJ, editors. Academic Press; Orlando: 1986. pp. S. 33–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.