Abstract
Tendon injuries, known as tendinopathies, are common musculoskeletal injuries that affect a wide range of the population. Canonical tendon healing is characterized by fibrosis, scar formation, and the loss of tissue mechanical and structural properties. Understanding the regenerative tendon environment is an area of increasing interest in the field of musculoskeletal research. Previous studies have focused on utilizing individual elements from the fields of biomechanics, developmental biology, cell and growth factor therapy, and tissue engineering in an attempt to develop regenerative tendon therapeutics. Still, the specific mechanism for regenerative healing remains unknown. In this review, we highlight some of the current approaches of tendon therapeutics and elucidate the differences along the tendon midsubstance and enthesis, exhibiting the necessity of location-specific tendon therapeutics. Furthermore, we emphasize the necessity of further interdisciplinary research in order to reach the desired goal of fully understanding the mechanisms underlying regenerative healing.
Keywords: tendon, regeneration, therapeutics, healing
Introduction
Tendons are complex musculoskeletal soft tissues that provide the joint with stability during locomotion by connecting and facilitating load transfer between muscle and bone. Given their role in joint function, tendon injuries, known as tendinopathies, are common musculoskeletal disorders that affect a wide spectrum of society.1 Overuse and subrupture damage accumulation are key contributors to the pathogenesis of tendinopathies that ultimately lead to tendon rupture.2
Canonical healing of ruptured tendons is characterized by a loss of mechanical properties, matrix disorganization, and scar tissue formation that lead to a high incidence of re-rupture.2 Furthermore, the variance in morphology between the midsubstance and the insertion site has shown that effective healing mechanisms depend on the location of the injury.2 The structural complexity and lack of understanding of this healing mechanism have been major obstacles for the development of current surgical methods and therapies.
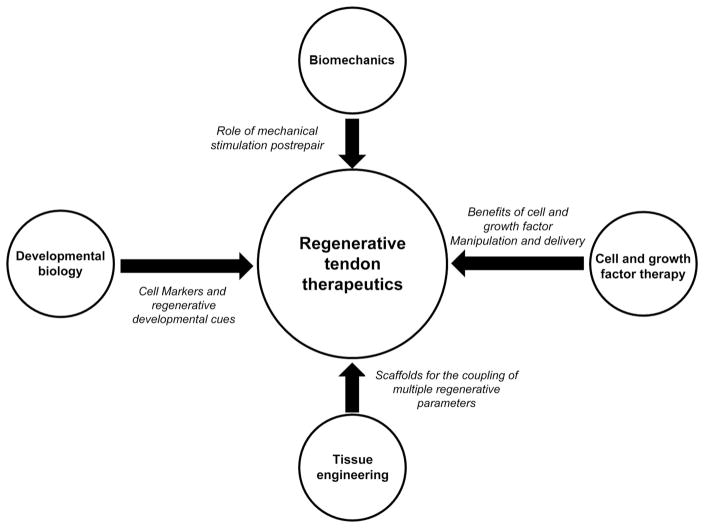
The current approach for the development of therapeutics has been influenced by key principles from a variety of fields, such as biomechanics, developmental biology, cell and growth factor therapy, and tissue engineering. This review will highlight valuable lessons from studies in these interdisciplinary fields and their applications to drive regenerative tendon healing (Fig. 1). The topics discussed here will include (1) the role of motion and mechanical stimulation throughout healing; (2) developmental cues that may elucidate key aspects of the regenerative healing environment during embryonic stages; (3) the effect of different growth factors on cell migration, proliferation, and matrix deposition; and (4) the use of different material scaffolds to replicate the functionality of naive tissue.
Figure 1.
Key principles from a variety of fields, such as biomechanics, developmental biology, cell and growth factor therapy, and tissue engineering provide valuable lessons necessary for the development of effective therapeutics that drive regenerative tendon healing.
Tendinopathies can be classified into two major subgroups: tendinosis or tendonitis, depending on the injury type. Commonly, clinical tendinopathies are presented as tendinosis, a disease state characterized by chronic injuries, primarily due to overuse, that over time may lead to rupture. However, because of the poor reproducibility of chronic injury in the laboratory, this review mainly examines tendon-healing studies from acute injury models, representative of the tissue damage and degeneration seen in blunt trauma, as well as the tendinitis disease state.
Lessons from biomechanics in tendon repair
Tendons experience variable magnitudes of loading throughout locomotion and require mechanical stimulation in order to maintain homeostasis and proper metabolism.5 Regular exercise has been shown to promote a positive anabolic effect on healthy musculoskeletal tissues, leading to enhanced mechanical properties in muscles, bones, ligaments, and tendons.3–6 These changes in bulk tendon properties occur as a result of dynamic changes in cell–matrix interactions during exercise.
For example, Zhang et al.7 showed that treadmill exercise led to an increased rate of proliferation of tendon stem cells and increased collagen synthesis in both Achilles and patellar tendons. Furthermore, Koskinen et al.8 showed that matrix metalloproteinases and tissue inhibitor metalloproteinases are upregulated after exercise, suggesting that mechanical simulation plays an important role in the matrix turnover and remodeling that is necessary for tendon strengthening.
Study of the benefits of exercise on healthy tissue has elucidated the importance of mechanical stimulation on tendon viability; however, healthy and damaged tendons demonstrate drastically different behavior. Several groups have studied whether the benefits of mechanical stimulation through exercise hold true for degenerated tendons; nevertheless, the complexity of the injured tendon environment has made it difficult to arrive at a conclusive answer. Kubota et al.9 showed that combinations of motion and tension applied to damaged chicken flexor tendons acted synergistically to improve tendon mechanical properties, such as ultimate load. This was in agreement with research done by Hitchcock et al.10 and Gelberman et al.11 who have also found that constrained motion is beneficial over immobilization (IM) for midsubstance tendon injuries. The therapeutic benefits of healthy exercise also extend to subrupture tendon damage due to fatigue loading. Bell et al.1 showed that delayed exercise, 14 days after injury, resulted in improved midsubstance properties and decreased levels of tissue damage, characterized by enhanced remodeling and matrix deposition, when compared to groups subject to immediate exercise.
A complication in the development of therapeutics lies in the fact that the anatomical location of the tendon injury affects the outcome of a therapeutic technique. Midsubstance tissue differs from the enthesis (insertion site) in composition and mechanical properties.12 In particular, the enthesis—the interface between two tissues of vastly different properties and functional roles—consists of four zones that form a structural gradient of decreasing elasticity from the inner tendon toward the bone.12–14
Thomopoulos et al.12 found that IM was preferred over motion when dealing with enthesis injuries. In this study, IM tendons showed a higher expression of collagen I, II, XII, and aggrecan, and a higher ratio of type I to type III collagen expression at 2 and 8 weeks when compared to tendons subjected to postoperative movement. Increased expression of aggrecan and collagen II, often found in articular cartilage, shows the potential of IM as a driving force for the development of cartilaginous and fibrocartilaginous zones seen in healthy tendon enthesis.12 IM also showed biomechanical benefits during healing, with IM tendons showing viscous properties closer to the uninjured controls when compared to the exercised group.12 Similar studies by Dagher et al.15 and Gimbel et al.16 have also shown the benefits of IM in healing of tendon enthesis.
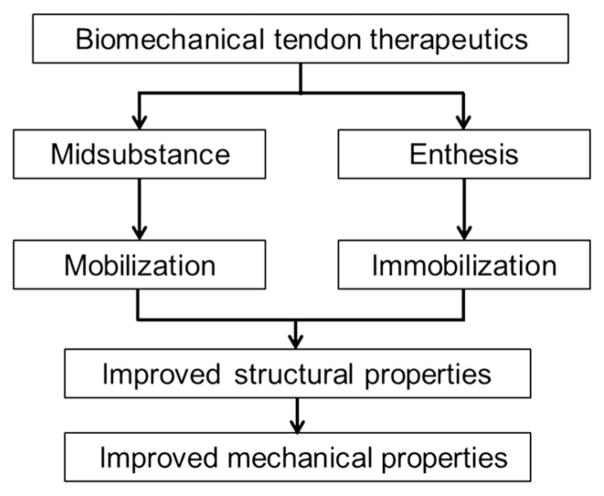
The structural and mechanical differences along the length of the tendon make location of the injury a key parameter in determining the proper method of treatment (Fig. 2). As previously mentioned, tendon IM has shown to be beneficial during enthesis healing; however, a number of groups have identified detrimental effects of this therapeutic method on the tissue’s midsubstance. Palmes et al. showed that IM tendons, healing from a midsubstance injury, did not regain mechanical properties similar to the uninjured controls as early as tissues that healed under constrained motion. Achilles tendons of IM mice did not show failure-load properties that resembled the uninjured controls until day 112 postinjury. Comparatively, tendons healing under constrained mobilization showed a failure load similar to controls by day 35 postinjury. The group also showed that tendon stiffness was significantly lower in IM tendons at day 112 compared to mobilized tendons, which showed stiffness similar to that of controls by this time.17 Similarly, Murrell et al.18 noted significantly lower tissue functionality of IM tendons when compared to tendons healing under motion and tendons of uninjured controls. Paw-print dimension analysis was done to obtain these functionality results. Stress shielding due to lack of mobility and mechanical stimulation could lead to tissue atrophy, in part explaining the diminished mechanical and functional properties of the midsubstance.19 These studies show that the mechanical environment plays a pivotal role in the tendon healing process. Determining the optimal magnitude and timing of mechanical stimulation that are effective during tendon healing would greatly enhance the ability of clinicians to prescribe a proper postrepair treatment.
Figure 2.
Structural and mechanical differences between the tendon midsubstance and enthesis make location of injury a key parameter in determining the appropriate method of treatment. Postoperative mobilization has been shown to encourage midsubstance healing; yet, immobilization shows beneficial results as a therapeutic for enthesis injuries.
The contradictory results related to the effectiveness of motion and loading between the midsubstance and the enthesis demonstrate that the benefits of mechanical stimulation are not universal, further revealing the challenges of developing locally specific therapeutics across the tendon length. Current therapeutic approaches are based on glimpses of information, making the lack of understanding behind the tendon healing mechanism a major constraint. Although controlling levels of exercise can improve mechanical properties after injury when comparing within injured groups, it does not restore mechanical properties when comparing with naive tissue. Understanding the reasoning behind the effectiveness of different therapeutic methods is imperative for future research.
Models incorporating techniques such as synergistic ablation of the Achilles tendon have been utilized to investigate the effect of repetitive mechanical loading on tendons, such as the plantaris tendon. Using this model, Gumucio et al.20 showed that there is an increase in the total cross-sectional area of stimulated tendons. The group found that tissue growth originates from cells localized in membranes surrounding the tendons, such as the epitenon and peritenon. Progenitor cells localized to these regions of the tissue showed increased proliferation and matrix deposition compared to cells within the core of the tissue, resulting in the formation of neo-tendon near the tendon outer layers.20 Although models such as this one provide insight into tendon behavior during mechanical stimulation, the specific cell behavior and growth-factor cascades that lead to this tissue formation remain largely unknown. To address these uncertainties, models of regenerative tendon healing are being interrogated to provide mechanistic insight to drive development of effective therapeutics. Recent studies have begun to utilize the naturally regenerative Murphy Roths Large mice to provide a template for the development of effective therapeutics.21 Similarly, the regenerative healing response that has been observed in tendons during developmental stages has long been highly insightful. This has led a number of musculoskeletal researchers to turn their attention to the field of developmental biology in search of promising mechanistic cues that may drive effective therapeutics.
Lessons from development
Tendon injuries heal without scar tissue during development; however, key steps involved in the early stages of healing are still not well understood.2,22 A better understanding of the developmental mechanisms of tendon healing can lead to the improvement of functional therapeutic techniques that drive tendon regeneration.
The study of developmental components, such as stem cells, progenitor cells, and the factors that control their differentiation along tenocyte lineage, has shown promise in illuminating some of the unknown aspects of regenerative healing. These cells are characterized by their ability to differentiate along diverse cell lineages and can be easily obtained from a variety of adult tissues, such as adipose or bone marrow. The potential for the use of stem cells and progenitor cells in tendon regeneration is vast; however, further research must still be done in order to understand their specific role in a tendon-healing environment.
Scleraxis as a tendon cell marker
Understanding the initiation of a tendon progenitor cell population and having the ability to study and translate tendon progenitor cell behavior from development into adulthood are necessary features for the advancement of regenerative tendon therapeutics.22 The identification of a tendon-specific cell marker would facilitate the process of achieving these goals.
Tendon markers, such as tenascin, have been previously utilized to study tendon cell behavior. However, the issue with this glycoprotein as a cell marker is that, although it is expressed in both tendon progenitor and mature tenocytes, it is not specific to soft connective tissues.22,23 Pacifici et al.24 and Morgan et al.25 found that tenascin expression extended to tissues, such as cartilage and bone, respectively. The localization of this marker in multiple musculoskeletal tissues limits the applications of tenascin for studying healing mechanisms that are exclusive to tenocyte lineage.
Developmental biology has been crucial in characterizing scleraxis, another promising tendon cell marker. The scleraxis gene (Scx) is expressed in tendon progenitor cells as early as embryonic day 9.5 and in differentiated mature tenocytes, showing its possible role in translating regenerative developmental cues into adulthood.22,26–28 Additionally, Scx expression is specific to connective tissues after day 9 of development, overcoming a major limitation seen in markers lacking tissue specificity, such as tenascin.22
Researchers have focused on further characterizing the properties of scleraxis to conduct a more in-depth evaluation of its benefits in tendon research. Schweitzer et al. showed that the location of tendon cell differentiation is limited by antagonistic signals, such as bone morphogenic proteins (BMPs) from surrounding tissues, and used this finding to further explore the specificity of Scx. The group utilized Noggin, a BMP antagonist, in an attempt to drive ectopic expression of Scx. Although Noggin upregulated early scleraxis expression when applied to nonconnective tissues, it was not enough to cause ectopic tendon formation. This elucidated an important factor in the differentiation of tendon progenitor cells: although scleraxis expression is necessary for tendon development, its mere manifestation does not guarantee cellular differentiation along the tendon lineage, emphasizing that a highly complex system of mechanisms is necessary for tenocyte formation.22 Similar results were shown by Pryce et al. who showed that TGFβ2−/− TGFβ3−/− mutant embryos maintained a wild-type cell phenotype and expressed normal levels of scleraxis until embryonic day 11.5; however, by embryonic day 12.5, both cell phenotype and scleraxis expression were lost. Loss of scleraxis expression did not culminate in cell death, supporting the idea that, although mature tenocytes express scleraxis, not all scleraxis-positive cells differentiate along tendon lineage.27
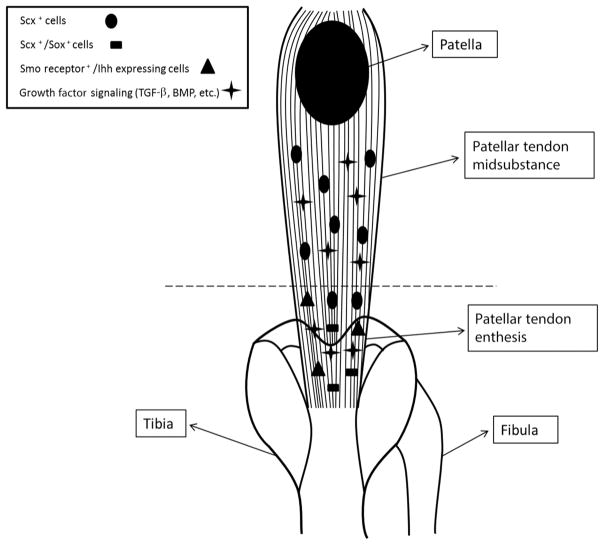
Overall, the benefits of scleraxis may be important for identifying some of the unknown mechanisms that lead to developmental regenerative tendon healing; however, it is not the only factor that must be investigated. Morphological differences between the enthesis and midsubstance play an important role in tissue behavior as early as the embryonic stages (Fig. 3). Understanding the effects of these environmental gradients throughout the tendon length is necessary in order to fully understand the regenerative environment seen during development.
Figure 3.
Location-specific signaling cascades from proteins and cytokines, such as hedgehog, TGF-β, and BMP, are essential to the differences in the development between tendon midsubstance and the enthesis. Differential expression of transcription factors by tenocytes from the midsubstance versus enthesis elucidates the location-specific cell niche necessary for the development of the compositional gradient seen in mature tendons.
Enthesis mechanism of development
Recognizing the differences in the development of the midsubstance and tendon enthesis can provide important therapeutic information. For example, hedgehog signaling has been shown to be essential to the development of the enthesis, but its overexpression is detrimental to the midsubstance.29,30 Ectopic expression of the Smoothened (Smo) protein receptor, normally localized to the tendon enthesis and fundamental for hedgehog signaling, resulted in the increased midsubstance expression of the enthesis markers tenascin-C, biglycan, and collagen II.29 Smo knockout mice, on the other hand, showed detrimental changes in the biomechanical properties of the enthesis, resulting in decreased linear stiffness when compared to controls.29 Breidenbach et al.30 presented supporting results, reporting a 34% decrease of linear stiffness and a 36% increase in failure displacement compared to controls. Decreases in enthesis mineralization of the Smo knockout mice could play a substantial role in these decreased mechanical properties, further supporting the importance of hedgehog signaling in enthesis development.29,30
Interestingly, the interplay between scleraxis expression and regulation of the BMP-4 signaling pathway also seems to play an important role in enthesis development.31 Blitz et al.31 indicated that BMP-4 is necessary for the differentiation of scleraxis-expressing progenitor cells into enthesis cells by showing that BMP-4 knockout mice displayed an inadequate formation of the tendon insertion site during development.
Enthesis localization of Sox9-expressing tendon cells during late stages of development additionally confirms the existence of a differential developmental mechanism along the tendon length.32 In a study by Soeda et al. that utilized Sox9CreERT2/+ R26R mice to trace Achilles tendon cell lineage, Sox9 was found to be expressed in Achilles tendon cells on embryonic days 11.5, 12.5, and 13.5 before tendon formation, localized at the insertion site by day 14.5, and disappeared once the tendon was formed. These results highlight the possibility that a population of mature tendon cells comes from a Sox9-expressing cell lineage.32 Further supporting the importance of Sox9 on enthesis development, Sugimoto et al.33 showed that a unique population of Scx+/Sox9+ cells is highly localized to the tendon insertion site. Structurally, the enthesis is made up of fibrocartilaginous zones, supporting the likelihood that Sox9 expression, a characteristic feature of cartilage development, may play a role in the developmental mechanism of the tendon insertion site population.32,33
Localized manipulation of signaling pathways, such as for hedgehog and BMP-4, and gene expression, such as of Sox9, could be used to develop location-specific treatments for tendon injuries (Fig. 3). Genetic developmental healing cues could elucidate aspects of the environment necessary for the recapitulation of natal tendon regeneration into adult healing, thus helping to overcome some of the challenges seen in current tendon therapeutics. In attempts to further understand cellular behavior and the healing cascade necessary for tendon regeneration, researchers have begun applying some of these important lessons in the fields of cell therapy and tissue engineering.
Cell and growth therapy without complex delivery scaffolds
Mesenchymal stem cell delivery
The use of mechanical stimulation as a therapeutic for tendon healing and the lessons from the regenerative tendon environment found during development have provided valuable insight for the advancement of tendon regeneration research. Nevertheless, advancements in the field of cell and growth factor delivery are necessary for the development of regenerative tendon therapeutics.
Mesenchymal stem cells (MSCs) have become a popular cell source in the field of musculoskeletal regeneration, owing to their ability to differentiate into different cell lineages and their relative ease of collection, expansion, and storage.34 Bone marrow MSCs (BMSCs) and adipose-derived MSCs are some of the commonly used cell lines in musculoskeletal regeneration research.35,36
Specifically, the effect of MSC delivery has been investigated as a potential driving force for regenerative tendon healing. For instance, Chong et al. used a rabbit animal model with a full-thickness midsubstance laceration to show the structural and mechanical effects of exogenous BMSC cell therapy during early tendon healing. The treatment groups received BMSCs in a fibrin carrier, while the control received the carrier alone. At 3 weeks, the treatment group showed improved alignment, as determined by nuclear orientation in relation to the longitudinal axis, and improved mechanical properties, as determined by a larger modulus when compared to the control group. BMSCs show a relatively positive effect in early tendon healing; however, a major limitation of this study is that no difference could be seen between experimental and control groups in the long term.35
Furthermore, Uysal et al. used a rabbit animal model to study the effects of adipose stem cells (ASCs) delivered through platelet-rich plasma (PRP), a biologically active delivery system. ASC treatment decreased levels of TGF-β1, TGF-β2, and TGF-β3, key mediators of fibrosis, as well as increased the tensile strength and levels of collagen I, when compared to controls. Other important cytokines, vascular endothelial growth factor and fibroblast growth factor (FGF), were also upregulated in the experimental groups.36 These results showed an overall positive effect of this treatment on tendon repair; however, the interactions between the cells and the delivery mechanism may be responsible for these results since PRP contains a cocktail of bioactive molecules that are thought to have advantageous effects during tendon healing.37 A limitation of this experiment is that levels of adhesions were not studied, and an increased level of adhesions in the experimental group could have resulted in a reduced range of motion.
Several high-quality trials have explored the benefits of using PRP as a delivery mechanism for tendon cell therapies. The use of collagen stimulatory delivery systems, such as PRP, could enhance tendon healing by reducing excessive inflammation and encouraging cell proliferation and maturation. Nevertheless, conflicting results on these benefits exist across different tendons and injury types. Although the vastly different environments found during chronic versus acute injuries may explain some of these discrepancies, the benefits of using delivery mechanisms such as PRP remain inconclusive, owing to the lack of knowledge on appropriate concentrations, timing, and administrations protocols.36–38
Optimal delivery time of these cells is not yet known; however, these studies show that the delivery mechanism might play an important role in tendon regeneration. Most importantly, they show that, although the use of stem cells to drive regeneration is important, it may not be sufficient on its own. More efficient and consistent methods to isolate, culture, and deliver MSCs are needed to synthesize a more organized and mechanically sound matrix.
Growth factor/gene therapy
A number of growth factors, such as platelet-derived growth factor (PDGF), basic FGF (bFGF), insulin-like growth factor (IGF-1), BMP-12, and cartilage-derived morphogenetic proteins (CDMPs), play key roles throughout the wound in healing responses of various tissues. Upregulation of these factors during healing affects cellularity, cell migration, cell proliferation, and regulates the immune response.39–45 Manipulating the concentration and timing of delivery of these growth factors at the injury site has become a popular research target. Therefore, understanding the response of tenocytes to these growth factors individually may bring light to the specific benefits of each growth factor for driving tendon regeneration (Table 1).
Table 1.
Benefits and limitations of cell and growth factor therapy for tendon injury
| Delivery factor | Carrier/control | Group | Tendon treated | Result | Limitation |
|---|---|---|---|---|---|
| BMSCs | Fibrin sealant | Chong et al.35 | Rabbit Achilles tendon |
|
|
| ADSCs | Platelet-rich plasma | Uysal et al.36 | Rabbit Achilles tendon |
|
|
| PDGF | Heparin-based delivery system | Thomopoulos et al.41 | Canine flexor tendon |
|
|
| bFGF | Heparin-based delivery system | Thomopoulos et al.42 | Canine flexor tendon |
|
|
| IGF-1 | 4% methylcellulose gel Carrageenan (inflammation analysis) |
Kurtz et al.43 | Rat Achilles tendon |
|
|
| BMP-12 | β-Galactosidase recombinant adenovirus | Lou et al.44 | Chicken flexor profundus tendon |
|
|
| CDMP-1 CDMP-2 CDMP-3 |
50-μL acetate buffer | Forslund et al.52 | Rat Achilles tendon |
|
Compared to untreated controls.
Platelet-derived growth factor
Exogenous delivery of PDGF-BB has shown promising results enhancing structural and morphological properties of damaged tendons.40 The ability of this growth factor to affect tenocyte proliferation and collagen deposition in vitro has marked PDGF as an appealing therapeutic method. Some research groups have started to characterize the in vivo effects of using PDGF. For example, Thomopoulos et al. found a positive effect of PDGF in the functional properties of canine flexor tendon healing. The PDGF-BB treatment group showed increased cell proliferation and improved range of motion when compared to groups that received only the heparin-based delivery system or surgical repair. Increased levels of hyaluronan were also seen in the experimental group when compared to the surgical repair group, which could be responsible for the increased range of motion, since hyaluronan plays an important lubricating role in joint mechanics.42 Gelberman et al.46 showed similar results, further supporting the benefits of this growth factor in tendon healing.
A limitation of using PDGF as a therapeutic method is that it does not improve the mechanical or structural properties of the injured tendon. Important considerations, such as the effective delivery mechanism and dosage of this growth factor, must be further characterized in order to overcome these limitations.42
Basic fibroblast growth factor
Basic FGF has been noted to play an interesting role during wound healing by increasing angiogenesis, stimulating cell proliferation, and driving enhanced matrix deposition at the injury site.41 In a study by Thomopoulos et al. that examined some of the effects of using this growth factor in an in vivo canine flexor tendon model, vascular and inflammatory cell responses were found to be increased in the bFGF groups. Likewise, cell proliferation increased with high concentrations of the growth factor (1000 ng) when compared to the heparin delivery system controls. The research group also highlighted certain limitations of this therapeutic method, such as that matrix production at the repair site was primarily scar tissue noted by the levels of collagen III, the factor’s inability to recapitulate native mechanical properties, and increased adhesions in the experimental group when compared to the surgical repair control.41 Still, bFGF remains a promising growth factor for the development of tendon therapeutics; however, further research is required to understand the specific mechanisms that are involved with this growth factor during healing.
Insulin-like growth factor-1
IGF-1 also plays a significant role in the wound healing response.39 Previous studies have shown that upregulation of this factor throughout the canonical tendon healing cascade is essential for various tenocyte functions, such as matrix deposition and proliferation.47,48 Kurtz et al.43 studied the effects of IGF-1 in the recovery of Achilles tendon injury in a rat model, where the experimental group received IGF-1 mixed with a 4% methylcellulose gel for the functional deficit study and IGF-1 mixed with the carrageenan injections for the inflammation analysis. Carrageenan injections were used to deliberately induce an inflammatory response in tendons. A functional deficit, a decrease in paw print dimensions due to injury, was used to analyze the effects of IGF-1 compared to surgical repair alone and to determine the anti-inflammatory effects of this growth factor. The IGF-1–treated rats showed improved functional deficit when compared to controls for both the surgical repair group and the nontreated carrageenan group. However, biomechanical analysis showed no significant effect of IGF-1 in increasing the failure loads of the tendons when compared to surgical repair controls.43 Although the exact mechanism for IGF-1 is not fully known, Kurtz et al.43 speculates that the inflammatory response could be IGF-1 mediated through a negative feedback loop. Therefore, increasing the levels of IGF-1 may be enough to reduce inflammation at the injury site.43 Further research must be conducted to further characterize the efficiency of using IGF-1 as an anti-inflammatory therapeutic.
Bone morphogenic protein-12
BMPs were first exposed as key factors in bone formation, owing to their osteogenic properties.49 However, a number of studies have shown the involvement of these proteins in a variety of organ systems as early as developmental stages.50 Recently, BMP-12 has become an interesting target in tendon tissue regeneration because of its ability to promote connective tissue formation.51 Lou et al. found structural and mechanical benefits of adenoviral (Adv) BMP-12 gene transfection in tendon repair using a chicken model. More specifically, the results showed a 30% increase of type I collagen and significantly enhanced mechanical properties, such as stiffness and ultimate force after Adv-BMP-12 treatment when compared to untreated cells.44 Although BMPs are not often associated with soft tissue regeneration, BMP-12 may hold an important role in tendon therapeutics. Further characterizing the effects of this growth factor on the tendon healing mechanism will be necessary to determine its efficiency and viability as a treatment for tendon injury.
Cartilage-derived morphogenetic proteins
CDMPs are less osteogenic members of the BMP family, present in cartilaginous tissues, and are involved in embryonic development of joints.45 However, their function in tendon metabolism has not been fully characterized. In a study by Forslund et al.52 that used a rat model to study how CDMPs affected injured Achilles tendon, no difference was found in mechanics between tendons treated with CDMP-1, -2, or -3, but force and stiffness were found to be increased at higher dosage of the proteins. Structural improvements, such as a decrease in fibrotic tissue and increased fiber orientation, were seen after 4 weeks. A main limitation in the use of CDMPs as tendon therapeutics is the inevitable formation of bone and cartilage throughout the healing tendon. CDMP-2 demonstrated to be the least osteogenic; however, all groups showed some level of cartilage or bone formation.52
Limitations of growth factor use in tendon regeneration
Although tendon therapeutics based on gene therapy and growth factor delivery show great promise, they do not come without limitations (Table 1). For example, many of the benefits of using these methods revolve around their ability to increase or accelerate cell proliferation, cell migration, and matrix deposition; however, improper timing of growth factor manipulation often results in the increased deposition of collagen III and the formation of a disorganized matrix, a characteristic of fibrosis and scar tissue formation.40,41,53 Controlled delivery of these factors, as well as delivery vehicles to the injury, can also be limitations of these types of therapeutics.41,42 Although current research has focused on using biocompatible delivery systems, such as the ones used in the previous studies, this does not discard the possibility of an increased inflammatory response at the implantation site due to the host’s natural response to foreign bodies. The effects of individual growth factor or gene therapy have also shown a lack of consistency in improving mechanical properties of injured tissue. A number of studies have shown that these types of therapeutics may show enhanced mechanical properties when compared to untreated groups; however, these remain far inferior to those of native tissue.52–54
Many of the growth factors that play an important role in canonical tendon healing are also responsible for a number of other aspects of tendon homeostasis and cell viability. The complexity of their signaling pathways makes it challenging to isolate the therapeutic effect of individual growth factors on tendon regeneration. The lack of knowledge in this field has driven many research groups to attempt to characterize the effects of individual and bundles of key growth factors by manipulating their concentrations or expression through trial and error. Although results seem promising, the limitations of these studies reinforce the idea that a number of factors must work together to achieve the goal of tendon regeneration.
Regenerative approaches in tissue engineering
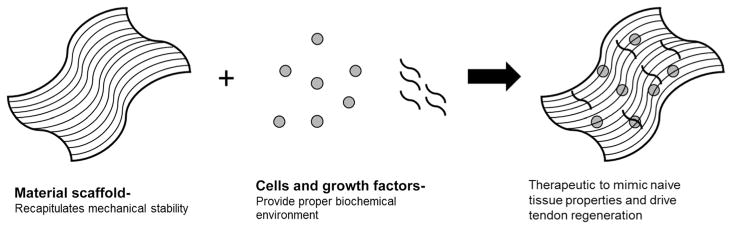
Coupling parameters such as mechanical integrity with cell and growth factor delivery has been a common approach to tissue engineering. A number of researchers have focused on the development of scaffolds to mimic multiple healing cues necessary to drive improved healing of tendon injuries. These constructs must provide an appropriate mechanical and biochemical environment in which tenocytes can maintain their phenotype while encouraging tissue regeneration.55 Although there are large numbers of different tissue-engineered tendon scaffolds, this review will focus on a few examples of synthetic constructs that incorporate aspects of mechanics and cell/growth factor delivery in order to emphasize the variety of manufacturing techniques, materials, and biological components used to replicate different aspects of the tendon extracellular environment (Fig. 4).
Figure 4.
Coupling multiple elements, such as tissue-engineered material scaffolds, with cells and biologically active growth factors shows promise in the ability to recapitulate naive tendon properties and drive tissue regeneration of tendon injuries.
Synthetic tissue-engineered constructs for cell and growth factor delivery
The ability to combine a variety of synthetic constructs with the lessons and applications gathered from biomechanics, developmental biology, and cell and growth factor therapy has made these scaffolds attractive options in the field of tendon tissue engineering. For instance, braided constructs have shown promise as therapeutic scaffolds, owing to their ability to act as effective biological carriers. Moreover, manipulation of the number of braided fibers leads to controlled variability of the mechanical properties of these constructs, an important quality in tendon tissue-engineered scaffolds.56 Vuornos et al.57 used polylactide, a biodegradable polyester, to develop a braided scaffold with the goal of driving tenogenic differentiation of human ASCs. The group found that cell-seeded scaffolds responded positively when cultured under media containing GDF-5 and L-ascorbic acid, showing increased cell proliferation and collagen synthesis when compared to culture under media without L-ascorbic acid. Additionally, scaffold culture under the combined media resulted in increased expression of tenocyte markers, such as scleraxis and tenomodulin, supporting the idea that the construct’s environment and culture medium may be able to influence stem cell differentiation along tendon lineage.57 Overall, this study showed the benefits of combining mechanical and biological elements to increase the efficiency of tendon tissue-engineered constructs.
Alternatively, other groups have shown that combining bioactive factors with other scaffold manufacturing techniques and materials can also lead to improved tendon healing. Namely, Shen et al.58 showed that a knitted silk-collagen sponge scaffold integrated with stromal cell–derived factor-1α (SDF-1α) resulted in increased expression of collagens I and III and a reduced inflammatory response during healing. The anti-inflammatory properties of SDF-1α may be important in driving regenerative healing since an enhanced inflammatory response is thought to contribute to scar formation during wound healing.59 Furthermore, Sahoo et al. presented another approach to enhancing the effects of knitted silk scaffolds through the addition of a surface layer composed of electrospun PLGA fibers coated with bFGF. Rabbit BMSC-seeded PLGA fibers infiltrated into the knit silk portion of the scaffold—a desired trait since matrix deposition must occur throughout the biomaterial to mimic native tissue. The addition of bFGF led to increased expression of the tendon compositional markers collagen, fibronectin, and biglycan, as well as enhanced mechanical properties when compared to scaffold alone.54 Combining manufacturing methods, such as the knitted silk scaffold and electrospun fibers, attempts to reproduce the mechanical strength and surface properties of a healthy tendon, respectively.60
Tissue engineering techniques have also been employed to develop scaffolds for the tendon enthesis. However, owing to the difference in mechanical and structural properties between the enthesis and midsubstance of the tendon, alternative manufacturing methods may be required to mimic insertion site properties. Li et al.,61 for example, used 10-times concentrated simulated bodily fluid solution (SBFS), coupled with PLGA and PCL scaffolds, to mimic the graded extracellular matrix seen in this section of the tissue. The group deposited 10-times concentrated SBFS, containing high levels of calcium phosphate, onto a container enclosing PLGA and PCL electrospun polymers, and the solution was continuously added to the container, causing the liquid levels to increase until the polymers were completely covered. The differences in submersion time along the scaffold resulted in the formation of a mineral gradient. Mechanical testing showed that the spatial difference in concentration generated different stiffness along the PLGA scaffold, with higher stiffness corresponding to higher mineral content. To analyze the effect of bioactive factors on this construct, the group seeded the scaffolds with MC3T3 preosteoblastic cells. The results showed that there was a positive relation between levels of calcification along the matrix and cell adhesion and density, further supporting the construct’s potential for simulating the insertion site environment.61 This study indicates the diverse approaches that must be taken in order to accommodate for the differences between tendon midsubstance and the enthesis. However, it also shows a promising future for the development of targeted tendon therapeutics for enthesis injuries.
The ability of treated tissues to mimic the structural and mechanical properties of native tendon long after treatment is a key indicator of therapeutic effectiveness. The use of tissue-engineered constructs with controlled release of growth factors, mechanics, and biocompatibility has elucidated the potential of combining biological and physical factors for improved tendon healing. However, current tissue-engineered constructs have only been able to depict basic components of the native tendon matrix. Many mechanisms necessary for tendon regeneration are still unknown. Future work studying cell–matrix interactions during healing and developing new materials to drive multiple regenerative cues is important for enhancing the field of tendon therapeutics.
Conclusion
The set of studies presented throughout this review highlights the importance of multidisciplinary research for the development of tendon therapeutics. We have shown that (1) biomechanical principles, such as mobilization versus IM postrepair, provide distinctive results, depending on the location of the tendon injury; (2) developmental cues, such as scleraxis expression throughout the tendon cell line, and differences in developmental mechanisms across the tendon length could play key roles in translating the regenerative tendon healing seen during embryonic stages into adulthood; (3) cells and growth factors play an intricate role in tendon healing, and developing therapeutics that allow for the synergistic behavior of these elements at the optimal time of delivery is necessary for the formation of regenerative tendon therapeutics; and (4) the variety of manufacturing techniques and materials that can be utilized to develop tissue-engineered constructs makes it a promising field for the coupling of mechanical, structural, and biological factors necessary to efficiently overcome tendon injury and pathology. Tendon injuries and tendinopathies are common musculoskeletal disorders that account for 30% of musculoskeletal consultations.62 However, because of the lack of knowledge on the mechanisms necessary for tendon regeneration, there are currently no effective tendon therapeutic methods that efficiently drive proper tendon healing. Future research focusing on the combination of some of the elements discussed in this review may help in improving tendon repair and bridging the gap in knowledge necessary for the development of therapies for regenerative tendon healing.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Bell R, Boniello MR, Gendron NR, et al. Delayed exercise promotes remodeling in sub-rupture fatigue damaged tendons. J Orthop Res. 2015;33:919–925. doi: 10.1002/jor.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andarawis-Puri N, Flatow EL, Soslowsky LJ. Tendon basic science: development, repair, regeneration, and healing. J Orthop Res. 2015;33:780–784. doi: 10.1002/jor.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tipton KD, Wolfe RR. Exercise, protein metabolism, and muscle growth. Int J Sport Nutr Exerc Metab. 2001;11:109–132. doi: 10.1123/ijsnem.11.1.109. [DOI] [PubMed] [Google Scholar]
- 4.Dook JE, James C, Henderson NK, et al. Exercise and bone mineral density in mature female athletes. Med Sci Sports Exerc. 1997;29:291–296. doi: 10.1097/00005768-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Magnusson SP, Hansen P, Kjær M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports. 2003;13:211–223. doi: 10.1034/j.1600-0838.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 6.Viidik A. The effect of training on the tensile strength of isolated rabbit tendons. Scand J Plast Reconstr Surg. 1967;1:141–147. doi: 10.3109/02844316709022844. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Pan T, Liu Y, et al. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res. 2010;28:1178–1183. doi: 10.1002/jor.21123. [DOI] [PubMed] [Google Scholar]
- 8.Koskinen SOA, Heinemeier KM, Olesen JL, et al. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol. 2004;96:861–864. doi: 10.1152/japplphysiol.00489.2003. [DOI] [PubMed] [Google Scholar]
- 9.Kubota H, Manske PR, Aoki M, et al. Effect of motion and tension on injured flexor tendons in chickens. J Hand Surg. 1996;21:456–463. doi: 10.1016/S0363-5023(96)80363-7. [DOI] [PubMed] [Google Scholar]
- 10.Hitchcock TF, Light TR, Bunch WH, et al. The effect of immediate constrained digital motion on the strength of flexor tendon repairs in chickens. J Hand Surg Am. 1987;12:590–595. doi: 10.1016/s0363-5023(87)80213-7. [DOI] [PubMed] [Google Scholar]
- 11.Gelberman RH, Woo SLY, Lothringer K, et al. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg Am. 1982;7:170–175. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 12.Thomopoulos S, Williams GR, Gimbel JA, et al. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin M, Kumai T, Milz S, et al. The skeletal attachment of tendons—tendon ‘entheses.’. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 14.Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagher E, Hays PL, Kawamura S, et al. Immobilization modulates macrophage accumulation in tendon–bone healing. Clin Orthop Relat Res. 2009;467:281–287. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimbel JA, Van Kleunen JP, Williams GR, et al. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–404. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 17.Palmes D, Spiegel HU, Schneider TO, et al. Achilles tendon healing: long-term biomechanical effects of postoperative mobilization and immobilization in a new mouse model. J Orthop Res. 2002;20:939–946. doi: 10.1016/S0736-0266(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 18.Murrell GA, Lilly EG, III, Goldner RD, et al. Effects of immobilization on Achilles tendon healing in a rat model. J Orthop Res. 1994;12:582–591. doi: 10.1002/jor.1100120415. [DOI] [PubMed] [Google Scholar]
- 19.Hettrich CM, Gasinu S, Beamer BS, et al. The effect of immobilization on the native and repaired tendon-to-bone interface. J Bone Joint Surg Am. 2013;95:925–930. doi: 10.2106/JBJS.K.01329. [DOI] [PubMed] [Google Scholar]
- 20.Gumucio JP, Phan AC, Ruehlmann DG, et al. Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol. 2014;117:1287–1291. doi: 10.1152/japplphysiol.00720.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sereysky JB, Flatow EL, Andarawis-Puri N. Musculoskeletal regeneration and its implications for the treatment of tendinopathy. Int J Exp Pathol. 2013;94:293–303. doi: 10.1111/iep.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 23.Salter DM. Tenascin is increased in cartilage and synovium from arthritic knees. Br J Rheumatol. 1993;32:780–786. doi: 10.1093/rheumatology/32.9.780. [DOI] [PubMed] [Google Scholar]
- 24.Pacifici M, Iwamoto M, Golden EB, et al. Tenascin is associated with articular cartilage development. Dev Dyn. 1993;198:123–314. doi: 10.1002/aja.1001980206. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JM, Wong A, Yellowley CE, et al. Regulation of tenascin expression in bone. J Cell Biochem. 2011;112:3354–3363. doi: 10.1002/jcb.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda T, Sakabe T, Sunaga A, et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pryce BA, Watson SS, Murchison ND, et al. Recruitment and maintenance of tendon progenitors by TGFβ signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JP, V, Finley G, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J Biomech. 2014;47:214–222. doi: 10.1016/j.jbiomech.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CF, Breidenbach A, Aschbacher-Smith L, et al. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS One. 2013;8:e65411. doi: 10.1371/journal.pone.0065411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breidenbach AP, Aschbacher-Smith L, Lu Y, et al. Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J Orthop Res. 2015;33:1142–1151. doi: 10.1002/jor.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blitz E, Sharir A, Akiyama H, et al. Tendon–bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. 2013;140:2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- 32.Soeda T, Deng JM, de Crombrugghe B, et al. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis. 2010;48:635–644. doi: 10.1002/dvg.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimoto Y, Takimoto A, Akiyama H, et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- 34.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 35.Chong AK, Ang AD, Goh JC, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J Bone Joint Surg Am. 2007;89:74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 36.Uysal CA, Tobita M, Hyakusoku H, et al. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65:1712–1719. doi: 10.1016/j.bjps.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Mishra A, Woodall J, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113–125. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Cole BJ, Seroyer ST, Filardo G, et al. Platelet-rich plasma: where are we now and where are we going? Sports Health. 2010;2:203–210. doi: 10.1177/1941738110366385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gartner MH, Benson JD, Caldwell MD. Insulin-like growth factors I and II expression in the healing wound. J Surg Res. 1992;52:389–394. doi: 10.1016/0022-4804(92)90121-f. [DOI] [PubMed] [Google Scholar]
- 40.Thomopoulos S, Harwood FL, Silva MJ, et al. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Thomopoulos S, Kim HM, Das R, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am. 2010;92:2285–2293. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomopoulos S, Das R, Silva MJ, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurtz CA, Loebig TG, Anderson DD, et al. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med. 1999;27:363–369. doi: 10.1177/03635465990270031701. [DOI] [PubMed] [Google Scholar]
- 44.Lou J, Tu Y, Burns M, et al. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 45.Chang SC, Hoang B, Thomas JT, et al. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227–28234. [PubMed] [Google Scholar]
- 46.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, et al. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg Am. 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Abrahamsson SO, Lundborg G, Lohmander LS. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J Orthop Res. 1991;9:495–502. doi: 10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- 48.Abrahamsson SO. Similar effects of recombinant human insulin-like growth factor-I and II on cellular activities in flexor tendons of young rabbits: experimental studies in vitro. J Orthop Res. 1997;15:256–262. doi: 10.1002/jor.1100150215. [DOI] [PubMed] [Google Scholar]
- 49.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 50.Wang RN, Green J, Wang Z, et al. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu SC, Wong YP, Chan BP, et al. The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci. 2013;72:2965–2974. doi: 10.1016/s0024-3205(03)00169-3. [DOI] [PubMed] [Google Scholar]
- 52.Forslund C, Rueger D, Aspenberg P. A comparative dose–response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res. 2003;21:617–621. doi: 10.1016/S0736-0266(03)00010-X. [DOI] [PubMed] [Google Scholar]
- 53.Chan BP, Fu S, Qin L, et al. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand. 2000;71:513–518. doi: 10.1080/000164700317381234. [DOI] [PubMed] [Google Scholar]
- 54.Sahoo S, Toh SL, Goh JC. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31:2990–2998. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Kuo CK, Marturanom JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barber JG, Handorf AM, Allee TJ, et al. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng Part A. 2013;19:1265–1274. doi: 10.1089/ten.tea.2010.0538. [DOI] [PubMed] [Google Scholar]
- 57.Vuornos K, Björninen M, Talvitie E, et al. Human adipose stem cells differentiated on braided polylactide scaffolds is a potential approach for tendon tissue engineering. Tissue Eng Part A. 2016;22:513–523. doi: 10.1089/ten.tea.2015.0276. [DOI] [PubMed] [Google Scholar]
- 58.Shen W, Chen X, Chen J, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–7249. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 59.Stramer BM, Mori R, Martin P. The inflammation–fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 60.Sahoo S, Ouyang H, Goh JC, et al. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12:91–99. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Xie J, Lipner J, et al. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009;9:2763–2768. doi: 10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCormick A, Charlton J, Fleming D. Assessing health needs in primary care. Morbidity study from general practice provides another source of information. BMJ. 1995;310:1534. doi: 10.1136/bmj.310.6993.1534d. [DOI] [PMC free article] [PubMed] [Google Scholar]