Abstract
Summary
A performance algorithm can be successfully used by bone density technologists at the time of a bone density test to identify patients with an indication for vertebral fracture assessment (VFA). Doing so appropriately increases physician prescription of fracture prevention medication.
Introduction
Densitometric spine imaging (vertebral fracture assessment, VFA) can identify prevalent vertebral fracture but is underutilized. We developed an algorithm by which DXA technologists identify patients for whom VFA should be performed. Following this algorithm, VFA was performed in patients whose lowest T-score (lumbar spine, total hip, or femoral neck) was between −1.5 and −2.4 inclusive and with one of the following: age, ≥65 years; height loss, ≥1.5 in.; or current systemic glucocorticoid therapy. Our main objectives were to assess change in VFA utilization at two other healthcare organizations after algorithm implementation, and to estimate the association of VFA results with prescription of fracture prevention medication.
Methods
The proportions of patients with an indication for VFA who had one performed before and after algorithm implementation were compared. Logistic regression was used to estimate the multivariable-adjusted association of VFA results with subsequent prescription of fracture prevention medication adjusted for healthcare organization (study site).
Results
After algorithm introduction, appropriate VFA use rose significantly Patients with a VFA positive for vertebral fracture had an odds ratio of 3.2 (95 % C.I., 2.1–5.1) for being prescribed new fracture prevention medication, adjusted for age, sex, prior clinical fracture, use of glucocorticoid medication, femoral neck bone mineral density T-score, and study site.
Conclusions
An algorithm to identify those for whom VFA is indicated can successfully be implemented by DXA technologists. Documentation of vertebral fracture increases prescription of fracture prevention medication for patients who otherwise lack an apparent indication for such therapy.
Keywords: Bone densitometry, Vertebral fracture assessment, VFA
Introduction
Prevalent vertebral fractures are a strong predictor of incident fractures [1–7] and are an indication for pharmacologic fracture prevention therapy [8–10]. Moreover, they are present in a substantial minority of older adults who do not have osteoporosis by bone density criteria [11] and who otherwise lack indications for fracture prevention therapy. However, most vertebral fractures are not clinically recognized at the time of their occurrence [12, 13]. Lateral spine imaging is required to identify these patients and facilitate appropriate use of fracture reducing therapy.
Bone density measurement is indicated for women age 65 and older [14, 15] and men age 70 and older [15, 16]. Hence, densitometric lateral spine imaging (vertebral fracture assessment [VFA]) at the time of a bone density test may be the best comprehensive population-based strategy to identify those with unrecognized vertebral fracture. Densitometric VFA is very accurate in detecting moderate to severe vertebral fractures [17–19]. However, a physician request is required in some countries for a VFA to be done. This can be secured in one of three ways: (a) encourage (and then expect) primary care providers to recognize when a VFA ought to be requested along with bone densitometry; (b) incorporate VFA as a routine part of all DXA tests; or (c) have DXA technicians apply criteria to determine who should have a VFA at the time of their bone density test. Option (a) is impractical as providers will not know their patient's most current bone mineral density (BMD) value to assist in the VFA decision. Similarly, option (b) seems inappropriate as patients with low likelihood of prevalent vertebral fracture will be imaged. As such, at a large urban healthcare delivery organization, we designed a VFA performance algorithm that has been implemented by technologists at the time of DXA scanning.
However, it remains unclear how well other DXA technologists can implement such a performance algorithm, whether this approach can be successfully applied in a variety of healthcare delivery organizations, and what impact VFA results have on physician behavior with respect to additional spine imaging and prescribing of fracture prevention medication. The purposes of our study were to assess (a) how well DXA technologists apply a VFA performance algorithm; (b) how well the algorithm is adopted at other healthcare delivery organizations; (c) the association between VFA performance and use of additional subsequent spine imaging (standard radiography, computed tomography [CT], and magnetic resonance imaging [MRI]); and (d) the association between the reported results of VFA on subsequent prescribing of fracture prevention medication.
Methods
Introduction of the performance algorithm for VFA
At the first healthcare delivery organization (HC-1), the DXA request form was revised in March 2005 such that VFA was authorized by the clinician requesting the bone density test if the worst T-score at the femoral neck, total hip, or lumbar spine was −1.5 or worse, and the patient also had one of the following: (a) age 65 years or older, (b) historical height loss of ≥1.5 in., or (c) was taking systemic glucocorticoid therapy (prednisone more than 5 mg per day) at the time of their DXA test. These criteria, simplified from the ISCD 2007 VFA position statements [20], were chosen as a literature review suggests that at least 10 % of individuals meeting one or more of these criteria would have a prevalent vertebral fracture on VFA. This algorithm was subsequently implemented in a large rural community healthcare delivery organization (HC-2) and in an academic health center (HC-3) on 1 January 2011, with one change; the worst T-score at the lumbar spine, total hip, and femoral neck had to be between −1.5 and −2.4 inclusive. This change was made because T-scores of −2.5 or lower are indications for fracture prevention therapy in the USA, regardless of prevalent vertebral fracture status. The Genant semi-quantitative criteria were used to assess VFA images for prevalent vertebral fracture by readers at all three institutions.
Electronic health record data extraction
All individuals who had DXA testing were identified from the electronic health record (EHR) based on a CPT code encounter for bone densitometry (see Appendix) at HC-1 between 1 July 2005 and 30 June 2010, and at HC-2 and HC-3 between 1 January 2009 and 30 April 2012. Patient sex, current use of systemic glucocorticoid therapy (oral prednisone, oral or IV methylprednisolone, and/or oral or IV dexamethasone) at the date of DXA, and patient birth date were extracted from the EHR. Individuals were considered to have had hip, clinical vertebral, wrist, humerus, or pelvic fractures within the past 5 years if they had an inpatient hospital stay with the primary or secondary discharge diagnosis code corresponding to that fracture (see Appendix) or an outpatient clinical encounter with an appropriate primary or secondary fracture diagnosis code within the 5 years preceding the date of the bone density test. Age was calculated as the date of the DXA test minus the birth date.
A patient was considered to be on a fracture prevention medication at the time of their DXA if they had an active prescription for a Food and Drug Administration-approved osteoporosis treatment in the EHR. Active prescriptions were those that were new or renewed within the 12-month period prior to the date of DXA with sufficient refills such that the patient would have medication available on the date of the DXA even if they were 100 % compliant. New fracture prevention medication for treatment naïve individuals was defined as a new prescription entered into the medical record for calcitonin, raloxifene, an oral or intravenous bisphosphonate, denosumab, or teriparatide within the 5-month period after the DXA test. Patients on pre-existing calcitonin and raloxifene were considered to have started new medication if they were switched to more potent therapy (a bisphosphonate, denosumab, or teriparatide) within 5 months after the test, by which time nearly all had a follow-up encounter with the provider who requested the bone density test. Similarly, patients on bisphosphonates or denosumab at the time of their bone density test were also considered to have started new medication if they switched to teriparatide within 5 months of their DXA.
Subsequent imaging of the thoracic or lumbar spine by standard spine radiography, CT, or MRI within the 5-month period following each DXA test was identified from the EHR by appropriate CPT code (see Appendix).
Extraction of axial bone mineral density values
BMD was measured on Hologic Discovery densitometers at HC-1 and GE Lunar Prodigy or iDXA densitometers at HC-2 and HC-3. At all three institutions, lumbar spine, left and/or right total hip, and left and/or right femoral neck BMD data were extracted from the densitometer databases. For HC-2 and HC-3, the densitometer databases also had current height and recalled young adult tallest height values, and these were extracted. At all three institutions, data from the densitometers were merged with EHR data using medical record number as the merge variable.
Calculation of utilization of VFA in all three healthcare delivery organizations
At HC-1, the proportion of all of those with an identifiable indication for VFAwho had VFA performed was calculated for each year between 1 July 2005 and 30 June 2010. For HC-2 and HC-3, the same proportion was originally calculated for four time periods; three 8-month time periods preceding introduction of the performance algorithm (time periods 1, 2, and 3), and one 8-month time periods after introduction of the performance algorithm (time periods 4). Based on the results of the uptake at the two institutions, a post hoc assessment of uptake over a subsequent fifth 8-month time period was added.
Ascertainment of results of VFA
At all three institutions, manual review of the EHR was required to ascertain whether or not the VFA was interpreted as showing one or more prevalent vertebral fractures. Rather than evaluating the VFA images themselves, we believed that the dictated VFA report would be most influential on the prescribing provider who requested the DXA test. All VFA reports done in time periods 3 and 4 at HC-2 and HC-3 through 31 August 2011 were reviewed. At HC-1, a nested sample for a case–control analysis was created, with 150 individuals with worst T-score −1.5 to −2.4 who had a VFA done who also had a new fracture prevention medication prescribed within 5 months of the VFA, and 150 individuals randomly from among those with a worst T-score between −1.5 and −2.4 who did not have a new fracture prevention medication prescribed following their VFA.
Statistical analyses
The healthcare organizations whose data were used for specific analyses are indicated in Table 1. Characteristics of those having a DXA at HC-1 who also had a VFAwere compared to those not having a VFA using t tests for continuous variables and chi-square tests for categorical variables. The significance of the associations of changes in proportions of those with an indication for a VFA who had one and time period was assessed with chi-square statistic.
Table 1.
Analyses performed at three participating healthcare organizations
| Analysis | HC-1 | HC-2 | HC-3 |
|---|---|---|---|
| Uptake of VFA after introduction of performance algorithm | X | X | |
| Association of VFA results with prescription of fracture prevention medication | X | X | X |
| Association of VFA use with additional downstream spine imaging | X |
The proportion of VFA tests positive for vertebral fracture at HC-2 and HC-3 were tabulated. At HC-1, the proportion with a VFA positive for vertebral fracture was first tabulated for the subsets in the nested case–control sample who did and who did not start a new fracture prevention medication after their VFA. Among all of those who had a VFA with a worst T-score of −1.5 to −2.4, the proportion that started a new fracture prevention medication was also calculated. From all three of these proportions, the percentage with a vertebral fracture was estimated among the entire population at HC-1 who had a VFA and a worst T-score of −1.5 to −2.4.
Because the same data elements with the same variable definitions were extracted for individuals undergoing VFA at all three institutions, data for those whose VFA results were extracted by manual medical record review were pooled. The association between VFA results and subsequent prescription of fracture prevention medication was assessed using data from all three healthcare organizations with logistic regression models adjusting for age, sex, current glucocorticoid use, prior fracture, study site, and femoral neck T-score. To test whether or not the association between VFA results and prescription of fracture prevention medication varied by study site, an interaction term between study site and VFA results was added as a predictor. Although bone density was measured on different densitometers at the three sites from different manufactures, we considered adjustment for femoral neck T-score to be valid for two reasons: (a) both Hologic and GE/Lunar densitometers calculate femoral neck T-scores according to NHANES III reference data [21] and (b) we also included study site as a fixed effect covariate in the regression models.
The significance of the association of performance of VFA with downstream spine imaging using data only from HC-1 was assessed with chi-square statistic.
Results
Utilization of VFA at HC-1
At HC-1, 38,646 unique individuals had 47,775 DXA testing between 1 July 2005 and 30 June 2010. Among these, BMD results could be identified for 46,703 tests in the densitometer database. We were able to match the BMD data for 45,889 tests (96 %) to the EHR data and among these 13,780 (30 %) had a VFA done at the time of their DXA test. Those who had VFA at the time of DXA were older, slightly more likely to be male, had lower body mass index, were much more likely to have had an encounter for a fracture of the spine, hip, proximal humerus, wrist, or pelvis within the prior 5-year period before the DXA (all p <0.001; Table 1). Among those who had a VFA, 72 % had an identifiable appropriate indication for the test (age or current glucocorticoid use), whereas only 7.9 % of patients with no identifiable indication for VFA had the test performed. The proportion of patients who had VFA performed for appropriate indications rose from 53 % (2005) to 78 % (2010) over the course of algorithm implementation and data collection. Among all who had a DXA, 22 % had an indication for a VFA and osteopenia (worst T-score of the femoral neck, total hip, and lumbar spine between −1.5 and −2.4). An additional 13 % had a worst T-score of ≤−2.5 and at HC-1 were considered to have an indication for VFA.
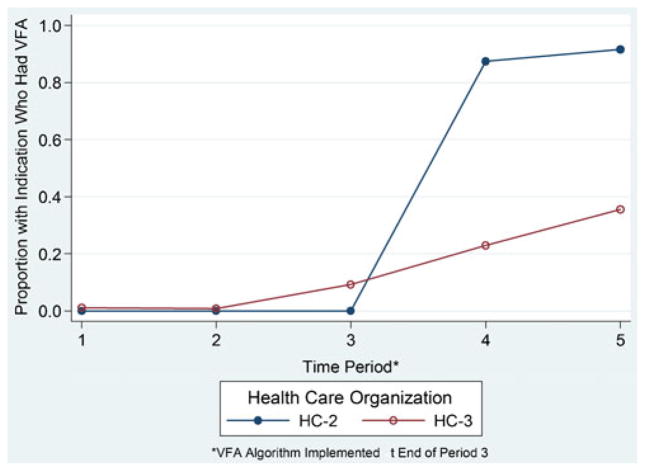
Change of utilization of VFA after implementation of the performance algorithm at HC-2 and HC-3
No VFA was performed during the first or second 8-month period at HC-2 and few were performed at HC-3 during the third 8-month period before implementation of the performance algorithm (Fig. 1). Among all who had a DXA, at HC-2 and HC-3, 28 and 34 %, respectively, had an indication for a VFA consistently across all time periods. After implementation, VFA use rose dramatically at HC-2, such that 87 and 92 % of patients with an appropriate VFA indication had the test during the first and second 8-month post-implementation periods respectively. At HC-3, 23 and 36 % of patients with an appropriate VFA indication had the test during the first and second 8-month post-implementation periods, respectively.
Fig. 1.
Uptake of VFA Utilization in healthcare organizations 2 and 3; proportions of those with an indication for VFAwho had one done in each time period
Association of reported results of VFA with prescription of fracture prevention medication and appropriateness of follow-up
At HC-1, 27 % (81/299) of those in the nested case–control sample had a VFA positive for vertebral fracture. However, those started on new or intensified fracture prevention medication were over-represented in this sample, and an estimated 21 % had a positive VFA when adjusted for the proportion started on new fracture prevention medication. At HC-2 and HC-3, respectively, 13 % (57/437) and 21 % (73/341) of VFA images were positive for vertebral fracture. Among the 70 who were started on a new fracture prevention medication, 59 were treatment naïve at the time of their bone density test and 11 were switched to a more potent agent. Those with a VFA positive for vertebral fracture had an odds ratio of 3.5 (95 % C.I., 2.0–6.2) for starting or intensifying fracture prevention medication compared to those with a VFA negative for vertebral fracture, adjusted for age category, sex, current use of glucocorticoid medication, femoral neck T-score, prior clinical fracture of the spine, hip, proximal humerus, wrist, or pelvis, study site, and interaction between study site and VFA results (Table 2). The association between VFA results and medication prescription did not vary by study site (interaction term p values>0.3).
Table 2.
Characteristics of patients having DXA alone vs. DXA plus VFA at time of bone density test (HC-1)
| Characteristic | DXA without VFA (n =32,109) | DXA with VFA (n =13,780) | p value |
|---|---|---|---|
| Age, years (SD) | 61.7 (10.5) | 73.8 (9.6) | <0.001 |
| Sex | 95.3 % female | 93.2 % female | <0.001 |
| 4.7 % male | 6.8 % male | ||
| Body mass index, kg/m2 (SD) | 27.0 (5.8) | 25.4 (4.7) | <0.001 |
| Current use of glucocorticoid Rx (> 5 mg ) | 4.4 % | 5.7 % | <0.001 |
| Prior fracture | 4.9 % | 14.3 % | <0.001 |
The absolute proportion of those started on new medication among those who had a VFA positive for vertebral fracture at the three institutions was nonetheless low. At HC-1, adjusting for the difference in the proportions prescribed new medication among osteopenic patients having a VFA in the nested case–control sample versus the parent population, an estimated 17 % of osteopenic patients with a VFA positive for vertebral fracture were started on new medication. At HC-2 and HC-3, respectively, an estimated 28 and 17 % of osteopenic individuals with a VFA positive for vertebral fracture were started on new medication. Among the 114 with a VFA positive for vertebral fracture who were not prescribed fracture prevention medication, the reasons for not starting or intensifying medication were documented in the medical record for 61 individuals. These included already being on fracture prevention medication (41), follow-up spine imaging showing no evidence of vertebral fracture [14], or the patient declining new medication [6]. At HC-1, adjusting for the difference in the proportions prescribed new medication among osteopenic patients having a VFA in the nested case–control sample versus the parent population, no adequate explanation for not prescribing fracture prevention medication was evident for 21 % of osteopenic patients with a VFA positive for vertebral fracture, At HC-2 and HC-3, respectively, these percentages were 36 % and 22 %.
Impact of VFA on downstream spine imaging
VFA performance was statistically associated with downstream spine imaging with standard radiography, CT, or MRI at HC-1 (Table 3), but the proportions of these who had additional spine imaging following a VFA were very low.
Table 3.
Association of VFA results with new prescription for fracture prevention medication (HC-1, HC-2 and HC-3)
| Predictor | Odds ratio (95 % C.I.) |
|---|---|
| VFA positive for vertebral fracture | 3.5 (2.0–6.2) |
| Age: <65 (ref.) | 1.0 |
| 65–69 | 1.4 (0.7–2.7) |
| 70–74 | 1.1 (0.6–2.2) |
| 75–79 | 0.9 (0.4–1.8) |
| 80 or older | 1.5 (0.8–3.0) |
| Sex (male vs. female) | 1.5 (0.9–2.5) |
| Prior fracture | 0.8 (0.5–1.3) |
| Current glucocorticoid | 1.7 (1.0–2.8) |
| Femoral neck BMD T-score (per increase of 1.0) | 0.56 (0.37–0.84) |
| Study sitea | HC-2: reference |
| HC-1: 1.9 (1.3–2.8) | |
| HC-3: 0.4 (0.2–0.7) | |
| Study site—VFA results interaction | HC-2: reference |
| HC-1: 0.66 (0.3–1.6) | |
| HC-3: 1.5 (0.5–5.0) |
n =945; data missing on one or more covariates for 132 individuals
The reasons for the differences for fracture prevention medication among osteopenic individuals between the three sites is unclear; since these odds ratios are adjusted for results of the VFA, it is possible that these odds reflect a higher likelihood of using medication to prevent bone loss among osteopenic individuals at HC-1, but a further manual medical record review study would be required to investigate this
Discussion
Vertebral fractures are common in older adults and are a marker of future fracture risk. Because 75 % of incident vertebral fractures are not recognized clinically at the time that they occur, spine imaging is essential for adequate detection of their presence, but such imaging is infrequently performed [11]. This study demonstrates that DXA requests that include a performance algorithm for VFA can be successfully implemented by DXA technologists in diverse healthcare systems. These VFA images reveal prevalent vertebral fractures in nearly one of five patients undergoing DXA and, importantly, reporting these fractures back to the requesting provider influences their prescribing of fracture prevention medication. Moreover, contrary to the fear that diagnostic tests have potential to increase subsequent test performance, we found very little additional downstream spine imaging following VFA (Table 4).
Table 4.
Downstream use of spine imaging studies in those who have compared to those who do not have VFA (HC-1 only)
| Imaging modality | Proportion with additional spine imaging | p value (chi-square) | |
|---|---|---|---|
|
| |||
| VFA not done (N =18,810) | VFA done (N =6,277) | ||
| Spine X-rays | 1.6 % | 3.2 % | <0.001 |
| CT of spine | 0.1 % | 0.2 % | 0.14 |
| MRI of spine | 1.3 % | 2.0 % | <0.001 |
However, uptake of the performance algorithm was suboptimal at HC-3 in our study, in which a parallel mechanism existed in the EHR by which a bone density test could be requested that did not include authorization for VFA. Successful implementation of this algorithm in any healthcare organization requires that all providers in that health organization who request DXA tests be made aware of the algorithm and its rationale, and that a DXA test request option that includes authorization for the performance algorithm be clearly visible to the provider. Further studies that delineate the factors within the practice environments of different healthcare organizations that explain the variability of speed with which new care processes are adopted are needed.
The absolute proportion of patients with a VFA positive for vertebral fractures who had a change in therapy was low (17–28 %). We were unable to document appropriate reasons for not starting fracture prevention medication in a significant minority of those with a VFA positive for vertebral fracture. Further study of patient and provider attitudes regarding diagnosis of vertebral fractures through spine imaging, is needed. However, some individuals with a positive VFA were already being treated with a fracture prevention medication, and the choice not to intensify therapy in these instances may have been reasonable. It may be appropriate that the algorithm used to select patients for VFA exclude those who are already on fracture prevention medication.
For practitioners selecting patients for treatment on the basis of BMD based criteria, prior studies have shown 14–18 % of women having a bone density test who have a worst T-score between −1.0 and −2.4 have a prevalent vertebral fracture on VFA [22–24], similar to the overall prevalence in our study. If selection of candidates for fracture prevention therapy is done on the basis of absolute fracture risk, then VFA still has substantial potential value, since the risk of subsequent fracture (vertebral and nonvertebral ) increases with the number and severity of prevalent vertebral fractures independent of BMD [6].
Alternative approaches to vertebral fracture screening include performing VFA on all who are referred for DXA, or having the requesting clinician (typically a primary care provider) appropriately identify which of their patients should receive VFA in addition to DXA. Performing VFA on all individuals referred for DXA would result in excessive spine imaging in persons in whom the pre-test probability of a prevalent vertebral fracture is very low. We believe this to be unjustified even though the cost and radiation exposure of a VFA are low.
Primary care providers will not have the benefit of the most up to date bone density values to help them decide who should have a VFA. Additionally, it may not be realistic to expect primary care physicians to sort out who among their patients for whom they are requesting a bone density test should also have a VFA, considering the time constraints under which they practice. One study has estimated that a primary care physician with a typically sized panel of patients a would require over 10 h per day just to follow the current practice guidelines for management of ten common chronic diseases, with no time left over for the acute care needs of those patients or care of other patients without those chronic conditions [25]. Hence, in the USA, adding additional screening procedures that require time and cognitive resources to implement is problematic, and will be even more so if an additional shortage of primary care providers materializes as many have projected [26].
Notably, we did not assess the potential utility and impact of VFA in those who have osteoporosis by BMD criteria. Performing VFA in those with osteoporosis by T-score may also be appropriate. Primary and secondary adherence to fracture prevention medication remains poor even among those with osteoporosis [27–31], and prevalent vertebral fracture is associated with a higher patient perceived need for fracture prevention medication even after adjustment for BMD and other fracture risk factors [32]. Hence, it is possible that VFA could improve medication acceptance and adherence among patients with both osteoporosis by T-score and prevalent vertebral fracture, but additional research is needed to address this.
The major strengths of this study are that it was conducted in the context of actual clinical practice in diverse clinical settings, including a large urban healthcare organization, a rural healthcare organization, and an academic healthcare center, and our relatively large sample sizes. Hence, our results are generalizable to a sizable proportion of practice settings in the USA. All three centers had electronic health record systems, allowing efficient capture of data elements essential to carry out the aims of the study.
There are important limitations of this study. First, we did not capture utilization that occurred outside of our institutions. If an individual was prescribed fracture prevention medication outside of our healthcare organizations, we would have misclassified that individual as not having been prescribed such medication. This would bias our estimated association of reported VFA results and prescription of fracture prevention medication toward the null, and we believe that our conclusions are robust to this limitation.
Second, medical records do not always capture all information relevant to patients' care decisions, and there may have been good reasons for not prescribing medication for some with a vertebral fracture that were not documented in the EHR. Third, it is not clear why the proportion of those who had a VFA with a reported vertebral fracture was lower at HC-2 than the other sites. Vertebral fracture identification with VFA or standard spine radiography is imperfect. Although all of the VFA interpreters have considerable training in vertebral fracture diagnosis on lateral spine images using the Genant semiquantitative method, we did not specifically test inter-rater reliability among readers, and there may be some variability in VFA readings.
In conclusion, an algorithm to select those who should have a VFA at the same time they have a DXA can be successfully implemented in clinical practice and executed by bone density technologists. This approach is an efficient way to screen for unrecognized vertebral fracture among the population of older adults, and increase the appropriate prescribing of fracture prevention medication without inducing undue additional spine imaging.
Acknowledgments
This study was funded by the University of Minnesota and University of Clinical Translational Science Institutes T2 Partnership Collaborative Grant under grant number CTSI-OCEH-2010-001.
We would also like to acknowledge Derek E. Fuerbringer, CNMT, at the University of Wisconsin and Matthew K. Breitenstein, MS, at Park Nicollet Clinic for their work retrieving bone density and electronic health record data for this study.
Appendix: Diagnosis and procedure codes used to characterize the study population at all three institutions
Those who had a bone density test were identified in EHR billing record by a CPT-4 code for a bone density test using dual energy X-ray absorptiometry (76075 or 76076 between 1 July 2005 and 31 December 2006, and 77080 or 77081 between 1 January 2007 and 30 June 2010). Those who also had a VFA were identified from encounters that also had a CPT-4 billing code of 76077 (on or before 31 December 2006) or 77080 (after 1 January 2007).
Those who had a clinical vertebral fracture were identified by any outpatient encounter with the one of the following ICD-9 diagnosis codes, or any inpatient hospital stay with one of the following primary or secondary discharge codes;
Spine 805.2, 805.4, 805.8, 733.13
Hip 820–820.32, 733.14
Pelvis 805.6, 808.0, 808.2, 808.4x, 808.8
Proximal humerus 812.0x, 812.2x, 733.11
Distal radius 813.4x, 813.5x, 813.8x, 733.12.
Downstream utilization of additional spine imaging was identified in EHR billing records as any encounter with one of the following CPT-4 codes;
Thoracic spine radiographs: 72070, 72072,72074,72080
Lumbar spine radiographs: 72100, 72110, 72114, 72120
CT scan of thoracic spine: 72128, 72129, 72130,
CT scan of lumbar spine: 72131, 72132, 72133
MRI thoracic spine: 72146, 72147, 72157
MRI lumbar spine: 72148, 72149, 72158
Footnotes
Conflicts of interest Dr. Schousboe is the president of the International Society for Clinical Densitometry; Dr. McKiernan received a research grant from OPKO Health, Alexion; Mr. Fuehrer has nothing to disclose; and Dr. Binkley received research support from Amgen, Lilly, Merck, OPKO. Advisory board, Merck, Lilly.
Contributor Information
J. T. Schousboe, Health Research Center, Park Nicollet Clinic, 3800 Park Nicollet Blvd., Minneapolis, MN 55416, USA, Division of Health Policy and Management, University of Minnesota, Minnesota, MN, USA
F. McKiernan, Marshfield Clinic Research Foundation, Marshfield, WI, USA
J. T. Fuehrer, Marshfield Clinic Research Foundation, Marshfield, WI, USA
N. Binkley, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
References
- 1.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821–828. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- 3.Ferrar L, Roux C, Felsenberg D, Gluer CC, Eastell R. Association between incident and baseline vertebral fractures in European women: vertebral fracture assessment in the Osteoporosis and Ultrasound Study (OPUS) Osteoporos Int. 2012;23:59–65. doi: 10.1007/s00198-011-1701-3. [DOI] [PubMed] [Google Scholar]
- 4.Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–532. doi: 10.1016/s8756-3282(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ, 3rd, Atkinson EJ, Cooper C, O'Fallon WM, Riggs BL. Vertebral fractures predict subsequent fractures. Osteoporos Int. 1999;10:214–221. doi: 10.1007/s001980050218. [DOI] [PubMed] [Google Scholar]
- 6.Siris ES, Genant HK, Laster AJ, Chen P, Misurski DA, Krege JH. Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int. 2007;18:761–770. doi: 10.1007/s00198-006-0306-8. [DOI] [PubMed] [Google Scholar]
- 7.McCloskey EV, Vasireddy S, Threlkeld J, Eastaugh J, Parry A, Bonnet N, Beneton M, Kanis JA, Charlesworth D. Vertebral fracture assessment (VFA) with a densitometer predicts future fractures in elderly women unselected for osteoporosis. J Bone Miner Res. 2008;23:1561–1568. doi: 10.1359/jbmr.080515. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E. Risk of new vertebral fracture in the year following a fracture. Jama. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 9.Briggs AM, Greig AM, Wark JD. The vertebral fracture cascade in osteoporosis: a review of aetiopathogenesis. Osteoporos Int. 2007;18:575–584. doi: 10.1007/s00198-006-0304-x. [DOI] [PubMed] [Google Scholar]
- 10.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ, 3rd, Lane AW, Cooper C, Eastell R, O'Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–119. doi: 10.1007/BF01623271. [DOI] [PubMed] [Google Scholar]
- 12.Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res. 2005;20:1216–1222. doi: 10.1359/JBMR.050314. [DOI] [PubMed] [Google Scholar]
- 13.Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Preventive Services Task Force. Screening for Osteoporosis: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2011;154:356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 15.National OF. Clinician's Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; 2008. [Google Scholar]
- 16.Leslie WD, Schousboe JT. A review of osteoporosis diagnosis and treatment options in new and recently updated guidelines on case finding around the world. Curr Osteoporos Rep. 2011;9:129–140. doi: 10.1007/s11914-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 17.Fuerst T, Wu C, Genant HK, von Ingersleben G, Chen Y, Johnston C, Econs MJ, Binkley N, Vokes TJ, Crans G, Mitlak BH. Evaluation of vertebral fracture assessment by dual X-ray absorptiometry in a multicenter setting. Osteoporos Int. 2009;20:1199–1205. doi: 10.1007/s00198-008-0806-9. [DOI] [PubMed] [Google Scholar]
- 18.Hospers IC, van der Laan JG, Zeebregts CJ, Nieboer P, Wolffenbuttel BH, Dierckx RA, Kreeftenberg HG, Jager PL, Slart RH. Vertebral fracture assessment in supine position: comparison by using conventional semiquantitative radiography and visual radiography. Radiology. 2009;251:822–828. doi: 10.1148/radiol.2513080887. [DOI] [PubMed] [Google Scholar]
- 19.Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17:281–289. doi: 10.1007/s00198-005-2010-5. [DOI] [PubMed] [Google Scholar]
- 20.Schousboe JT, Vokes T, Broy SB, Ferrar L, McKiernan F, Roux C, Binkley N. Vertebral Fracture Assessment: the 2007 ISCD official positions. J Clin Densitom. 2008;11:92–108. doi: 10.1016/j.jocd.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 22.Greenspan SL, von Stetten E, Emond SK, Jones L, Parker RA. Instant vertebral assessment: a noninvasive dual X-ray absorptiometry technique to avoid misclassification and clinical mismanagement of osteoporosis. J Clin Densitom. 2001;4:373–380. doi: 10.1385/jcd:4:4:373. [DOI] [PubMed] [Google Scholar]
- 23.Schousboe JT, DeBold CR, Bowles C, Glickstein S, Rubino RK. Prevalence of vertebral compression fracture deformity by X-ray absorptiometry of lateral thoracic and lumbar spines in a population referred for bone densitometry. J Clin Densitom. 2002;5:239–246. doi: 10.1385/jcd:5:3:239. [DOI] [PubMed] [Google Scholar]
- 24.Olenginski TP, Newman ED, Hummel JL, Hummer M. Development and evaluation of a vertebral fracture assessment program using IVA and its integration with mobile DXA. J Clin Densitom. 2006;9:72–77. doi: 10.1016/j.jocd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodenheimer T, Pham HH. Primary care: current problems and proposed solutions. Health Aff (Millwood) 2010;29:799–805. doi: 10.1377/hlthaff.2010.0026. [DOI] [PubMed] [Google Scholar]
- 27.Yood RA, Mazor KM, Andrade SE, Emani S, Chan W, Kahler KH. Patient decision to initiate therapy for osteoporosis: the influence of knowledge and beliefs. J Gen Intern Med. 2008;23:1815–1821. doi: 10.1007/s11606-008-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon MD, Majumdar SR. Primary non-adherence of medications: lifting the veil on prescription-filling behaviors. J Gen Intern Med. 2010;25:280–281. doi: 10.1007/s11606-010-1286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solomon DH, Brookhart MA, Tsao P, Sundaresan D, Andrade SE, Mazor K, Yood R. Predictors of very low adherence with medications for osteoporosis: towards development of a clinical prediction rule. Osteoporos Int. 2011;22:1737–1743. doi: 10.1007/s00198-010-1381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman SL, Nasser K, Nattrass S, Drinkwater B. Impact of bone turnover markers and/or educational information on persistence to oral bisphosphonate therapy: a community setting-based trial. Osteoporos Int. 2012;23:1069–1074. doi: 10.1007/s00198-011-1721-z. [DOI] [PubMed] [Google Scholar]
- 31.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 32.Schousboe JT, Davison ML, Dowd B, Thiede Call K, Johnson P, Kane RL. Predictors of patients' perceived need for medication to prevent fracture. Med Care. 2011;49:273–280. doi: 10.1097/MLR.0b013e318202915e. [DOI] [PubMed] [Google Scholar]