Abstract
Carpal tunnel syndrome (CTS), caused by entrapment of the median nerve in the carpal tunnel, impairs hand function including dexterous manipulation. The purpose of this study was to investigate the effects of CTS on force coordination and muscle coherence during low-intensity sustained precision pinch while the wrist assumed different postures. Twenty subjects (10 CTS patients and 10 asymptomatic controls) participated in this study. An instrumented pinch device was used to measure the thumb and index finger forces while simultaneously collecting surface electromyographic activities of the abductor pollicis brevis (APB) and first dorsal interosseous (FDI) muscles. Subjects performed a sustained precision pinch at 10% maximum pinch force for 15 sec with the wrist stabilized at 30° extension, neutral, or 30° flexion using customized splints. The force discrepancy and the force coordination angle between the thumb and index finger forces were calculated, as well as the β-band (15-30 Hz) coherence between APB and FDI. The index finger applied greater force than the thumb (p < 0.05); this force discrepancy was increased with wrist flexion (p < 0.05), but was not affected by CTS (p > 0.05). The directional force coordination was not significantly affected by wrist posture or CTS (p > 0.05). In general, digit force coordination during precision pinch seems to be sensitive to wrist flexion, but is not affected by CTS. The β-band muscular coherence was increased by wrist flexion for CTS patients (p < 0.05), which could be a compensatory mechanism for the flexion-induced exacerbation of CTS symptoms. This study demonstrates that wrist flexion negatively influences muscle and force coordination in CTS patients supporting the avoidance of flexion posture for symptom exacerbation and functional performance.
Keywords: carpal tunnel syndrome, force coordination, muscle coherence, precision pinch
1 Introduction
Carpal tunnel syndrome (CTS) is a common compression neuropathy of the upper extremity, with high prevalence in the general population. Due to entrapment of the median nerve in the carpal tunnel, CTS patients experience symptoms of hand tingling, numbness, and pain. These symptoms are exacerbated in the extreme wrist flexion position (i.e. Phalen’s maneuver), which is commonly used as a provocative test for diagnostic purposes [1,2]. The median nerve supplies sensory input to the palmar side of the thumb, index finger, middle finger, and the radial half of the ring finger. The motor branch of the median nerve innervates the opponens pollicis, abductor pollicis brevis (APB) and superficial head of flexor pollicis brevis, as well as first and second lumbricals. CTS is known to impair sensory functions of the hand as commonly evaluated by two-point discrimination, Semmes Weinstein monofilament testing, and sensory latency [3,4]. Motor function of the hand is also shown to be affected by CTS as demonstrated by weakness of grasp and pinch strength [5-7], although motor capability of the thumb has been found to be relatively preserved [8,9].
Precision pinch with the thumb and index finger is a dexterous manual task involving sensorimotor coordination of the two digits. CTS patients commonly demonstrate lack of dexterity in activities of daily living, such as inexplicably dropping objects. As a potential compensatory strategy to overcome sensorimotor deficits and prevent objects from unintentionally slipping, patients with CTS apply excessive pinch force while lifting objects [10-12]. CTS also impairs digit force accuracy and stability during precision pinch, especially when the force application lacks accompanying visual feedback [13].
During precision pinch, extrinsic and intrinsic hand muscles work together to ensure successful manipulation, and therefore, muscle coordination is necessary for successful pinch performance. Coherence analysis of electromyographic (EMG) signals has been used to quantify muscle coordination in specific frequency ranges, which provides a representative measure of rhythmic activities of underlying motor unit in synergic muscles [14] Specifically for pinching tasks, EMG-EMG coherence among hand and forearm muscles in the 15-30 Hz range (β-band) was shown to be associated with maintaining a steady force [15,16]. Impaired sensory input by digital nerve anaesthesia and deafferentation was also shown to modulate hand muscle coherence in the β-band [17,18]. Due to the sensorimotor deficits of the thumb and index finger associated with CTS, muscular coherence may be impaired in CTS patients completing precision pinch tasks.
Although grip and pinch strength have been extensively investigated in CTS patients, there is limited understanding of precision pinch forces and associated muscle activities. Therefore, the purpose of this study was to investigate the effects of CTS on force coordination and muscle coherence during precision pinch. For muscle coordination and coherence, the APB and the first dorsal interosseous (FDI) were chosen as they are key intrinsic hand muscles involved in pinching tasks and convenient access by surface EMG. In addition, force and muscle coordination were examined with the wrist in different flexion/extension positions to understand the postural effects on hand function. We hypothesized that the CTS patients would exhibit impaired coordination of thumb and finger forces and lack of APB-FDI muscle coherence during precision pinch, and that wrist deviation would further exacerbate the force coordination and muscle coherence.
2 Materials and Methods
2.1 Participants
A total of 20 right-hand dominant participants were recruited for this study, including 10 CTS patients (50.8 ± 9.6 years old; 8 females and 2 males) and 10 asymptomatic controls (47.9 ± 13.3 years old; 8 females and 2 males). All participants provided informed consent prior to study participation in accordance with the Institutional Review Board at Cleveland Clinic.
The inclusion criteria for the CTS group included satisfying at least three of the following criteria: (1) history of pain and/or numbness in the median-innervated territory of the right hand for at least 3 months; (2) positive provocative maneuvers with Tinel’s sign, Phalen’s maneuver, and/or median nerve compression test; (3) abnormal electrodiagnostic test results demonstrating median nerve neuropathy in the right hand; (4) an overall Boston Carpal Tunnel Syndrome Questionnaire score greater than 1.5 [19]; and (5) confirmation of CTS according to clinical discretion [20]. For the control group, the inclusion criteria included absence of CTS-like symptoms. The exclusion criteria for the CTS and control groups were: (1) left-hand dominance; (2) existence of any central nervous system disease; (3) diabetes; (4) pregnancy; (5) arthritis in the right hand or wrist; (6) steroid injection to the right hand within three months of study participation; and (7) history of musculoskeletal injury or surgery to the right hand or wrist.
2.2 Experimental Set-up
A pinch apparatus consisting of two six-component force/torque transducers (Mini40, ATI Industrial Automation, Inc., Apex, NC, USA) was used to measure the thumb and index finger forces. Each transducer was attached to an aluminum mounting support which was rigidly fixed to a stainless steel plate. The pinch contact surfaces were covered with 100-grit sandpaper and the pinch span was 1.8 cm. The force/torque signals were amplified and multiplexed using a custom interface box (ATI Industrial Automation, Inc., Apex, NC, USA) and converged to an 18-bit analog digital converter (PXI-6289, National Instruments, Austin, TX, USA). A surface EMG system (MyoSystem 1400, Noraxon USA, Inc., Scottsdale, AZ, USA) was used to record the activity of the APB and FDI muscles. The system had a 12-bit resolution and a hardware band pass filter of 10-500 Hz. In addition, a 22-inch computer monitor was positioned 50 cm in front of the participant to graphically provide real-time force information.
2.3 Experimental Protocol
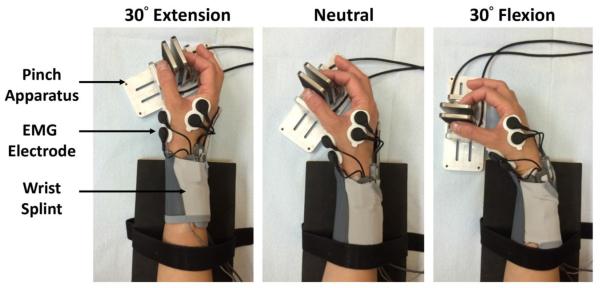
Participants washed their hands with soap and water prior to the experiment. In addition, the skin on the right hand was prepared using sandpaper and an alcohol swab before the application of EMG electrodes. A dual Ag/AgCl electrode with a center-to-center distance of 2.75 cm (Noraxon U.S.A., Inc., Scottsdale, AZ) was attached to the skin surface above both the APB and FDI muscles according to literature recommendations [21]. A ground electrode was also attached to the styloid process of radius. Then, each participant was seated comfortably on a height-adjustable chair by the testing table with their right arm abducted 30° in the frontal plane and flexed 30° in the sagittal plane. The forearm was rested on the table with the elbow flexed 90°. Customized splints were worn by the subject to stabilize the wrist in postures of 30° extension, anatomical neutral (0°), and 30° flexion. The pinch apparatus was fixed to the testing table at predetermined orientations so that the angle between the palm and pinch contact surfaces were 60°, allowing participants to perform pinching comfortably at each wrist posture (Fig. 1). The participants were instructed not to place the long, ring and little fingers against the pinch device or the index finger.
Fig. 1.
Experimental setup for measuring digit forces and muscle activities during precision pinch at various wrist postures
The study consisted of two tasks. First, the participants were instructed to pinch the apparatus using the thumb and index finger with their maximum effort. Verbal encouragement was given, guiding participants to reach their maximum pinch force within 5 sec. Three maximum pinch trials were performed for each wrist posture. For each trial, the maximum pinch force was defined as the maximum value of the averaged thumb and index finger normal forces. Then, the three maximum pinch force values for each posture were averaged and 10% of the average value was set as the target force for the subsequent submaximal pinch trials. During the submaximal pinch task, graphical information of the real-time pinch force, including a target line, was provided on the computer monitor. Participants were asked to match their pinch force to the target line as accurately as possible. Each submaximal pinch trial was 15 sec in duration and the participants were encouraged to match the target as soon as possible. A total of 10 trials were performed for each wrist posture. The three wrist postures were randomized, and a 1-minute rest was given between consecutive trials. A customized LabVIEW (National Instruments, Austin, TX, USA) program collected the force and EMG data at a sampling rate of 1000 Hz.
2.4 Data Processing
For the submaximal task, the force and EMG data in the first 5 sec of each trial were excluded from analyses to avoid the effects of non-stationarity. The remaining 10 sec of data for each trial were analyzed using customized MATLAB (The MathWorks, Natick, MA, USA) programs.
To quantify the force matching accuracy, the root mean square error (RMSE) between the measured force and the target force [13] was calculated as:
| (1) |
where n is the number of force samples, xi is the instant mean of the thumb and index finger normal forces, and xt is the target force (i.e. 10% of the averaged maximum pinch force). The force discrepancy between the two digits was calculated as the percentage difference between the resultant force magnitudes of the index finger and the thumb normalized by the force magnitude of the thumb. The 3D force vectors of the thumb and index finger were transformed to a common coordinate system [22,23], and then the angle between the two force vectors was calculated using the following equation:
| (2) |
where and are the force vectors of the thumb and index finger, respectively. The angle between the thumb and index finger was defined as the force coordination angle, which ranges from 0 to 180°, where 0° means the two force vectors are in phase and 180° means that they are in opposite directions The coordination angle between the digits was averaged over the 10-sec period.
To calculate the coherence within the β-band between APB and FDI, first the EMG data were filtered and rectified using a 4th order band-pass (5-100 Hz) Butterworth filter. Then, the signals were further processed using a bivariate autoregressive model and a boxcar window to generate coefficients for coherence estimation [24]. The coherence between two EMG signals was calculated as:
| (3) |
where f is a given frequency, Sxy is the cross spectrum of associated signals, and Sxx and Syy are the auto spectra of the associated signals. The estimated coherence was then transformed using a Fisher Z-transformation. The mean coherence between APB and FDI in the β-band was calculated. The coherence value ranges from 0 to 1, where higher value means greater coupling between the muscles.
2.5 Statistical Analysis
Two-way repeated measures ANOVAs, with one factor repeated, were performed to test the main effects of group (CTS and control) and posture (extension, neutral, and flexion), as well as the interaction effect of group and posture. The effects on the maximum pinch force, force matching accuracy, force discrepancy, force coordination angle, and mean β-band coherence were examined. Post hoc Tukey's tests were completed for pairwise comparisons. Statistical analyses were performed using SigmaStat 3.5 (Systat Software, San Jose, CA) and the significance level of α = 0.05.
3 Results
The demographic data of the 10 participants in the CTS group are shown in Table 1. Their CTS symptom duration ranged from 10 months to 21 years. The Boston CTS Questionnaire scores of the patients ranged from 1.58 to 3.58. Eight of them received the provocative maneuver tests, and they presented positive results with Tinel’s sign or Phalen’s Test.
Table 1.
The age, gender, symptom duration, Boston CTS Questionnaire score, and the result of the provocative maneuver tests of 10 CTS participants
| Subject # | Age (yr) | Gender | CTS Symptom Duration |
Boston CTS Questionnaire Score |
Tinel’s Sign / Phalen’s Test |
|---|---|---|---|---|---|
| CTS 01 | 64 | Female | 5 years | 2.21 | Positive |
| CTS 02 | 56 | Female | 16 years | 3.11 | Positive |
| CTS 03 | 55 | Female | 1.5 years | 2.89 | Positive |
| CTS 04 | 46 | Female | 21 years | 2.79 | N/A |
| CTS 05 | 28 | Male | 10 months | 2.16 | N/A |
| CTS 06 | 58 | Male | 1 year | 2.47 | Positive |
| CTS 07 | 50 | Female | 1 year | 2.00 | Positive |
| CTS 08 | 50 | Female | 6 years | 3.58 | Positive |
| CTS 09 | 53 | Female | 1.5 years | 1.58 | Positive |
| CTS 10 | 48 | Female | 1.5 years | 2.63 | Positive |
The maximum pinch force and force matching accuracy are presented in Table 2. The maximum pinch force was significantly affected by the factor of wrist posture (p < 0.001), but not by the group factor (p = 0.514) or the posture × group interaction (p = 0.077). The maximum pinch force in the flexed wrist posture was significantly less than that at the neutral (p = 0.002) and extended (p = 0.003) postures. The force matching accuracy for submaximal pinching was not significantly affected by group (p = 0.436), posture (p = 0.956), or the posture × group interaction (p = 0.199).
Table 2.
Maximum pinch force and submaximal force accuracy for the control and CTS groups at different wrist postures (mean ± standard deviation)
| Control (n = 10) |
CTS (n = 10) |
|||
|---|---|---|---|---|
| Maximum (N) | Accuracy (N) | Maximum (N) | Accuracy (N) | |
| Extension | 37.9 ± 14.5 | 0.12 ± 0.06 | 39.4 ± 11.5 | 0.13 ± 0.09 |
| Neutral | 37.0 ± 14.1 | 0.10 ± 0.04 | 40.7 ± 9.1 | 0.15 ± 0.11 |
| Flexion | 32.9 ± 13.0 | 0.11 ± 0.05 | 38.3 ± 8.1 | 0.13 ± 0.08 |
For the submaximal pinch task, there existed a force discrepancy between the digits with the index finger resultant force being greater than that of the thumb for both the control and CTS groups (p < 0.05). This force discrepancy was significantly affected by posture (p < 0.05), but not by group (p = 0.916). Wrist flexion led to an increased force discrepancy between the digits. In the wrist extension posture, the force discrepancies were 19.0 ± 23.5% for the control group and 20.0 ± 29.5% for the CTS group. At the neutral posture, the discrepancies were 24.4 ± 29.0% and 23.4 ± 34.2% for the control and CTS groups, respectively. As the wrist deviated to a more flexed posture of 30°, the force discrepancy for the control group was 28.2 ± 32.7% and for the CTS group was 23.7 ± 36.4%.
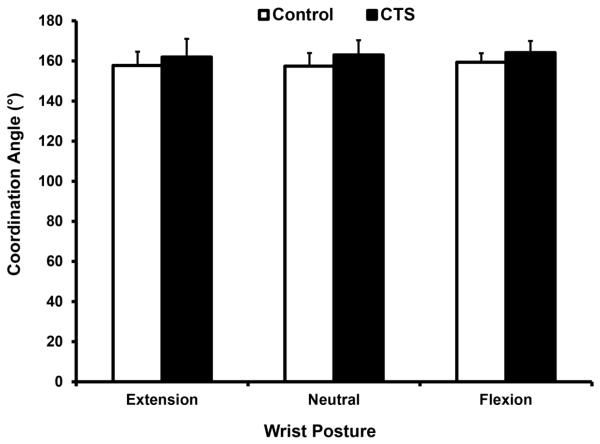
The force coordination angle between the digits ranged from 157° to 164° (Fig. 2). The angle was not significantly affected by group (p = 0.096), posture (p = 0.191), or the group × posture interaction (p = 0.828). The average coordination angle across the three wrist postures was 158.2 ± 5.9° for the control group and 163.0 ± 7.3° for the CTS group.
Fig. 2.
Force coordination angles (mean ± standard deviation) at difference wrist postures for the CTS and control groups
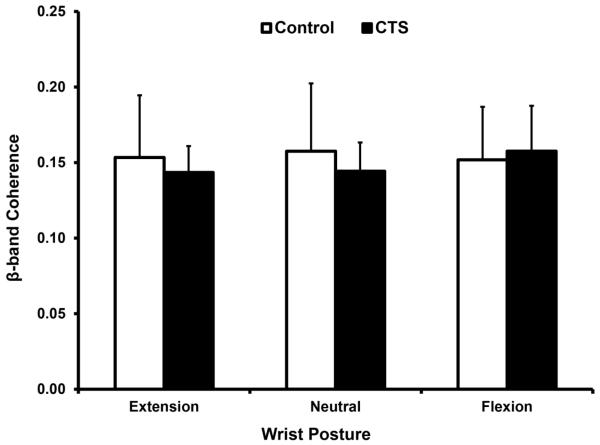
The coherence in the β-band was not significantly affected by group (p = 0.684) or posture (p = 0.269); however, there was a significant group × posture interaction (p < 0.05). The coherence values for the control group were 0.153 ± 0.041, 0.158 ± 0.045, and 0.152 ± 0.035 at wrist extension, neutral, and flexion, respectively. For the CTS group, the coherence values were 0.144 ± 0.018 for extension, 0.144 ± 0.019 for neutral, and 0.158 ± 0.03 for flexion. The interaction effect was associated with a significant difference of pairwise comparisons within the CTS group (Fig. 3).
Fig. 3.
β-band coherence at different wrist postures for CTS and control groups
4 Discussion
Our finding of decreased maximal pinch force at a flexed wrist posture is consistent with previous reports that pinch/grip strength is sensitive to wrist posture, particularly in the direction of flexion/extension with weak strength in flexion [25-29]. The weakness can be explained by the length-tension relationship of the extrinsic flexors of the digits. The muscular compartments are at an optimal length for maximum active force production at the slightly extended, functional wrist position. As the wrist joint flexes, the associated muscular compartment becomes less optimal, leading to an impairment of pinch force production. The result that CTS patients had similar maximal pinch force to healthy controls corroborates with previous findings that motor capability is relatively preserved in the CTS patient population [8,9].
For the sustained submaximal pinch, the CTS-associated sensory and motor deficits were expected to increase the force matching error. However, no statistical difference in the force matching accuracy was observed between the two groups or among wrist postures. In the current study, the precision pinch was performed on a stabilized object and visual feedback of force application was provided. It is possible that the sensorimotor deficits associated with CTS were compensated by the visual information to generate accurate pinch force [13]. In addition, 10% of the maximum pinch force was set as the target force in this study to minimize fatigue and discomfort for CTS patients, especially at the flexed posture. It is possible that higher pinch force may reveal force or muscle dyscoordination to a greater extent.
The result that the index finger generated more force than the thumb during submaximal precision pinch is consistent with a previous study [13]. Force discrepancy has been postulated as a consequence of anatomical structure and neural control. In the current study, no group effect was found on the inter-digit force difference, but there was a wrist posture effect. Wrist flexion caused increased force discrepancy between the thumb and index finger. The increase in the index finger contribution may be due to the more diminished thumb flexor function in a flexed wrist position [30].
The directional coordination of the precision pinch forces was not influenced by CTS or wrist posture. Biomechanically, the thenar muscles tend to abduct the thumb and generate shear force, making the thumb force vector deviated from the opposing direction perpendicular to the pinching surface. Alteration of thenar muscle function implicated by median nerve dysfunction could change directional force coordination. The fact that coordination angle was not changed by CTS might be explained by (a) the task required relatively low exertion effort and (b) the preservation of motor capability in the patient group. It is possible that pinch force dyscoordination will be more salient in patients with more severe CTS, especially during maximal pinching force production. The coordination angle, regardless of wrist posture and subject group, deviated about 20° from perfect opposition of 180°, which is consistent with previous findings [23,22]. The individual digits did not apply forces perpendicular to the pinch surface, nor did they orient their forces parallel in space. For pinching on a stabilized object without the requirement of force equilibrium, each digit may apply a force that favors its own anatomical structure and independent neural control.
We found that CTS patients demonstrated different β-band coherence of the APB and FDI muscles in flexion from that in extension. Coherence of hand muscles was shown in the β-band while maintaining steady pinch force [16], and increased coherence was observed when manipulating more compliant objects because of the increased sensorimotor integration required to adjust digit force and position for compliant object control [15]. It has also been shown that muscle coherence is affected by impaired sensory inputs due to deafferentation [18] and digital nerve anaesthesia [17]. In addition to sensory loss, the changes in somatosensory feedback due to varied wrist posture impacts muscle coherence across intrinsic and extrinsic muscles [31]. Furthermore, extrinsic muscles were found to have a stronger coherence than the intrinsic muscles [32,33]. The lower coherence has been interpreted as a benefit for intrinsic muscle function [34]. Intrinsic muscles are specifically important for fine modulation of digit forces, for this reason, a less degree of coupling might be better for individual muscle control. In the current study, participants performed an isometric pinch on a stabilized object, thus the independence of muscle activation may not be required for this task. Although varied wrist posture does not change the length of the APB and FDI muscles, wrist flexion may exacerbate the symptoms of CTS. In contrast to the reduced coherence after digital nerve block [17], we observed that the CTS patients had increased coherence in wrist flexion. Compared to the acute sensory loss after digital nerve anaesthesia, patients with CTS experience altered sensation in a longer process. Different modulations in the muscle coherence may reflect the different natures of sensory modifications. It could be postulated that the increase in β-band coherence in CTS patients with flexed wrists is a compensatory mechanism for the flexion-induced exacerbation of symptoms.
Some limitations in this study should be considered. First, moderate changes in wrist posture were applied. Slight wrist extension (10-30°) has been widely used for functional positioning [35,36] and no significant change was found in the carpal tunnel pressure between the neutral wrist position and 30° extension [37]. These may explain the findings in the current study that the hand has similar functionality at the neutral position and 30° extension. Although hand function is more sensitive to wrist flexion, the 30° wrist flexion posture employed in the current study was relatively moderate in comparison to the clinically used wrist flexion for Phalen’s maneuver. Perhaps a more flexed wrist position would reveal greater differences between CTS patients and controls. Second, this study had a relatively small sample size and the CTS patients were not classified by severity of symptoms. Though most CTS patients have sufficient thenar muscle function, the wasting or weakness of thenar muscles could be demonstrated in patients with more severe symptoms [38]. The force coordination between digits and the muscle coherence between APB and FDI could be more affected in CTS cases with thenar muscle atrophy. At last, a stabilized, non-deformable pinch device was used in the current study for the investigation of force coordination and muscle coherence. However, translating and manipulating movable objects are the skills often required in daily life. As greater muscle coherence was found in handling a more complaint object [15], neuromuscular coordination could be affected by the compliance of objects. Also, the disturbance of gravity when holding objects in the air could be another factor to influence force and muscle coordination. Therefore, different task designs and object materials may lead to varied force coordination and muscle coherence. The target was set as 10% of the maximum pinch force and a 1-minute rest was given between trials, the effect of muscle fatigue on force coordination and muscle coherence was assumed to be minor although this fatigue factor was not evaluated in this study.
In conclusion, this study observed the wrist posture effect on the force discrepancy that the index finger generated significantly higher force than the thumb when the wrist was flexed. Also, the wrist posture × group interaction effect was observed that, within the CTS group, the β-band coherence was significantly higher in the wrist flexion condition than in the wrist extension condition. This study demonstrates that wrist flexion negatively influences muscle and force coordination in CTS patients supporting the avoidance of flexion posture for symptom exacerbation and functional performance.
Acknowledgments
This publication was made possible by Grant R01AR056964 from NIAMS/NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
6 References
- 1.MacDermid JC, Wessel J. Clinical diagnosis of carpal tunnel syndrome: a systematic review. J Hand Ther. 2004;17(2):309–319. doi: 10.1197/j.jht.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Wiesman IM, Novak CB, Mackinnon SE, Winograd JM. Sensitivity and specificity of clinical testing for carpal tunnel syndrome. Can J Plast Surg. 2003;11(2):70–72. doi: 10.1177/229255030301100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havton LA, Hotson JR, Kellerth JO. Correlation of median forearm conduction velocity with carpal tunnel syndrome severity. Clin Neurophysiol. 2007;118(4):781–785. doi: 10.1016/j.clinph.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Szabo RM, Gelberman RH, Dimick MP. Sensibility testing in patients with carpal tunnel syndrome. J Bone Joint Surg Am. 1984;66(1):60–64. [PubMed] [Google Scholar]
- 5.Baker NA, Moehling KK, Desai AR, Gustafson NP. Effect of carpal tunnel syndrome on grip and pinch strength compared with sex- and age-matched normative data. Arthritis Care Res (Hoboken) 2013;65(12):2041–2045. doi: 10.1002/acr.22089. [DOI] [PubMed] [Google Scholar]
- 6.Gehrmann S, Tang J, Kaufmann RA, Goitz RJ, Windolf J, Li ZM. Variability of precision pinch movements caused by carpal tunnel syndrome. J Hand Surg Am. 2008;33(7):1069–1075. doi: 10.1016/j.jhsa.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Tamburin S, Cacciatori C, Marani S, Zanette G. Pain and motor function in carpal tunnel syndrome: a clinical, neurophysiological and psychophysical study. J Neurol. 2008;255(11):1636–1643. doi: 10.1007/s00415-008-0895-6. [DOI] [PubMed] [Google Scholar]
- 8.Agabegi SS, Freiberg RA, Plunkett JM, Stern PJ. Thumb abduction strength measurement in carpal tunnel syndrome. J Hand Surg Am. 2007;32(6):859–866. doi: 10.1016/j.jhsa.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Li ZM, Harkness DA, Goitz RJ. Thumb strength affected by carpal tunnel syndrome. Clin Orthop Relat Res. 2005;441:320–326. doi: 10.1097/01.blo.0000181143.93681.0d. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HY, Kuo LC, Kuo YL, Chiu HY, Jou IM, Wu PT, et al. Feasibility of a novel functional sensibility test as an assisted examination for determining precision pinch performance in patients with carpal tunnel syndrome. PLoS One. 2013;8(8):e72064. doi: 10.1371/journal.pone.0072064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe BD, Freivalds A. Effect of carpal tunnel syndrome on grip force coordination on hand tools. Ergonomics. 1999;42(4):550–564. doi: 10.1080/001401399185469. [DOI] [PubMed] [Google Scholar]
- 12.Yen WJ, Kuo YL, Kuo LC, Chen SM, Kuan TS, Hsu HY. Precision pinch performance in patients with sensory deficits of the median nerve at the carpal tunnel. Motor Control. 2014;18(1):29–43. doi: 10.1123/mc.2013-0004. [DOI] [PubMed] [Google Scholar]
- 13.Li K, Evans PJ, Seitz WH, Jr., Li ZM. Carpal tunnel syndrome impairs sustained precision pinch performance. Clin Neurophysiol. 2015;126(1):194–201. doi: 10.1016/j.clinph.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501(Pt 1):225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20(23):8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15-30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516(Pt 2):559–570. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher RJ, Galea MP, Brown P, Lemon RN. Digital nerve anaesthesia decreases EMG-EMG coherence in a human precision grip task. Exp Brain Res. 2002;145(2):207–214. doi: 10.1007/s00221-002-1113-x. [DOI] [PubMed] [Google Scholar]
- 18.Kilner JM, Fisher RJ, Lemon RN. Coupling of oscillatory activity between muscles is strikingly reduced in a deafferented subject compared with normal controls. J Neurophysiol. 2004;92(2):790–796. doi: 10.1152/jn.01247.2003. [DOI] [PubMed] [Google Scholar]
- 19.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Keith MW, Masear V, Chung KC, Maupin K, Andary M, Amadio PC, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2009;91(10):2478–2479. doi: 10.2106/jbjs.i.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basmajian JV, Blumenstein R. Electrode placement in EMG biofeedback. Williams & Wilkins; 1980. [Google Scholar]
- 22.Marquardt TL, Li ZM. Quantifying Digit Force Vector Coordination during Precision Pinch. J Mech Med Biol. 2013;13(2):1350047. doi: 10.1142/s0219519413500474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K, Nataraj R, Marquardt TL, Li ZM. Directional coordination of thumb and finger forces during precision pinch. PLoS One. 2013;8(11):e79400. doi: 10.1371/journal.pone.0079400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasluosta CF, Domalain MM, Fang Y, Yue GH, Li ZM. Influence of nerve supply on hand electromyography coherence during a three-digit task. J Electromyogr Kinesiol. 2013;23(3):594–599. doi: 10.1016/j.jelekin.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imrhan SN. The influence of wrist position on different types of pinch strength. Appl Ergon. 1991;22(6):379–384. doi: 10.1016/0003-6870(91)90079-w. [DOI] [PubMed] [Google Scholar]
- 26.Lamoreaux L, Hoffer MM. The effect of wrist deviation on grip and pinch strength. Clin Orthop Relat Res. 1995;(314):152–155. [PubMed] [Google Scholar]
- 27.Mathur K, Pynsent PB, Vohra SB, Thomas B, Deshmukh SC. Effect of wrist position on power grip and key pinch strength following carpal tunnel decompression. Journal of hand surgery. 2004;29(4):390–392. doi: 10.1016/j.jhsb.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 28.O'Driscoll SW, Horii E, Ness R, Cahalan TD, Richards RR, An KN. The relationship between wrist position, grasp size, and grip strength. J Hand Surg Am. 1992;17(1):169–177. doi: 10.1016/0363-5023(92)90136-d. [DOI] [PubMed] [Google Scholar]
- 29.Li ZM. The influence of wrist position on individual finger forces during forceful grip. J Hand Surg Am. 2002;27(5):886–896. doi: 10.1053/jhsu.2002.35078. [DOI] [PubMed] [Google Scholar]
- 30.Harvey L, Herbert RD, Stadler M. Effect of wrist position on thumb flexor and adductor torques in paralysed hands of people with tetraplegia. Clin Biomech (Bristol, Avon) 2010;25(3):194–198. doi: 10.1016/j.clinbiomech.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Jesunathadas M, Laitano J, Hamm TM, Santello M. Across-muscle coherence is modulated as a function of wrist posture during two-digit grasping. Neurosci Lett. 2013;553:68–71. doi: 10.1016/j.neulet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston JA, Winges SA, Santello M. Neural control of hand muscles during prehension. Adv Exp Med Biol. 2009;629:577–596. doi: 10.1007/978-0-387-77064-2_31. [DOI] [PubMed] [Google Scholar]
- 33.Poston B, Danna-Dos Santos A, Jesunathadas M, Hamm TM, Santello M. Force-independent distribution of correlated neural inputs to hand muscles during three-digit grasping. J Neurophysiol. 2010;104(2):1141–1154. doi: 10.1152/jn.00185.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winges SA, Kornatz KW, Santello M. Common input to motor units of intrinsic and extrinsic hand muscles during two-digit object hold. J Neurophysiol. 2008;99(3):1119–1126. doi: 10.1152/jn.01059.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lannin NA, Horsley SA, Herbert R, McCluskey A, Cusick A. Splinting the hand in the functional position after brain impairment: a randomized, controlled trial. Arch Phys Med Rehabil. 2003;84(2):297–302. doi: 10.1053/apmr.2003.50031. [DOI] [PubMed] [Google Scholar]
- 36.Pizzi A, Carlucci G, Falsini C, Verdesca S, Grippo A. Application of a volar static splint in poststroke spasticity of the upper limb. Arch Phys Med Rehabil. 2005;86(9):1855–1859. doi: 10.1016/j.apmr.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Coppieters MW, Schmid AB, Kubler PA, Hodges PW. Description, reliability and validity of a novel method to measure carpal tunnel pressure in patients with carpal tunnel syndrome. Man Ther. 2012;17(6):589–592. doi: 10.1016/j.math.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Ebata T, Imai K, Tokunaga S, Takahasi Y, Abe Y. Thumb opposition in severe carpal tunnel syndrome with undetectable APB-CMAP. Hand Surg. 2014;19(2):199–204. doi: 10.1142/s0218810414500208. [DOI] [PubMed] [Google Scholar]