Abstract
Constitutive B-cell receptor signaling leads to overexpression of the anti-apoptotic BCL-2 protein and is implicated in the pathogenesis of many types of B-cell Non-Hodgkin Lymphoma (B-NHL). The BCL-2 small molecule inhibitor venetoclax shows promising clinical response rates in several lymphomas, but is not curative as monotherapy. Radiotherapy (RT) is a rational candidate for combining with BCL-2 inhibition, as DNA damage caused by RT increases the activity of pro-apoptotic BCL-2 pathway proteins, and lymphomas are exquisitely sensitive to radiation. We tested B-NHL responses to venetoclax combined with either external beam RT or radioimmunotherapy (RIT), which joins the selectivity of antibody targeting with the effectiveness of irradiation. We first tested cytotoxicity of cesium-137 irradiation plus venetoclax in 14 B-NHL cell lines representing five lymphoma sub-types. Combination treatment synergistically increased cell death in ten of 14 lines. Lack of synergy was predicted by resistance to single-agent venetoclax and high BCL-XL expression. We then assessed the efficacy of external beam RT plus venetoclax in murine xenograft models of mantle cell (MCL), germinal-center diffuse large B-cell (GCB-DLBCL), and activated B-cell (ABC-DLBCL) lymphomas. In each model, external beam RT plus venetoclax synergistically increased mouse survival time, curing up to 10%. We finally combined venetoclax treatment of MCL and ABC-DLBCL xenografts with a pretargeted RIT (PRIT) system directed against the CD20 antigen. Optimal dosing of PRIT plus venetoclax cured 100% of mice with no detectable toxicity. Venetoclax combined with RT may be a promising treatment for a wide range of lymphomas.
Keywords: Venetoclax, Radioimmunotherapy, Lymphoma, CD20, Pretargeting
Introduction
Non-Hodgkin Lymphoma (NHL) developed in an estimated 72,580 Americans in 2016, and over 20,000 will die despite the abundance of treatment options available (1). Over 75% of these NHL cases will be of B-cell origin. Constitutive B-cell receptor (BCR) signaling has been implicated in the pathogenesis of many types of B-NHLs, including chronic lymphocytic leukemia (CLL), follicular lymphoma (FL), the activated B-cell (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL). Tonic signaling downstream of the BCR ultimately up-regulates anti-apoptotic BCL-2 pathway proteins including BCL-2 itself (2,3). Constitutively high levels of BCL-2 are separately produced by the t(14;18)(q32;q21) translocation, which is found in up to 85% of FL and 34% of the germinal center (GCB) subtype of DLBCL, and by over-expression of the un-rearranged BCL2 gene (2,3). These findings have made BCL-2 an important therapeutic target in NHL, leading to the development of several novel drugs. The BCL-2 inhibitor venetoclax is one of the most promising new agents, as evidenced by a breakthrough status designation in 2015 followed by full Food and Drug Administration (FDA) approval in 2016 for the treatment of CLL. Despite this promise, venetoclax is not curative as monotherapy (4), and here we examine the efficacy of combining venetoclax with radiotherapy.
Radiotherapy (RT) is among the oldest cancer treatments in the modern era and remains an effective tool both as external beam RT (5), and as radioimmunotherapy (RIT), which combines the selectivity of antibody targeting with the effectiveness of irradiation (6–11). Radiation causes DNA strand breaks that ultimately increase the activity of pro-apoptotic members of the BCL-2 family (12,13), making RT a rational candidate for combination with BCL-2 inhibiting drugs. Such drugs have been shown to synergize with DNA-damaging drugs (14) and apoptosis promoters (15–17) in several cancers, yet to our knowledge no study has combined BCL-2 inhibition with RT. Lymphomas are uniquely sensitive to radiation (18), increasing the promise of this combination. While venetoclax is most intensively studied in CLL, it promotes apoptosis in a variety of NHL subtypes (4), and we hypothesized that venetoclax would combine synergistically with either external beam RT or targeted RIT to treat a range of B-NHL diseases.
To test this hypothesis, we studied in vitro cytotoxicity resulting from cesium-137 (137Cs) irradiation combined with venetoclax, using 14 B-NHL cell lines representing five lymphoma subtypes (Table 1). As predicted, the combination treatment synergistically increased cell mortality in the majority of cell lines. We then performed in vivo studies using three murine xenograft models, Rec-1 (MCL), U2932 (ABC-DLBCL) and SU-DHL-6 (GCB-DLBCL), chosen for their divergent single-agent sensitivities to venetoclax. For these experiments, we investigated combining venetoclax with external beam RT using a 137Cs irradiator, and with RIT using a two-step “pretargeted” system (PRIT) directed against the CD20 antigen. PRIT dissociates the slow, antibody distribution phase of RIT from the administration of the radionuclide, and typically delivers an order of magnitude greater tumor-to-normal organ ratio of RT than single-step RIT (11,19,20). In all three in vivo models, optimal dose combinations of venetoclax plus external beam RT, and venetoclax plus PRIT, caused synergistic reduction or eradication of lymphoma.
Table 1.
B-NHL cell lines
| Cell Line | NHL sub-type | Relevant characteristics |
|---|---|---|
| OCI-Ly3 | ABC-DLBCL | Amplified BCL-2 (copy no. 3.8)a, low BCL-XL and high MCL-1 expressionb |
| Ri-1 | ABC-DLBCL | Amplified BCL-2 (copy no. 14.7)a, high BCL-2 expressionb |
| U2932 | ABC-DLBCL | Amplified BCL-2 (copy no. 14.9)a, high BCL-2 and low BCL-XL expressionb |
| HT | GCB-DLBCL | Low BCL-2b and high MCL-1 expressiona |
| OCI-Ly19 | GCB-DLBCL | t(14;18) translocation, amplified BCL-2 (copy no. 3.3)a |
| Pfieffer | GCB-DLBCL | High BCL-XL and MCL-1 expressionb, t(14;18) translocationc |
| SU-DHL-4 | GCB-DLBCL | High BCL-2 expressionb, t(14;18) translocationa |
| SU-DHL-6 | GCB-DLBCL | Low BCL-2 and high MCL-1 expressionb, t(14;18) translocationa |
| SU-DHL-8 | GCB-DLBCL | Low BCL-2 and high BCL-XL expressionb |
| Jeko-1 | MCL | Overexpresses BCL-2, Bcl-1/J(H) gene rearrangementc |
| JVM2 | MCL | High BCL-XL expressionb,d |
| Rec-1 | MCL | High BCL-XL expression d, p53 oncogenec |
| Ramos | Burkitt’s | Low BCL-2 expressionb,e |
| WSU-FSCCL | Trans. follicular | t(14;18) translocationf |
Sources:
Souers et al. 2013. Nat. Med. 19(2): 202–8;
current study, high and low expression defined as values outside 95% confidence intervals of the mean, n = 14 lines;
ATCC;
Chiron et al. 2015. Oncotarget 6(11): 8750–9;
Shan et al. 2000. Cancer Immunol. Immunother. 48(12): 673–83;
DSMZ.
Materials and Methods
Cell lines
Human cell lines Ramos, Jeko-1, JVM-2, Rec-1, SU-DHL-4, and SU-DHL-8 were obtained from the American Type Culture Collection (ATCC) between 2006 and 2014; OCI-Ly3 and OCI-Ly19 were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) in 2014; and HT, Pfeiffer, Ri-1, SU-DHL-6, U2932, and WSU-FSCCL were generous gifts from Gilead Sciences in 2014. Cell line characteristics are shown in Table 1. Cells were maintained in log-phase growth at >95% viability (trypan-blue exclusion) in RPMI 1640 or alpha-MEM (OCI-Ly19) media supplemented with 10–20% fetal bovine serum, 50 U/ml penicillin G, and 50 μg/ml streptomycin sulfate, and studied within 6 weeks of thawing. All cells were tested for mycoplasma and authenticated by DNA profiling (ATCC kit 135-XV).
In vitro cytotoxicity of radiation combined with venetoclax
For each cell line, single agent dose-response tests were conducted to identify an incubation period and dose range that provided ~0, 20, 40, 60, 80, and 100% mortality attributable to 660 keV gamma rays from 137Cs (Gammacell 1000 irradiator, MDS Nordion) or to venetoclax (donated by AbbVie). Cells (1–2 × 105/ml) were treated with drug or irradiated, incubated 24–120 hours, and assayed for mortality using the Celltiter-Glo assay (Promega, G7571). Optimized incubation and dose parameters were subsequently used to test the efficacy of agent combinations in 6 × 6 dose matrices. Radiation was administered at time 0 and venetoclax added 24–48 hrs later. All tests were performed in triplicate and the computed means used in further analysis. To determine synergy, additivity, or antagonism, combination indexes (CI) were calculated with Calcusyn software (Biosoft) using the median effect equation, from mortalities in the 3 × 3 matrix section of each assay centered on 50% mortality (considered most accurate) (21–24).
In vitro BCL-2, BCL-XL, MCL-1 expression
BCL-2 pathway expression was characterized in B-NHL cell lines by flow cytometry. Cells were fixed in 4% formaldehyde, permeabilized in 90% methanol and stained with PE-conjugated BCL-2, BCL-XL, MCL-1, or isotype control mAbs (Cell Signaling Technology, 26295, 13835, 65617, 6899, 5742). All cell lines were examined by flow cytometry in a single assay. Protein expression was estimated as controlled median fluorescence index (MFI), calculated for each sample by subtracting isotype control MFI from target mAb MFI. This complete assay was replicated on a different day by a different person. Assay repeatability was examined by linear regression of controlled MFI from the first versus the second assay, for each protein. Results were highly repeatable (r2 > 0.7, p < 0.01).
Mice
NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ mice ([NRG], from the Jackson Laboratory or Fred Hutchinson Cancer Research Center [FHCRC]) and FoxN1Nu athymic nude mice (Envigo) were maintained under standard protocols approved by the FHCRC Institutional Animal Care and Use Committee (IACUC). Individual studies used either all female mice or 50:50 sex ratios in all experimental groups and controlled for gender in statistical analyses. Genders were housed separately.
Antibodies, pretargeting reagents, and radiolabeling
1F5, a murine immunoglobulin G2a anti-human CD20 mAb, and HB8181, an IgG2a isotype control, were produced from hybridomas using a hollow-fiber bioreactor system in the Biological Production Facility at FHCRC. The hybridoma cell line expressing 1F5 was a gift from Clay Siegall (Seattle Genetics), and the HB8181 hybridoma was purchased from ATCC. In all immunotherapy experiments, mice were co-injected with 400 μg of HB8181 to block nonspecific binding of 1F5 to Fc receptors. 1F5-streptavidin conjugates, DOTA-biotin reagents, and biotin-galactose clearing agent ([CA] N-acetyl-galactosamine) (NeoRx) were prepared as previously described (11,25). Radiolabeling of DOTA-biotin with the pure beta-emitter yttrium-90 (90Y) (Perkin Elmer, NEZ306) was performed as described (11,25) with labeling efficiencies >86%.
In vivo radiotherapies combined with venetoclax
NRG or athymic nude mice were injected subcutaneously in the right flank with 1×107 Rec-1 or SU-DHL-6 cells or 0.5×107 U2932 cells 8–16 days prior to therapy to generate lymphoma xenografts, depending on growth kinetics of the individual cell line. Athymic mice were injected intraperitoneally with anti-asialoGM1 antibody according to manufacturer recommendations (Wako, 986-10001) to attenuate tumor rejection via natural killer cell activity. Injections were given one day prior to tumor implantation, five days later and weekly thereafter. When tumors were ~50 mm3, mice were randomized into groups of 8–10 with equivalent mean tumor volumes (sample size determined by power analysis). To examine interactions between external RT and venetoclax, mice were treated with either the drug diluent (60% Phosal 50PG, 30% PEG 400, 10% EtOH, oral gavage once daily for 10–28 days), venetoclax (100–200 mg/kg, same schedule), RT (single, total body dose of 6–10 Gy 137Cesium from JL Shepard Mark I irradiator), or a combination of venetoclax and RT in which venetoclax treatment began one day after RT. Mice receiving >6 Gy RT underwent syngeneic bone marrow transplantation (BMT) 4 hrs after RT, receiving 5×106 donor bone marrow cells without T-cell depletion, as described previously (26). PRIT studies used the same experimental design, but in place of external beam RT, mice were initially co-injected with 1.4 nmol (300 μg) unlabeled 1F5-SA conjugate and 2 mg/mL (400 μg) HB8181 (11). Twenty-one hours later, 5.8 nmol CA was administered, followed 3 hours later by 1.2 nmol of 90Y-DOTA-biotin labeled with 400, 800, or 1200 μCi of 90Y (14.8, 29.6, or 44.4 MBq, respectively). The total amount of antibody delivered was the same for every animal, regardless of the radioactive dose of 90Y-DOTA-biotin administered. In combination groups venetoclax treatment began 2 days after 90Y-DOTA-biotin administration. Tumor size and body weight were measured three times a week following treatment and continued through day 120. Mice were euthanized when they experienced excessive weight loss, hind limb paralysis, or exceeded tumor volume limits per institutional guidelines.
Statistical analyses
In vitro responses to venetoclax plus RT were analyzed using t-tests to determine if the mean combination index (CI, see “In vitro cytotoxicity” methods) of a cell line differed from 1, with CI values <1 indicating synergy and values >1 indicating antagonism. Correlations between CI and venetoclax LD50, 137Cs LD50, and BCL-2, BCL-XL and MCL-1 expression were determined using simple and multiple linear regression. In vivo treatment effects on mouse survival were determined by log-rank comparisons of Kaplan-Meier survival functions. All analyses were performed using JMP 12.2.0 (SAS Institute, Cary, NC).
Results
In vitro cytotoxicity of radiation combined with venetoclax
The 14 B-NHL cell lines studied showed a wide range of sensitivities to single-agent external beam RT (LD50 = 0.3–49 Gy) and venetoclax (LD50 = 0.002–13 μM). Therefore, to optimally evaluate cytotoxicity of RT combined with venetoclax, each cell line was assayed using a unique 6 × 6 dose combination. The resulting cytotoxicity data (Fig. 1A) were evaluated with isobolographic analyses (Fig. 1B) which provided a combination index (CI) for each dose pair (Fig. 1C), where values <1 indicate synergy, values of 1 signify additivity, and values >1 indicate antagonism. Overall, 10 of 14 NHL lines responded synergistically to combined RT and venetoclax (Fig. 1D, p <0.003 for CI <1), including 4 of 6 GCB-DLBCL cell lines, 3 of 3 ABC-DLBCL cell lines, and 2 of 3 MCL lines. The remaining four lines responded antagonistically (Fig. 1D, p <0.027 for CI >1). We examined two possible predictors of treatment response: single-agent sensitivity and BCL-2 pathway protein expression. Single-agent sensitivity to venetoclax strongly predicted the CI: cell lines that were more responsive to single-agent venetoclax were more likely to respond synergistically to combining the drug with radiation (Fig. 2A, r2 = 0.73, p = 0.0001). In contrast, single-agent sensitivity to radiation had no predictive power (Fig. 2A, r2 = 0.03, p = 0.6). Among the BCL-2 pathway proteins, lower expression of BCL-XL predicted a more synergistic response to combination treatment (Fig. 2B, r2 = 0.51, p = 0.004), while levels of BCL-2 and MCL-1 were not predictive. Multivariate analysis, examining all possible correlations among venetoclax LD50, RT LD50, BCL-2, BCL-XL and MCL-1 expression, and CI, detected no further relationships among variables (multivariate, p > 0.22) and indicated that venetoclax LD50 (multivariate, p = 0.0002) and BCL-XL expression (multivariate, p = 0.006) had statistically independent influences on CI.
Figure 1. Venetoclax combined synergistically with 137Cs irradiation to increase mortality in the majority of B-NHL cell lines.
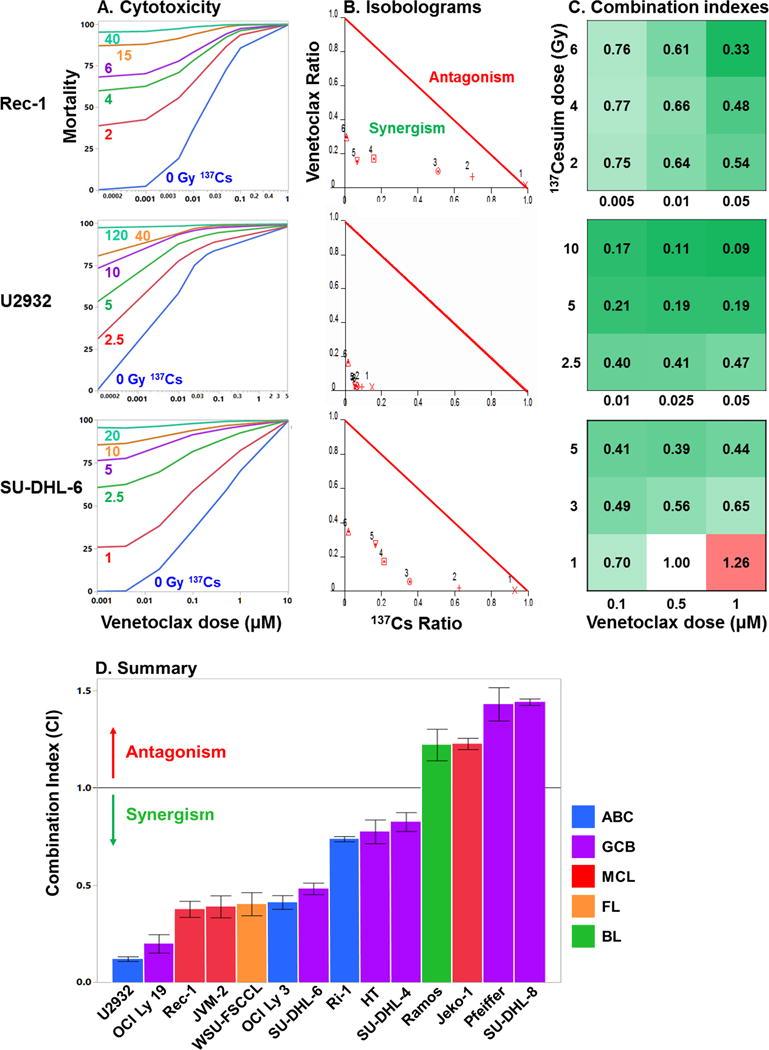
A. Representative cytotoxicity profiles for cells treated with 6 levels of 137Cesium irradiation combined with 6 dose levels of venetoclax. Treated cells were incubated 72–96 hrs, cytotoxicity assessed using Celltiter-Glo, and mortality calculated as percent of untreated control. Each assay was conducted in triplicate and the mean values used in further analyses. B. Normalized isobolograms were constructed from mortality data at each level of 137Cs treatment. Values below the red 1:1 line of additivity indicate synergy; values above the line indicate antagonism. Isobolograms shown are for the 4th 137Cs treatment level (purple lines in A). C. Matrices of combination indexes (CI) from the 3 × 3 section of the dose matrix centered on 50% mortality (considered most accurate). CI values < 1 indicate synergy (green), values of 1 indicate additivity (white), values > 1 indicate antagonism (red). The top row in each matrix derives from the isobologram in B. D. Summary of responses of B-NHL cell lines to 137Cs irradiation combined with venetoclax. Ten of 14 lines responded synergistically (CI < 1, p < .003). The remaining four lines responded antagonistically (CI > 1, p < 0.03). N = 9 CIs/cell line, CIs calculated from the means of 1–2 triplicate assays, error bars = 1 SEM.
Figure 2. Predictors of in vitro efficacy of RT plus venetoclax combination treatments in B-NHL.
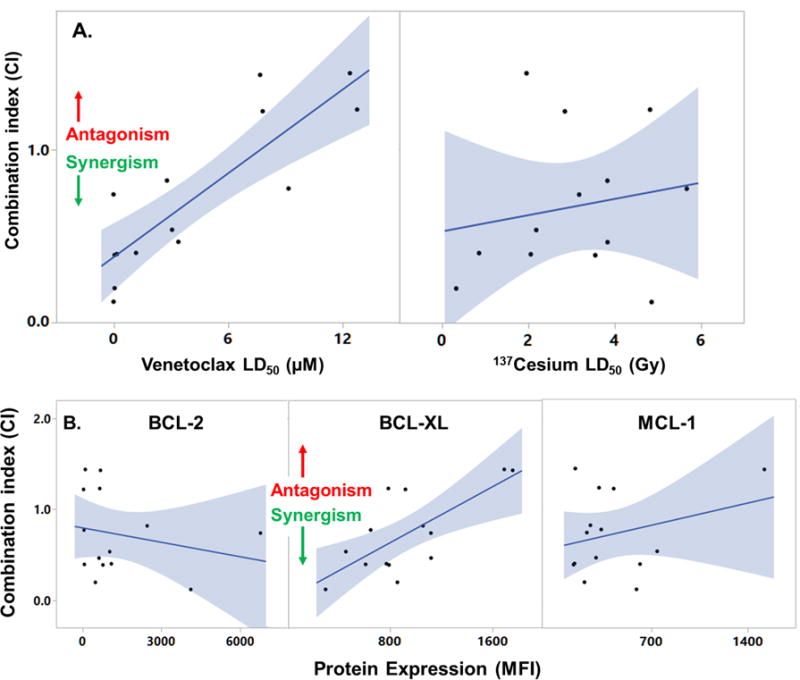
A. Single-agent cytotoxicities (LD50) were examined for ability to predict CI (data from Fig. 1). Regression analysis detected a significant positive correlation between venetoclax LD50 and CI (r2 = 0.73, p = 0.0001, n = 14 cell lines), thus greater sensitivity to single-agent venetoclax (lower LD50) predicted a more synergistic response (CI < 1) to combination treatment. Sensitivity to single-agent 137Cesium irradiation did not predict CI (r2 = 0.03, p = 0.6). B. Protein levels of BCL-2, BCL-XL and MCL-1 were assayed by flow cytometry in independent duplicate assays. Median florescence index (MFI) was highly correlated (repeatable) across assays (r2 > 0.7, p < 0.01). Regression analyses detected a positive correlation between BCL-XL protein levels and CI (r2 = 0.51, p = 0.004, n = 14), while BCL-2 and MCL-1 did not correlate with CI (r2 < 0.08, p > 0.3). Thus greater BCL-XL expression predicted a more antagonistic response to combination treatment. Multivariate analysis indicated that BCL-XL and venetoclax LD50 had independent influences on CI (see Results).
In vivo radiotherapies combined with venetoclax
Each of three in vivo lymphoma models, treated with either RT or PRIT in combination with venetoclax, responded synergistically to combination therapy without significant toxicity. These models were solid xenografts of Rec-1 (MCL), SU-DHL-6 (GCB-DLBCL) or U2932 (ABC-DLBCL), chosen to represent synergistic in vitro responders from different disease subtypes and with differing single-agent sensitivity to venetoclax, the primary predictor of in vitro synergistic response (Fig. 2).
Efficacy of external beam RT combined with venetoclax
In mice bearing Rec-1 tumors, venetoclax alone had no detectable effect on mouse survival (Fig 3A, p = 0.32 compared to controls), and 8 Gy external-gamma RT alone, a dose necessitating BMT rescue, increased mean survival time 44% over controls (p = 0.00002, Kaplan-Meier log-rank test). However, 8 Gy RT combined synergistically with venetoclax, nearly tripling mean survival time relative to controls while curing 1 of 9 mice (p = 0.00004 for combination group > RT alone; cure defined as mice surviving to end of study [120 days] with no signs of relapse; synergy defined as survival of the combination group being greater than the additive survival benefits of each agent administered alone [Supplementary Table S1]). Results for the SU-DHL-6 model were similar: venetoclax alone had no detectable benefit (Fig 3B, p = 0.22), 10 Gy RT extended mean survival time 48% over controls (p = 0.013), while combination therapy boosted survival time 156% and cured 1 of 10 mice (p = 0.008 for combination group > RT alone). Using the SU-DHL-6 and U2932 models, we additionally studied combination therapy using 6 Gy RT, the highest dose not requiring BMT rescue. As a single agent, 6 Gy RT increased survival time 36% over controls in SU-DHL-6 (p <0.001, Supplementary Table S1), but had no effect in U2932 (p = 0.7 for RT alone compared to control). However, combining 6 Gy RT with venetoclax increased mean survival time in SU-DHL-6 models an additional 18% beyond either single agent treatment (p <0.001, Supplementary Table S1), and in U2932 models the combination increased survival 58% beyond either single agent treatment (p <0.004).
Figure 3. External beam RT synergizes with venetoclax to lengthen survival of mice bearing B-NHL xenografts.
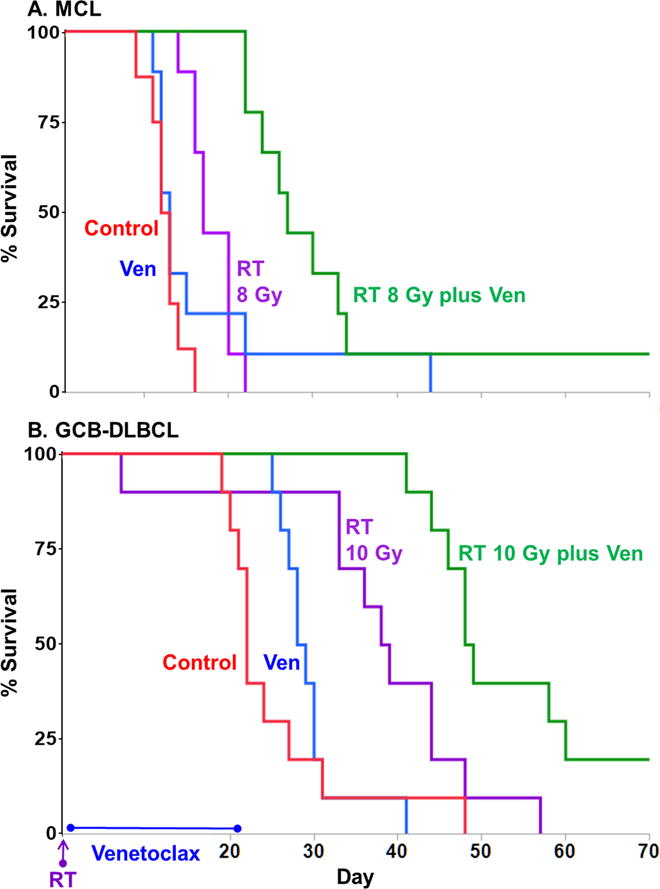
Mice implanted with subcutaneous xenografts of A. Rec-1 (MCL) or B. SU-DHL-6 (GCB-DLBCL) were treated with either drug diluent only (control), 8 or 10 Gy external beam 137Cs irradiation (RT), venetoclax (daily for 21 days), or RT plus venetoclax, when tumors were ~50mm3. Mouse survival was plotted on Kaplan-Meier curves. In both xenograft models, single-agent venetoclax (blue) did not significantly affect survival time (p > 0.2 compared with controls), but combining venetoclax with RT (green) extended mean survival times significantly beyond that provided by RT alone (purple) and cured at least 10% of mice (p < 0.007, combination groups > RT groups). Cure defined as survival to 120 days with no sign of relapse; synergy defined as survival of the combination group being greater than the additive survival benefits of each agent administered alone (Supplementary Table S1). N = 9–10 mice/group, additional statistics in text.
Efficacy of PRIT combined with venetoclax
Combination therapy using PRIT was also effective across models, and produced more cures than combinations using external beam RT. The PRIT studies examined Rec-1 and U2932 models, assaying two levels of PRIT activity in each. In Rec-1, venetoclax alone only marginally increased mean survival time (Fig. 4A, p = 0.05 compared to controls, Kaplan-Meier log-rank test), internal beta radiation from suboptimal doses (800μCi) of PRIT increased survival 111% beyond controls (p = 0.0001), while the combination synergistically extended survival 483% and included 25% cures (p = 0.001 for combination group > PRIT alone). In the same study, 400μCi PRIT alone increased survival time 46% over controls (p = 0.02, Supplementary Table S1) while combining PRIT and venetoclax increased survival by 106% over controls (p = 0.0001 for combination group > PRIT alone). The U2932 model proved more sensitive to all treatments (Fig. 4B). In this model, venetoclax alone doubled mean survival time but with no cures, while 800μCi and 1200μCi PRIT alone cured 10% and 30% of mice, respectively (p <0.0001 for any single-agent group > controls). At both PRIT doses, combination with venetoclax cured 100% of mice bearing U2932 tumors (Fig. 4B, p < 0.0006 for any combination group > any single agent group).
Figure 4. PRIT synergizes with venetoclax to cure up to 100% of mice bearing B-NHL xenografts.
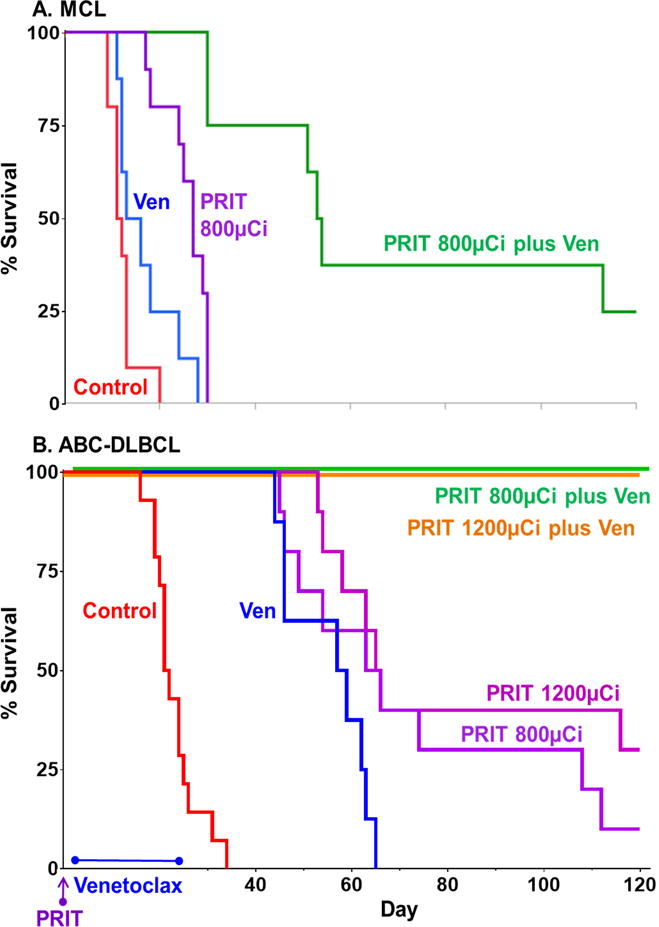
Mice implanted with subcutaneous xenografts of A. Rec-1 (MCL) or B. U2932 (ABC-DLBCL) were treated with drug diluent only (control), PRIT (CD20-pretargeted RIT using 90Y), venetoclax (daily for 21 days), or PRIT plus venetoclax, when tumors were ~50mm3. A. In Rec-1, our most drug and RT resistant model, single-agent venetoclax (blue) had only marginal effects (p = 0.05 compared to control, Kaplan-Meier log-rank test), and single-agent 800μCi PRIT (purple) induced some remission but all mice died from tumor burden by day 30. Yet combining venetoclax with PRIT (green) produced 75% disease-free survival through day 45 and 38% through day 100 (p = 0.001 for combination group > PRIT group). B. In U2932, single-agent venetoclax (blue) caused complete remission during treatment but no cures, and single-agent 800 and 1200μCi PRIT (purples) cured 10 and 30%, respectively. However combinations of venetoclax with 800 or 1200μCi of PRIT (orange and green, respectively, offset for visual clarity) each cured 100% of mice (p < 0.0006 for either combination group > any single agent group). Cure defined as survival to 120 days with no sign of relapse; synergy defined as survival of the combination group being greater than the additive survival benefits of each agent administered alone (Supplementary Table S1). N = 8–10/group, additional statistics in text.
Treatment toxicities
Single-agent venetoclax treatment caused no detectable weight loss nor non-tumor-related mortality in mice in any study at any dose tested. Single-agent RT treatments of 6, 8 and 10 Gy caused maximum weight loss (averaged over all mice/treatment) of 6, 12 and 13%, respectively, at four days after treatment, with full recovery to baseline weight within 12 days. Combining RT with venetoclax had no additional effects on weight. Single-agent PRIT treatments of 400, 800 and 1200 μCi caused maximum weight loss of 3, 6, and 4%, respectively, with full recovery within 14 days, and with no additional weight loss from combining PRIT with venetoclax. Non-tumor related mortality, prior to study day 105, was limited to 2 of 39 mice (5%) for all single-agent RT treatments, 3 of 47 (6%) for RT plus venetoclax treatments, 1 of 50 (2%) for single-agent PRIT treatments and 0 of 76 mice for PRIT plus venetoclax treatments. After study day 105, 3 single-agent PRIT mice (Fig. 4B), and 1 PRIT plus venetoclax treated mouse (Fig. 4A) died without apparent cause (body weights equivalent to healthy study-mates and no tumor evidence on necropsy). In age-matched controls for the Fig. 4 studies, 2 of 7 of these immunodeficient mice died without apparent cause in this same time frame, suggesting that late deaths among study mice may have been non-study-related.
Discussion
Finding cures for inoperable cancers will usually require identifying a combination of treatments that effectively targets multiple oncogenic mechanisms (27,28). Ideally targeted combinations will both minimize toxicity to healthy tissues and eradicate cancer quickly, as treatments that do not cure quickly can promote the evolution of resistant subclones. Our results demonstrate that the combination of radiotherapy and venetoclax may achieve these goals to treat B-NHL. Ten of 14 in vitro cell lines and 3 of 3 in vivo xenograft models (GCB-DLBCL, ABC-DLBCL and MCL) demonstrated synergistic responses to combination therapy. In the mouse models, combining venetoclax with RT or PRIT added no toxicity, and optimal dosing cured 100% of mice.
These results suggest a way to improve on the already notable clinical success of small molecule inhibitors. Venetoclax is a highly promising drug and the subject of at least 22 NIH-sponsored clinical trials. Nonetheless, the most impressive results using venetoclax as monotherapy are from a Phase 1 CLL study showing 79% overall (ORR) and 20% complete (CR) response rates (29). In relapsed/refractory (R/R) NHL, a Phase 1 trial of venetoclax monotherapy showed an ORR of 30% and a CR rate of 10% (30). These rates are typical of other promising drugs used as single agents: auspicious ORRs, but few if any durable cures. Another small molecule, the Bruton-tyrosine kinase inhibitor ibrutinib, is heralded as a top new MCL therapy (31), yet the best monotherapy results in R/R MCL show a CR of only 21% with a continuous pattern of relapse (32,33). Prognosis is dire when resistant disease evolves; median survival was only 2.9 months in MCL patients following ibrutinib failure (34,35). While preliminary data suggest a less grim prognosis when venetoclax resistance evolves (36), the above studies highlight the importance of identifying combination treatments capable of rapid disease eradication.
To this end, 19 of the aforementioned 22 venetoclax clinical trials, and multiple pre-clinical studies, combine venetoclax with other agents, but none to our knowledge has combined the drug with radiotherapy. We predicted this combination would synergize due to the complementary molecular mechanisms that underlie RT and venetoclax activity. Radiation damage to DNA creates a signaling cascade that interacts with BCL-2 family proteins via at least two pathways. In one, DNA damage activates the ATM/ATR kinases, which activate checkpoint kinase 2 (CHK2), phosphorylating tumor suppressor p53 that subsequently activates transcription of pro-apoptotic BCL-2 family proteins Bax, Noxa and PUMA (13). Increased levels of Bax directly promote apoptosis, while Noxa and PUMA inhibit multiple anti-apoptotic BCL-2 proteins including BCL-XL and MCL-1, important because BCL-XL and MCL-1 overexpression is implicated in venetoclax resistance (37,38). In a second pathway, DNA damage acts independently of p53 by activating the checkpoint protein RAD9, which inhibits anti-apoptotic BCL-XL (39). Hence, RT both directly promotes apoptosis and inhibits anti-apoptotic alternatives to BCL-2, thus complementing the selective BCL-2 inhibition of venetoclax (4,12,40) and suggesting that combining RT with venetoclax may be valuable even in venetoclax-resistant disease.
This multiplicity of mechanisms joining RT and venetoclax activities suggests that their combination might be effective across lymphomas with different molecular profiles. The cell line Rec-1 overexpresses BCL-XL (41) and is venetoclax resistant (Figs. 3A, 4A, (41)), yet consistent with the idea that RT plus venetoclax may be valuable in venetoclax-resistant disease, Rec-1 responded synergistically to combination therapy (Figs. 1D, 3A and 4A). U2932 has an opposite profile, with extremely high BCL-2 expression, low BCL-XL expression (Table 1, (4)) and a predictably extreme sensitivity to venetoclax (Fig 4B). Yet venetoclax alone was not curative, and U2932 showed the most synergistic responses to combination therapy in our study (Figs. 1D, 4B, Supplementary Table S1). Our final in vivo cell line, SU-DHL-6, had a yet different BCL-2 profile (Table 1) and a synergistic response to combination therapy (Figs. 1D, 3B). These data support the possibility that RT plus venetoclax may be effective in a broad range of NHL sub-types.
These results also suggest that treatment efficacy might result from different mechanisms in different diseases, posing a challenge for identification of biomarkers for responsiveness to RT plus venetoclax. Exhaustive studies identifying biomarkers were beyond the scope of this investigation, but may be important for clinical translation of our findings, as 4 of 14 cell lines tested in vitro responded antagonistically to RT plus venetoclax (Fig. 1D). The BCL-2 family may be the most promising source of biomarkers. Cell sensitivity to single-agent 137Cs irradiation was not correlated with the antagonistic response to RT plus venetoclax (Fig. 2A), perhaps because irradiation influences cell survival via multiple complex pathways, not all of which intersect with the BCL-2 family (13,40). Insensitivity to single-agent venetoclax did predict a more antagonistic response to combination therapy (Fig. 2A), as did higher BCL-XL expression levels (Fig. 2B). While high BCL-XL expression is a documented venetoclax escape mechanism, resistance to venetoclax correlates with different BCL-2 proteins in different diseases, and these correlations are not fully predictive (4,37,38,41–43). Similarly, in our study neither BCL-XL levels nor venetoclax sensitivity fully predicted response to combination therapy (Fig. 2). Identifying biomarkers of response to venetoclax plus RT merits further study, potentially including transcript and protein level examination of BCL-2 pathway, BCR signaling and DNA damage cascades, across a range of B-NHL subtypes.
Recent, comprehensive reviews confirm the ongoing importance of RT for treating NHL (20,44–46). Two types of radiotherapy are currently available for clinical use, RIT and external beam RT. While RIT generally provides a superior therapeutic index due to superior targeting, RIT is not recommended for all patients, and external beam modalities remain effective in many settings (5,47). In a study of DLBCL patients over the age of 60, RT consolidation after R-CHOP improved OS from 67 to 89% and PFS from 49 to 79% at five years, with no reported adverse effects (47). A review of NHL studies from 2004 through 2015 concluded that RT use in NHL has declined in the rituximab era, but that excluding RT decreases response rates and worsens toxicity in many disease sub-types (5). RT efficacy has improved with modified dosing and more nuanced approaches to identifying patients most likely to benefit (5,47). Our findings suggest that adding venetoclax might further improve the therapeutic index of RT, even at reduced RT doses, and that this combination deserves consideration.
In most situations however, radiolabeled antibodies will be therapeutically preferable to external beam RT, and our results support the benefits of this approach. In xenograft models, combinations of venetoclax with PRIT showed greater synergism and produced more cures than combinations of venetoclax with external beam RT (Fig. 3 vs. Fig. 4, Supplementary Table S1). PRITs’ superiority is likely the result of multiple factors that differentiate these delivery methodologies, including differences in both the dose rate and the total dose delivered to tumor. Prior dosimetry and biodistribution studies published by our group demonstrate that the enhanced therapeutic ratios of 90Y-SA PRIT allow delivery of the highest total radiation dose to lymphoma xenografts of any RT method (11,25,48–50). In the current study, we purposefully included lower, sub-optimal doses of RT to examine the range of synergistic responses, and demonstrate in both external beam RT and PRIT experiments that higher radiation doses produce greater synergistic responses in combinations with venetoclax (Supplementary Table S1). These results suggest that higher total radiation doses absorbed by tumor tissue with PRIT vs. external beam RT contribute, at least in part, to the greater efficacy and synergy of PRIT plus venetoclax.
We elected to study two-step PRIT rather than single-step RIT as our group and others have previously established the superior therapeutic index of PRIT. Our current findings are consistent with this prior experience, here demonstrating that adding venotoclax to PRIT greatly increases cure rates without adding detectable toxicity. Anti-CD20 PRIT is currently being studied in a phase I/II clinical trial for high-risk B-NHL (Trial NCT02483000, clinicaltrials.gov), raising the possibility of future clinical translation of our combination approach. Furthermore, our results suggest that combinations of venetoclax and conventional, single-step RIT may also lead to improved responses among patients with B-cell lymphoma. Single-step RIT is commercially available and effective against several hematological malignancies (20,44–46). While we did not directly test conventional RIT, the synergy observed between venetoclax and both external beam RT and PRIT strongly suggests venetoclax would also synergize with single-step RIT. Reasons for RIT efficacy include the presence of surface antigens largely restricted to specific hematological tissues, the availability of monoclonal antibodies that efficiently target these antigens, and the extreme radiosensitivity of leukemias and lymphomas. In studies of follicular lymphoma and DLBCL, conventional CD20-targeted RIT produced ORRs of 80–100% and CRs of 72–96% when used as consolidation after front-line treatments, and significantly improved treatment responses in R/R disease (20,45). In MCL, CD20 RIT contributed to ORRs of 88–100% and CRs of 67–100% in front-line treatments, and improved R/R treatment outcomes (45). In both DLBCL and MCL, RIT was additionally effective as a conditioning agent prior to hematopoietic cell transplant (HCT), improving response rates while reducing toxicity (20,44). Moreover, RIT may be more cost effective and simpler to administer than many treatment alternatives including prolonged, continuous drug therapies (46). Despite these benefits, conventional RIT is underutilized in current practice, and when used, is most effective when combined with toxic agents or HCT, making it important to identify safer treatment regimens. In our mouse models, combining venetoclax with radiotherapies ranging from low-dose, minimally-toxic PRIT to high-dose, myeloablative external beam RT consistently improved survival with no added toxicity (see “Treatment toxicities” in Results). We conclude that venetoclax plus PRIT, and likely venetoclax plus conventional single-step RIT, represent safe and promising therapeutic combinations. Our immunocompromised mouse model, and our use of single-dose rather than fractionated external beam RT, differ from clinical settings and should prompt caution when considering translation of this research. Well-designed clinical trials can address these issues, however, and we believe the combination of radiotherapy and venetoclax may offer a valuable treatment option in the large range of diseases constituting B-NHL.
Supplementary Material
Acknowledgments
We thank AbbVie for kindly donating venetoclax for this study.
Financial Support: This work was supported by grants from the US National Institutes of Health: P01CA044991 (O.W.Press), R01CA076287 (O.W.Press), R01CA136639 (O.W.Press), R01CA154897 (O.W.Press), K24CA184039 (A.K.Gopal), K23CA154874 (B.G.Till), K08CA151682 (D.J.Green), K01CA188151 (J.J.Orozko), by Fred Hutchinson Cancer Center Support Grant P30CA015704 (O.W.Press); and by the David and Patricia Giuliani Family Foundation (O.W.Press).
References
- 1.SEER Cancer Statistics Fact Sheets: Non-Hodgkin Lymphoma. National Cancer Institute; Bethesda, MD: 2016. [Google Scholar]
- 2.Shaffer AL, Young RM, Staudt LM. Pathogenesis of Human B Cell Lymphomas. In: Paul WE, editor. Annual Review of Immunology. Vol. 30. Palo Alto: Annual Reviews; 2012. pp. 565–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pon JR, Marra MA. Clinical impact of molecular features in diffuse large B-cell lymphoma and follicular lymphoma. Blood. 2016;127:181–6. doi: 10.1182/blood-2015-07-658401. [DOI] [PubMed] [Google Scholar]
- 4.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann M, Oehler C, Mey U, Ghadjar P, Zwahlen DR. Radiotherapy for Non-Hodgkin’s lymphoma: still standard practice and not an outdated treatment option. Radiation oncology (London, England) 2016;11:110. doi: 10.1186/s13014-016-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassaday RD, Stevenson PA, Gooley TA, Chauncey TR, Pagel JM, Rajendran J, et al. High-dose CD20-targeted radioimmunotherapy-based autologous transplantation improves outcomes for persistent mantle cell lymphoma. British journal of haematology. 2015;171:788–97. doi: 10.1111/bjh.13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reagan PM, Friedberg JW. Advancing radioimmunotherapy and its future role in non-Hodgkin lymphoma. Future oncology (London, England) 2015;11:1543–53. doi: 10.2217/fon.15.1. [DOI] [PubMed] [Google Scholar]
- 8.Hadid T, Raufi A, Kafri Z, Mandziara M, Kalabat J, Szpunar S, et al. Safety and efficacy of radioimmunotherapy (RIT) in treatment of non-Hodgkin’s lymphoma in the community setting. Nuclear medicine and biology. 2016;43:227–31. doi: 10.1016/j.nucmedbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Morschhauser F, Dreyling M, Rohatiner A, Hagemeister F, Bischof Delaloye A. Rationale for consolidation to improve progression-free survival in patients with non-Hodgkin’s lymphoma: a review of the evidence. The oncologist. 2009;14(Suppl 2):17–29. doi: 10.1634/theoncologist.2009-S2-17. [DOI] [PubMed] [Google Scholar]
- 10.Buchegger F, Press OW, Delaloye AB, Ketterer N. Radiolabeled and native antibodies and the prospect of cure of follicular lymphoma. The oncologist. 2008;13:657–67. doi: 10.1634/theoncologist.2008-0020. [DOI] [PubMed] [Google Scholar]
- 11.Press OW, Corcoran M, Subbiah K, Hamlin DK, Wilbur DS, Johnson T, et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood. 2001;98:2535–43. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 12.Jendrossek V. The intrinsic apoptosis pathways as a target in anticancer therapy. Curr Pharm Biotechnol. 2012;13:1426–38. doi: 10.2174/138920112800784989. [DOI] [PubMed] [Google Scholar]
- 13.Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23:2797–808. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Nakajima W, Seike M, Gemma A, Tanaka N. Cisplatin-induced apoptosis in non-small-cell lung cancer cells is dependent on Bax- and Bak-induction pathway and synergistically activated by BH3-mimetic ABT-263 in p53 wild-type and mutant cells. Biochem Biophys Res Commun. 2016;473:490–6. doi: 10.1016/j.bbrc.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 15.Bajpai R, Matulis SM, Wei C, Nooka AK, Von Hollen HE, Lonial S, et al. Targeting glutamine metabolism in multiple myeloma enhances BIM binding to BCL-2 eliciting synthetic lethality to venetoclax. Oncogene. 2016;35:3955–64. doi: 10.1038/onc.2015.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matulis SM, Gupta VA, Nooka AK, Hollen HV, Kaufman JL, Lonial S, et al. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2016;30:1086–93. doi: 10.1038/leu.2015.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppermann S, Ylanko J, Shi Y, Hariharan S, Oakes CC, Brauer PM, et al. High-content screening identifies kinase inhibitors that overcome venetoclax resistance in activated CLL cells. Blood. 2016;128:934–47. doi: 10.1182/blood-2015-12-687814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ott OJ, Rodel C, Gramatzki M, Niedobitek G, Sauer R, Grabenbauer GG. Radiotherapy for stage I-III nodal low-grade non-Hodgkin’s lymphoma. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2003;179:694–701. doi: 10.1007/s00066-003-1062-8. [DOI] [PubMed] [Google Scholar]
- 19.Pagel JM, Orgun N, Hamlin DK, Wilbur DS, Gooley TA, Gopal AK, et al. A comparative analysis of conventional and pretargeted radioimmunotherapy of B-cell lymphomas by targeting CD20, CD22, and HLA-DR singly and in combinations. Blood. 2009;113:4903–13. doi: 10.1182/blood-2008-11-187401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347–60. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 22.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Lee JJ, Kong M, Ayers GD, Lotan R. Interaction index and different methods for determining drug interaction in combination therapy. J Biopharm Stat. 2007;17:461–80. doi: 10.1080/10543400701199593. [DOI] [PubMed] [Google Scholar]
- 24.Chou TC, Hayball M, Lamble CW. Calcusyn - Windows software for dose-effect analysis and synergism/antagonism quantification, and User’s Manual. Biosoft; Cambridge, U.K.: 1996–2007. www.biosoft.com. [Google Scholar]
- 25.Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340–8. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 26.Orozco JJ, Kenoyer A, Balkin ER, Gooley TA, Hamlin DK, Wilbur DS, et al. Anti-CD45 radioimmunotherapy without TBI before transplantation facilitates persistent haploidentical donor engraftment. Blood. 2016;127:352–9. doi: 10.1182/blood-2014-12-617019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marquez RT, Tsao BW, Faust NF, Xu L. Apoptosis. Intech; 2013. Drug Resistance and Molecular Cancer Therapy: Apoptosis Versus Autophagy. < http://www.intechopen.com/books/apoptosis/drug-resistance-and-molecular-cancer-therapy-apoptosis-versus-autophagy>. [Google Scholar]
- 28.Melero I, Berman DM, Aznar MA, Korman AJ, Gracia JLP, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–72. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374:311–22. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerecitano JF, Roberts AW, Seymour JF, Wierda WG, Kahl BS, Pagel JM, et al. A Phase 1 Study of Venetoclax (ABT-199/GDC-0199) Monotherapy in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Blood. 2015;126:254. [Google Scholar]
- 31.Martin P. Ibrutinib–a new standard treatment for relapsed mantle cell lymphoma? Lancet. 2016;387:728–9. doi: 10.1016/S0140-6736(15)01040-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maddocks K, Blum KA. Ibrutinib in B-cell Lymphomas. Curr Treat Options Oncol. 2014;15:226–37. doi: 10.1007/s11864-014-0274-8. [DOI] [PubMed] [Google Scholar]
- 34.Martin P, Maddocks K, Leonard JP, Ruan J, Goy A, Wagner-Johnston N, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127:1559–63. doi: 10.1182/blood-2015-10-673145. [DOI] [PubMed] [Google Scholar]
- 35.Maddocks KJ, Ruppert AS, Lozanski G, et al. ETiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncology. 2015;1:80–7. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tam C, Anderson MA, Ritchie DS, Januszewicz EH, Carney D, Roberts AW, et al. Favorable Patient Survival after Failure of Venetoclax (ABT-199/GDC-0199) Therapy for Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) Blood. 2015;126:2939. [Google Scholar]
- 37.Khaw SL, Suryani S, Evans K, Richmond J, Robbins A, Kurmasheva RT, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood. 2016;128:1382–95. doi: 10.1182/blood-2016-03-707414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Punnoose EA, Leverson JD, Peale F, Boghaert ER, Belmont LD, Tan N, et al. Expression Profile of BCL-2, BCL-X-L, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Mol Cancer Ther. 2016;15:1132–44. doi: 10.1158/1535-7163.MCT-15-0730. [DOI] [PubMed] [Google Scholar]
- 39.Zhan Z, He K, Zhu D, Jiang D, Huang YH, Li Y, et al. Phosphorylation of Rad9 at Serine 328 by Cyclin A-Cdk2 Triggers Apoptosis via Interfering Bcl-xL. PloS one. 2012;7:12. doi: 10.1371/journal.pone.0044923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 41.Chiron D, Dousset C, Brosseau C, Touzeau C, Maiga S, Moreau P, et al. Biological rational for sequential targeting of Bruton tyrosine kinase and Bcl-2 to overcome CD40-induced ABT-199 resistance in mantle cell lymphoma. Oncotarget. 2015;6:8750–9. doi: 10.18632/oncotarget.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dousset C, Maiga S, Gomez-Bougie P, Le Coq J, Touzeau C, Moreau P, et al. BH3 profiling as a tool to identify acquired resistance to venetoclax in multiple myeloma. British journal of haematology. 2016 doi: 10.1111/bjh.14251. [DOI] [PubMed] [Google Scholar]
- 43.Al-Harbi S, Choudhary GS, Ebron JS, Hill BT, Vivekanathan N, Ting AH, et al. miR-377-dependent BCL-xL regulation drives chemotherapeutic resistance in B-cell lymphoid malignancies. Mol Cancer. 2015;14:17. doi: 10.1186/s12943-015-0460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali AM, Dehdashti F, DiPersio JF, Cashen AF. Radioimmunotherapy-based conditioning for hematopoietic stem cell transplantation: Another step forward. Blood reviews. 2016;30:389–99. doi: 10.1016/j.blre.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Mondello P, Cuzzocrea S, Navarra M, Mian M. 90 Y-ibritumomab tiuxetan: a nearly forgotten opportunityr. Oncotarget. 2016;7:7597–609. doi: 10.18632/oncotarget.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizzieri D. Zevalin((R)) (ibritumomab tiuxetan): After more than a decade of treatment experience, what have we learned? Critical reviews in oncology/hematology. 2016;105:5–17. doi: 10.1016/j.critrevonc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Cassidy RJ, Jegadeesh N, Switchenko J, Danish H, Esiashvili N, Flowers CR, et al. The role of radiotherapy for patients over age 60 with diffuse large B-cell lymphoma in the rituximab era. Leukemia & lymphoma. 2016;57:1876–82. doi: 10.3109/10428194.2015.1120866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frost SH, Frayo SL, Miller BW, Orozco JJ, Booth GC, Hylarides MD, et al. Comparative efficacy of 177Lu and 90Y for anti-CD20 pretargeted radioimmunotherapy in murine lymphoma xenograft models. PloS one. 2015;10:e0120561. doi: 10.1371/journal.pone.0120561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green DJ, Orgun NN, Jones JC, Hylarides MD, Pagel JM, Hamlin DK, et al. A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies. Cancer research. 2014;74:1179–89. doi: 10.1158/0008-5472.CAN-13-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subbiah K, Hamlin DK, Pagel JM, Wilbur DS, Meyer DL, Axworthy DB, et al. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med. 2003;44:437–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


