Abstract
Phaeochromocytomas are rare neuroendocrine tumours that produce catecholamines and numerous secretory proteins and peptides, including neuropeptide Y (NPY), a vasoactive peptide with influences on blood pressure. The production of catecholamines and NPY by phaeochromocytomas is highly variable. This study examined influences of hereditary factors and differences in catecholamine production on tumour expression of NPY, as assessed by quantitative PCR, enzyme immunoassay and immunohistochemistry. Phaeochromocytomas included hereditary adrenaline-producing tumours (adrenergic phenotype) in multiple endocrine neoplasia type 2 (MEN 2), predominantly noradrenaline-producing tumours (noradrenergic phenotype) in von Hippel–Lindau (VHL) syndrome, and other adrenergic and noradrenergic tumours where there was no clear hereditary syndrome. NPY levels in phaeochromocytomas from VHL patients were lower (P<0·0001) than in those from MEN 2 patients for both mRNA (84-fold difference) and the peptide (99-fold difference). These findings were supported by immunohistochemistry. NPY levels were also lower in VHL tumours than in those where there was no hereditary syndrome. Relative absence of expression of NPY in phaeochromocytomas from VHL patients when compared with other groups appears to be largely independent of differences in catecholamine production and is consistent with a unique phenotype in VHL syndrome.
Introduction
Phaeochromocytomas are rare neuroendocrine tumours of chromaffin cells characterised by synthesis, storage, release and local metabolism of catecholamines. Phaeochromocytomas also produce a diverse range of secretory proteins and peptides that are stored and processed within chromaffin granules from where they are coreleased with catecholamines, thereby potentially contributing to the clinical manifestations of these tumours (Parmer & Zinder 2002). Neuropeptide Y (NPY), a tyrosine-rich 36 amino acid peptide widely distributed throughout the central and peripheral nervous systems, including chromaffin cells of the adrenal medulla, is one such peptide that is produced in high quantities by phaeochromocytomas (Adrian et al. 1983, Lundberg et al. 1986, Grouzmann et al. 1989, O’Hare & Schwartz 1989, deS Senanayake et al. 1995, Bravo 2002, Spinazzi et al. 2005). NPY has powerful vasoconstrictor effects causing significant increases in blood pressure at plasma concentrations reached in several pathological conditions, including heart failure and phaeochromocytoma (Ullman et al. 2002). This has led some to suggest that approaches to antihypertensive management in phaeochromocytoma should take into account the possible effects of NPY, in addition to those of catecholamines (Bravo 2002).
Production of NPY by phaeochromocytomas is highly variable (Lundberg et al. 1986, Grouzmann et al. 1989, deS Senanayake et al. 1995), but the factors responsible for this variation are unclear. Previous studies suggested that differences in production of NPY may depend on whether tumours are malignant or benign (Grouzmann et al. 1989) or are derived from adrenal chromaffin cells or extra-adrenal paraganglia (Lundberg et al. 1986). Since adrenal and extra-adrenal tumours differ in expression of phenylethanolamine N-methyltransferase (PNMT), the enzyme that converts noradrenaline to adrenaline, lower expression of NPY in extra-adrenal when compared with adrenal tumours might reflect differences in adrenaline synthesis. Therefore, we examined whether the expression of NPY might be related to differences in catecholamine production. Four groups of tumours were examined, categorised by the presence or absence of a hereditary syndrome and by the production or lack of production of adrenaline (adrenergic vs noradrenergic phenotypes). Hereditary phaeochromocytomas included PNMT-expressing adrenergic tumours from patients with multiple endocrine neoplasia type 2 (MEN 2) and noradrenergic tumours that do not express PNMT from patients with von Hippel–Lindau (VHL) syndrome (Eisenhofer et al. 2001).
Materials and Methods
Patients and tumour specimens
Tumour specimens were from 56 patients with histologically confirmed phaeochromocytomas, including 16 patients with VHL syndrome, 12 with MEN 2A and 28 patients with phaeochromocytomas in whom there was no evidence of hereditary syndrome (Table 1). Tumour samples were obtained under clinical protocols approved by an Institutional Review Board, with informed consent from all patients.
Table 1.
Patient data
| MEN 2
|
VHL
|
No hereditary syndrome
|
|
|---|---|---|---|
| N | 12 | 16 | 28 |
| Gender (F/M) | 8/4 | 9/7 | 13/15 |
| Age (years; mean, range) | 40 (28–75) | 30 (11–60) | 52 (22–74) |
|
|
|||
Diagnosis of MEN 2 or VHL syndrome was confirmed by screening for germ-line mutations of the RET proto-oncogene or the VHL tumour suppressor gene. Approved clinical protocols allowed testing for germ-line mutations in 9 out of the 28 patients with phaeochromocytoma in whom there was no evidence of a hereditary syndrome. Among these nine patients, none was found to harbour germ-line mutations of the RET proto-oncogene, the VHL tumour suppressor gene, or the succinate dehydrogenase type D (SDHD) gene. One out of the nine patients was, however, found to have a variant of the succinate dehydrogenase type B (SDHB) gene, but as described elsewhere, this particular variant (p.Val140Phe) is not yet established to be a disease-causing mutation (Brouwers et al. 2006). Given varying reported frequencies of germ-line mutations of RET, VHL, SDHD and SDHB genes in patients with apparently sporadic phaeochromocytoma (Bornstein & Gimenez-Roqueplo 2006), it remains possible that 2–5 out of the 19 patients who were not tested may have harboured disease-causing mutations of those genes. For the purposes of this report, the 28 patients in whom there was no evidence of a hereditary syndrome are referred to as having non-syndromic phaeochromocytoma. All hereditary and non-syndromic adrenergic tumours had an adrenal location, whereas 5 out of the 15 non-syndromicnoradrenergic tumours had an extra-adrenal location.
Tissue procurement conditions
Samples of tumour tissue were obtained within 90-min surgical removal of phaeochromocytomas. Small samples of tumour tissue were dissected away, divided into smaller 5–100 mg pieces, placed on dry ice, or optimal cutting temperature blocks and then stored at −80 °C. Other samples were formalin-fixed and processed for paraffin embedding for subsequent immunohistochemistry or histopathology. Samples of adrenal medulla were obtained from resected tumours that included margins of normal adrenal tissue.
Determination of biochemical phenotypes
The biochemical phenotype of tumour specimens was determined by the analysis of tumour catecholamine concentrations, as described elsewhere (Eisenhofer et al. 2005). Tissue concentrations of noradrenaline and adrenaline were quantified by liquid chromatography with electrochemical detection (Eisenhofer et al. 2005). Samples of tumour tissue were weighed, frozen and homogenised in 5–10 vol. of 0·4 M perchloric acid containing 0·5 mM EDTA. Homogenates were centrifuged and supernatants collected for catecholamine determinations.
Quantitative PCR
RNA was extracted from frozen phaeochromocytoma tumour samples and reverse transcribed as previously described (Huynh et al. 2005). Quantitative PCR was performed with an ABI 7000 Sequence Detector (Applied Biosystems, Foster City, CA, USA) using PCR conditions consisting of 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Singleplex PCRs were performed using 20 ng cDNA template and 1× TaqMan Universal PCR MasterMix, with 0·9 μmol/l forward and reverse primers and 0·25 μmol/l probe for NPY (TaqMan Gene Expression Assays, Applied Biosystems), 0·3 μmol/l forward and reverse primers and 0·2 μmol/l probe for PNMT (Huynh et al. 2005) and with 0·15 μmol/l forward and reverse primers and 0·2 μmol/l probe for 18S RNA (TaqMan Endogenous Controls, Applied Biosystems). The expression of NPY and PNMT was normalised against 18S RNA and the levels of NPY and PNMT in phaeochromocytoma samples were then calculated relative to the expression levels of NPY or PNMT mRNA from normal human adrenal tissue according to previously described methods (Livak & Schmittgen 2001).
NPY peptide quantification and extraction
Small samples (5–100 mg) of frozen phaeochromocytoma tumour tissue were boiled in 750 μl of 0·1 M acetic acid for 10 min, homogenised for 25 s and centrifuged for 15 min at 4000 g. Supernatants were then collected and lyophilised. Dried residues were reconstituted in 250 μl assay buffer, with subsequent dilutions performed as necessary to enable quantification of NPY concentrations in 50 μl samples using a commercially available enzyme immunoassay (Bachem, King of Prussia, PA, USA). Concentrations of NPY were calculated relative to a standard curve generated using GraphPad Prism 4 software (GraphPad Software, San Diego, CA, USA) and converted into units of picomolar NPY per gram of tumour tissue according to sample dilution and the original wet weight of the sample of tumour tissue that was processed.
Immunohistochemistry
Immunohistochemical staining was performed on formalin-fixed paraffin-embedded (5 μm sections of phaeochromocytoma tumour tissue according to the previously described methods (Cleary et al. 2005). Samples examined for NPY staining included tumours from six patients with VHL syndrome, seven patients with MEN 2 and seven patients with tumours where there was no obvious hereditary basis or syndrome. The primary antibody consisted of a polyclonal rabbit anti-NPY antibody (1:100; Bachem), coupled with a donkey anti-rabbit Cy3 secondary antibody (1:500, Jackson Immuno, Westgrove, PA, USA). Negative control sections of phaeochromocytoma were also processed concurrently according to the same methods, with omission of the primary antibody. Sections were examined using a Zeiss LSM 510 ‘405’ confocal microscope at 543 nm excitation and with a LP 560 emission filter. All images were acquired with identical settings to allow for comparison. Images were processed using Zeiss LSM Image Browser software (Carl Zeiss, Oberkochen, Germany) without modifications to subsequent images. Staining was described as negligible, moderate or intense, and the distribution pattern of immunoreactive cells was noted.
Statistical analysis
NPY mRNA and peptide levels showed non-normal distributions. The data were therefore logarithmically transformed before statistical analysis and were presented as geometric means with standard errors calculated for either side of mean values. ANOVA, with post hoc tests by Sheffe’s method, was used to assess for differences among all four groups of tumours. Relationships between variables were examined by linear regression analysis, with significance determined using Pearson’s correlation coefficient.
Results
Tumour biochemical phenotypes
Among the 28 patients with MEN 2 and VHL syndrome, tumour tissue concentrations of adrenaline ranged widely over four orders of magnitude, from 0·002 to 91·8 μmol/g, while the concentrations of noradrenaline ranged less widely from 1·73 to 116·6 μmol/g. Tissue concentrations of adrenaline in tumours from VHL patients were <1% of the concentrations in tumours from MEN 2 patients, whereas tissue concentrations of noradrenaline in VHL tumours were a third of those in MEN 2 tumours (Fig. 1A).
Figure 1.
Tumour concentrations of adrenaline and noradrenaline (A) and relative contents of adrenaline and noradrenaline, expressed as percentage of total tumour catecholamine contents (B), in phaeochromocytomas from patients with VHL syndrome, MEN 2, and patients with no clear hereditary syndrome (non-syndromic tumours). Tumours in patients from the latter group were divided according to previously published criteria (Eisenhofer et al. 2004) into those that produced predominantly noradrenaline, designated with a noradrenergic (NA) phenotype, and those that produced significant amounts of adrenaline, designated with an adrenergic (A) phenotype. Results are arithmetic means±S.D. (A) *Higher (P<0·0001) adrenaline levels in either MEN 2 or non-syndromic adrenergic tumours than in either VHL or non-syndromic noradrenergic tumours; †higher (P=0·011) tissue noradrenaline concentrations in MEN 2 than in non-syndromic noradrenergic tumours. (B) *Higher (P<0·0001) percent contents of adrenaline in MEN 2 or non-syndromic adrenergic tumours when compared with either VHL or non-syndromic noradrenergic tumours. (B) *Also denotes lower (P<0·0001) percent contents of noradrenaline in MEN 2 or non-syndromic adrenergic tumours when compared with either VHL or non-syndromic noradrenergic tumours.
Among the 28 patients in whom there was no evidence of a hereditary syndrome, tumour tissue concentrations of adrenaline also varied widely over four orders of magnitude, from 0·002 to 36·2 μmol/g, while the concentrations of nor-adrenaline ranged from 0·105 to 34·6 μmol/g. Fifteen of those tumours contained <5% adrenaline and more than 95% noradrenaline and were defined with a noradrenergic phenotype. The other 13 tumours contained more than 11% adrenaline and were defined with an adrenergic phenotype.
For the tumours with a noradrenergic phenotype from the patients without evidence of a hereditary syndrome, the proportion of adrenaline averaged 1·5% (range 0·02–4·5%) of the total catecholamine content, and was similar to that (2·1%, range 0·1–9·8%) for the tumours from patients with VHL syndrome (Fig. 1B). For the tumours with an adrenergic phenotype, the proportion of adrenaline averaged 44·8% (range 11–86·1%), similar to that in the tumours from MEN 2 patients where the proportion of adrenaline was 43·2% (range 8·9–67·8%).
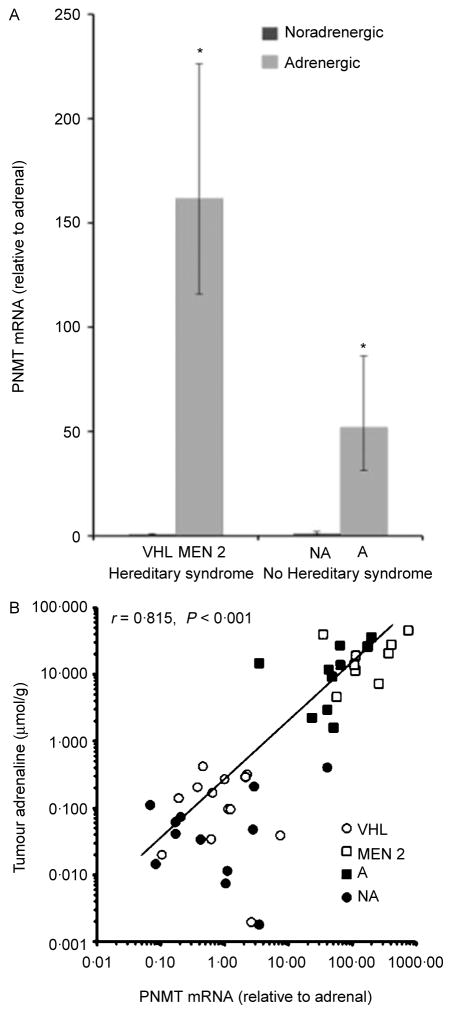
Quantitative PCR indicated that MEN 2 tumours possessed 200-fold higher (P<0·0001) levels of PNMT mRNA than VHL tumours (Fig. 2A). The PNMT mRNA levels in non-syndromic adrenergic tumours were also 64-fold higher (P<0·0001) than in VHL tumours. Tumour tissue PNMT mRNA levels were 50-fold higher (P<0·0001) in non-syndromic adrenergic tumours than in non-syndromic noradrenergic tumours. Tumours from MEN 2 patients had 153-fold higher (P<0·0001) PNMT mRNA levels than in non-syndromic noradrenergic tumours. Linear regression analysis indicated a strong positive relationship (r=0·815, P<0·001) between tumour PNMT mRNA levels and tumour tissue adrenaline levels (Fig. 2B).
Figure 2.
Expression of tumour tissue PNMT mRNA levels in phaeochromocytomas from patients with VHL syndrome, MEN 2, and patients with no clear hereditary syndrome (non-syndromic tumours) is shown in (A). Non-syndromic tumours include those with adrenergic (A) and noradrenergic (NA) phenotypes, as defined in Fig. 1. The relationship between tumour PNMT mRNA levels and adrenaline concentrations is shown in (B). Results in (A) are shown as geometric means with standard errors estimated from logarithmically transformed data. Levels of PNMT mRNA are expressed relative to levels in human adrenal and are derived from 15 VHL tumour samples, 9 MEN 2 tumours, 12 non-syndromic noradrenergic tumours and 11 adrenergic tumours. ***P<0·0001 denotes higher expression than in VHL tumours.
NPY mRNA and peptide levels
Similar to the findings for tumour contents of adrenaline and expression of PNMT mRNA, phaeochromocytoma tumour tissue levels of NPY mRNA and peptide varied considerably among tumour samples; NPY mRNA expression and peptide levels varied by four orders or more in magnitude among different tumour samples. Expression of NPY mRNA correlated positively (r=0·73, P<0·0001) with tumour tissue concentrations of NPY peptide (Fig. 3).
Figure 3.

Relationship between phaeochromocytoma tumour levels of NPY mRNA and peptide. (●) Noradrenergic hereditary and non-syndromic tumours and (■) adrenergic hereditary and non-syndromic tumours.
Quantitative PCR indicated an 84-fold higher (P<0·0001) level of NPY mRNA expression in phaeochromocytomas from MEN 2 patients than in those from VHL patients (Fig. 4A). NPY mRNA levels in adrenergic tumours, where there was no clear evidence of hereditary syndrome, were also 26-fold higher (P=0·003) than in VHL tumours. Tumour tissue NPY mRNA levels in non-syndromic adrenergic tumours also tended to be higher than in non-syndromic noradrenergic tumours (threefold difference), but this difference did not reach significance (P=0·55) and was much less than the 84-fold difference between hereditary adrenergic and noradrenergic tumours.
Figure 4.
Expression of tumour tissue NPY mRNA (A) and peptide (B) levels in noradrenergic (NA) and adrenergic (A) hereditary and non-syndromic phaeochromocytomas. Results are shown as geometric means with standard errors estimated from logarithmically transformed data. Levels of NPY mRNA are expressed relative to levels in human adrenal and are derived from 12 VHL tumour samples, 10 MEN 2 tumours, 11 non-syndromic noradrenergic tumours and 10 adrenergic tumours. Data for peptide levels are from 12 VHL tumours, 9 MEN 2 tumours, 10 non-syndromic noradrenergic tumours and 12 non-syndromic adrenergic tumours. Sample numbers reflect the availability of tissue extracts of mRNA or NPY peptide for each patient group. *P<0·0001, †P<0·001 and ‡P<0·01 denotes higher expression than in VHL tumours.
Similar to the results for levels of mRNA, tumour tissue NPY peptide levels were 99-fold higher (P<0·0001) in MEN 2 than in VHL tumours and 83-fold higher (P<0·0001) in non-syndromic adrenergic tumours than in VHL tumours (Fig. 4B). Tumour tissue NPY peptide levels were also 22-fold higher (P<0·001) in non-syndromic noradrenergic tumours than in VHL tumours. Tumour tissue levels of NPY peptide also tended to be higher (fourfold difference) in non-syndromic adrenergic than noradrenergic tumours, but again this difference did not reach significance (P=0·34) and was much less than the 99-fold difference between hereditary adrenergic (MEN 2) and noradrenergic (VHL) tumours.
Relationships of NPY with PNMT and adrenaline
Linear regression analysis indicated that tumour tissue levels of PNMT mRNA were positively related with tumour tissue levels of NPY mRNA (r=0·600, P<0·001) and peptide (r=0·626, P<0·001; Fig. 5A and B). Similarly, tumour tissue concentrations of adrenaline were positively related to both tumour tissue levels of NPY mRNA (r=0·425, P=0·0045) and tumour tissue concentrations of NPY peptide (r=0·603, P<0·0001; Fig. 5C and D).
Figure 5.
Relationships of tumour tissue levels of PNMT mRNA (A and B) or adrenaline (C and D) with NPY mRNA (A and C) or peptide (B and D). (●) Non-syndromic noradrenergic tumours; (■) non-syndromic adrenergic tumours; (○) VHL tumours; (□) MEN 2 tumours.
Relationships of tissue concentrations of adrenaline with the peptide remained significant when examined independently for tumours in patients with MEN 2 and VHL syndrome (r=0·679, P=0·0007) and for tumours in patients where there was no evidence of a hereditary syndrome (r=0·633, P=0·0016). There were no significant relationships between tumour tissue levels of NPY mRNA and tumour tissue concentrations of noradrenaline (r=0·022, P=0·889) or combined tissue concentrations of nor-adrenaline and adrenaline (r=0·087, P=0·557). Tumour tissue levels of NPY peptide were positively related to tumour tissue concentrations of noradrenaline (r=0·327, P=0·027) and combined tissue concentrations of noradrenaline and adrenaline (r=0·465, P=0·002), but these relationships were weaker than those with tissue concentrations of adrenaline.
NPY immunohistochemistry
Staining of tissue samples with antibodies against NPY demonstrated clear differences between MEN 2 and VHL tumours, but no obvious differences between adrenergic and noradrenergic tumours in patients with non-syndromic phaeochromocytoma (Fig. 6). Intense staining for NPY was observed in all sections from the seven MEN 2 tumours examined. Staining was uniformly distributed in six specimens from MEN 2 patients, while intense staining of large numbers of clusters of cells was observed in the seventh specimen. In contrast, all six VHL tumours examined showed negligible to moderate cytoplasmic staining in isolated clusters, with occasional clusters of intense staining. The staining pattern in non-syndromic noradrenergic tumours (n=3) ranged from moderate to intense staining in clusters of cells throughout the section, to intense immunoreactivity throughout the entire section. Staining in adrenergic tumours (n=4) was moderate to intense throughout all examined sections; however, overall no discernable differences were noted between these two groups of tumours.
Figure 6.
Immunohistochemical staining of NPY in representative samples of phaeochromocytomas from patients with MEN 2 (A), VHL syndrome (B) and non-syndromic adrenergic and noradrenergic phaeochromocytomas (C and D). Arrows in (B) point to isolated clusters of cells with intense NPY staining in VHL tumour specimen. Scale bar: 50 μm.
Discussion
This study demonstrating near absence of NPY in phaeochromocytomas from patients with VHL syndrome when compared with tumours from other groups of patients adds to an increasing body of evidence that the phaeochromocytomas in VHL syndrome have a unique phenotype (Eisenhofer et al. 2001, Koch et al. 2002, Huynh et al. 2005, Vogel et al. 2005).
The development of hereditary phaeochromocytomas with different catecholamine phenotypes has been suggested to reflect origins from different populations of PNMT-negative noradrenergic and PNMT-positive adrenergic chromaffin cells (Eisenhofer et al. 2001, 2004). Interestingly, the expression of NPY in the normal adrenal gland is confined to chromaffin cells that express PNMT and produce adrenaline (Lundberg et al. 1986, Henion & Landis 1990, Wolfensberger et al. 1995). The above observations, together with our findings of positive relationships between tissue PNMT mRNA or adrenaline levels with NPY expression and the differences in NPY expression between VHL and MEN 2 tumours, might suggest that the expression of PNMT and NPY is linked. Our additional finding of non-significant differences in NPY expression between adrenergic and noradrenergic non-syndromic tumours argues against this hypothesis. The significantly lower levels of NPY in tumours from VHL patients than in all other groups examined, including non-syndromic noradrenergic tumours, strongly indicates that the near 100-fold difference in NPY expression between hereditary tumours reflects the influence of a VHL gene mutation rather than an influence of adrenaline content or production.
Analysis of genes activated in hereditary phaeochromocytomas suggests that development of these tumours can be explained by a single pathway linking mutations in disease-causing genes to a failure of apoptosis after withdrawal of growth factors during chromaffin cell development (Lee et al. 2005). According to this proposal, inappropriate survival of embryonic chromaffin progenitor cells, arrested in their development, would lead, later in life, to a propensity for these cells to develop into tumours. The particular phenotype of the tumour would further depend on different susceptibilities of the pathway to the effects of mutations of specific genes during particular stages of chromaffin cell maturation. During development of the embryonic adrenal gland, NPY is expressed in precursor chromaffin cells as they invade the adrenal mesodermal anlagen, and before those precursor cells begin to express PNMT (Henion & Landis 1990). Relative lack of expression of both PNMT and NPY in VHL tumours might therefore reflect their development from more primitive chromaffin progenitor cells (i.e. those that do not yet express PNMT or NPY) than those giving rise to tumours in MEN 2, where NPY and PNMT are both expressed, or those in non-syndromic cases, where NPY and PNMT are coexpressed more variably.
The molecular events during development that link the various mutations to the diverse phenotypic variants of phaeochromocytoma are unknown, but could be explored in appropriately selected cell systems by experimental manipulations of key genes responsible for the disease or of the elements known to regulate downstream-affected genes. Expression of NPY in cells of the sympathetic nervous system is induced by nerve growth factor (NGF; Minth-Worby 1994). Abnormal NGF signalling during embryonic development of chromaffin cells with resulting failure of apoptosis is suggested to contribute to development of phaeochromocytomas (Lee et al. 2005). Interestingly, the inducing action of NGF on NPY expression is blocked in early embryonic adrenal chromaffin cells and in neurosecretory-deficient rat phaeochromocytoma (PC12) cell lines, indicating inhibition of NPY expression by an as yet unidentified repressor (Barreto-Estrada et al. 2003, Pance et al. 2006). Such a repressor, held active as a downstream consequence of mutations of the VHL gene, might also contribute to the relative lack of expression of NPY in phaeochromocytomas that subsequently develop from affected embryonic chromaffin cells. Such stem cells might provide the most appropriate system to test an effect of VHL mutations on NPY expression.
The results of this study are consistent with previous reports of considerable variability in the expression of NPY in phaeochromocytoma tumour tissue and in plasma levels of the peptide in patients with the tumour (Adrian et al. 1983, Lundberg et al. 1986, Grouzmann et al. 1989, O’Hare & Schwartz 1989, deS Senanayake et al. 1995). Variable tumour expression and plasma levels of the peptide have been suggested in other studies to contribute to differences in disease presentation, including presence of hypertension and left ventricular hypertrophy (Connell et al. 1987, deS Senanayake et al. 1995, Eurin et al. 2000, Bravo 2002, Kuch-Wocial et al. 2004). This possibility is supported by findings that the expression of NPY in phaeochromocytoma tumour tissue determines elevations in plasma levels of the peptide, which in some patients may reach 6·16 nmol/l (26 μg/l; deS Senanayake et al. 1995). Such levels are well in excess of those shown during i.v. infusion of NPY to cause significant increases in blood pressure (Ullman et al. 2002), indicating that the release of NPY from phaeochromocytomas may easily contribute to increased blood pressure.
During screening for phaeochromocytoma in VHL patients, Walther and colleagues (Walther et al. 1999) observed that only 16% of patients with the tumour had hypertension and 8% symptoms of catecholamine excess. Although hypertension in VHL patients with phaeochromocytoma was noted to be more prevalent (50%) when screening was not employed, this prevalence was still lower than in patients with other forms of phaeochromocytoma, where 92% were hypertensive. Other comparisons of patients screened for hereditary phaeochromocytoma further confirmed a lower prevalence of hypertension in VHL than in MEN 2 patients (18 vs 40%) and showed that this difference was independent of tumour size (Eisenhofer et al. 2001). Moreover, as a function of tumour size, VHL patients had larger increases in plasma catecholamines, yet had a lower prevalence of hypertension than MEN 2 patients with phaeochromocytoma.
The above observations suggest that factors other than increases in plasma catecholamines contribute to differences in clinical presentation of the tumour. The relative lack of expression of NPY in tumours from VHL patients observed here suggests one such alternative factor that might contribute to the lower prevalence of hypertension in VHL patients than in other patients with phaeochromocytoma. Establishing this possibility requires further studies with measurements of plasma concentrations of NPY in relation to presentation of signs and symptoms. Since the plasma half-life of NPY is only about 12 min (Grouzmann et al. 2001), and because the peptide is cosecreted with catecholamines, such studies will require accounting for the more episodic nature of catecholamine secretion in MEN 2 tumours when compared with the more continuous secretion in VHL tumours.
In summary, this study establishes much lower expression of NPY in phaeochromocytomas from VHL patients than in other forms of the tumour. This difference supports the presence of a distinct phenotype of phaeochromocytomas in VHL patients.
Acknowledgments
This work was supported by the intramural programs of the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, the National Human Genome Research Institute, the Center for Information Technology and the National Cancer Institute, Center for Cancer Research, at the National Institutes of Health.
Footnotes
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Adrian TE, Allen JM, Terenghi G, Bacarese-Hamilton AJ, Brown MJ, Polak JM, Bloom SR. Neuropeptide Y in phaeochromocytomas and ganglioneuroblastomas. Lancet. 1983;2:540–542. doi: 10.1016/s0140-6736(83)90570-6. [DOI] [PubMed] [Google Scholar]
- Barreto-Estrada JL, Medina-Ortiz WE, Garcia-Arraras JE. The morphological and biochemical response of avian embryonic sympatho-adrenal cells to nerve growth factor is developmentally regulated. Brain Research Developmental Brain Research. 2003;144:1–8. doi: 10.1016/s0165-3806(03)00129-9. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Gimenez-Roqueplo AP. Genetic testing in pheochromocytoma: increasing importance for clinical decision making. Annals of the New York, Academy of Sciences. 2006;1073:94–103. doi: 10.1196/annals.1353.010. [DOI] [PubMed] [Google Scholar]
- Bravo EL. Pheochromocytoma: an approach to antihypertensive management. Annals of the New York Academy of Sciences. 2002;970:1–10. doi: 10.1111/j.1749-6632.2002.tb04408.x. [DOI] [PubMed] [Google Scholar]
- Brouwers FM, Eisenhofer G, Tao JJ, Kant JA, Adams KT, Linehan WM, Pacak K. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. Journal of Clinical Endocrinology and Metabolism. 2006;91:4505–4509. doi: 10.1210/jc.2006-0423. [DOI] [PubMed] [Google Scholar]
- Cleary S, Brouwers FM, Eisenhofer G, Pacak K, Christie DL, Lipski J, McNeil AR, Phillips JK. Expression of the noradrenaline transporter (NAT) and phenylethanolamine N-methyltransferase (PNMT) in normal human adrenal gland and phaeochromocytoma. Cell and Tissue Research. 2005;322:443–453. doi: 10.1007/s00441-005-0026-y. [DOI] [PubMed] [Google Scholar]
- Connell JM, Corder R, Asbury J, Macpherson S, Inglis GC, Lowry P, Burt AD, Semple PF. Neuropeptide Y in multiple endocrine neoplasia: release during surgery for phaeochromocytoma. Clinical Endocrinology. 1987;26:75–84. doi: 10.1111/j.1365-2265.1987.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Walther MM, Huynh TT, Li ST, Bornstein SR, Vortmeyer A, Mannelli M, Goldstein DS, Linehan WM, Lenders JW, et al. Pheochromocytomas in von Hippel–Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. Journal of Clinical Endocrinololgy and Metabolism. 2001;86:1999–2008. doi: 10.1210/jcem.86.5.7496. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, Munson PJ, Mannelli M, Goldstein DS, Elkahloun AG. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel–Lindau syndrome. Endocrine-Related Cancer. 2004;11:897–911. doi: 10.1677/erc.1.00838. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Goldstein DS, Mannelli M, Csako G, Walther MM, Brouwers FM, Pacak K. Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of free metanephrines. Clinical Chemistry. 2005;51:735–744. doi: 10.1373/clinchem.2004.045484. [DOI] [PubMed] [Google Scholar]
- Eurin J, Barthelemy C, Masson F, Maistre G, Soualmia H, Noe E, Sarfati E, Eurin B, Carayon A. Release of neuropeptide Y and hemodynamic changes during surgical removal of human pheochromocytomas. Regulatory Peptides. 2000;86:95–102. doi: 10.1016/s0167-0115(99)00092-0. [DOI] [PubMed] [Google Scholar]
- Grouzmann E, Comoy E, Bohuon C. Plasma neuropeptide Y concentrations in patients with neuroendocrine tumors. Journal of Clinical Endocrinology and Metabolism. 1989;68:808–813. doi: 10.1210/jcem-68-4-808. [DOI] [PubMed] [Google Scholar]
- Grouzmann E, Fathi M, Gillet M, de Torrente A, Cavadas C, Brunner H, Buclin T. Disappearance rate of catecholamines, total metanephrines, and neuropeptide Y from the plasma of patients after resection of pheochromocytoma. Clinical Chemistry. 2001;47:1075–1082. [PubMed] [Google Scholar]
- Henion PD, Landis SC. Asynchronous appearance and topographic segregation of neuropeptide-containing cells in the developing rat adrenal medulla. Journal of Neuroscience. 1990;10:2886–2896. doi: 10.1523/JNEUROSCI.10-09-02886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh TT, Pacak K, Brouwers FM, Abu-Asab MS, Worrell RA, Walther MM, Elkahloun AG, Goldstein DS, Cleary S, Eisenhofer G. Different expression of catecholamine transporters in phaeochromocytomas from patients with von Hippel–Lindau syndrome and multiple endocrine neoplasia type 2. European Journal of Endocrinology. 2005;153:551–563. doi: 10.1530/eje.1.01987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CA, Mauro D, Walther MM, Linehan WM, Vortmeyer AO, Jaffe R, Pacak K, Chrousos GP, Zhuang Z, Lubensky IA. Pheochromocytoma in von Hippel–Lindau disease: distinct histopathologic phenotype compared to pheochromocytoma in multiple endocrine neoplasia type 2. Endocrine Pathology. 2002;13:17–27. doi: 10.1385/ep:13:1:17. [DOI] [PubMed] [Google Scholar]
- Kuch-Wocial A, Slubowska K, Kostrubiec M, Pasierski T, Januszewicz W, Switalska H, Wocial B, Pruszczyk P. Plasma neuropeptide Y immunoreactivity influences left ventricular mass in pheochromocytoma. Clinica Chimica Acta. 2004;345:43–47. doi: 10.1016/j.cccn.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, Farese RV, Freeman RS, Carter BD, Kaelin WG, Jr, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8:155–167. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hokfelt T, Hemsen A, Theodorsson-Norheim E, Pernow J, Hamberger B, Goldstein M. Neuropeptide Y-like immunoreactivity in adrenaline cells of adrenal medulla and in tumors and plasma of pheochromocytoma patients. Regulatory Peptides. 1986;13:169–182. doi: 10.1016/0167-0115(86)90224-7. [DOI] [PubMed] [Google Scholar]
- Minth-Worby CA. Transcriptional regulation of the human neuropeptide Y gene by nerve growth factor. Journal of Biological Chemistry. 1994;269:15460–15468. [PubMed] [Google Scholar]
- O’Hare MM, Schwartz TW. Expression and precursor processing of neuropeptide Y in human pheochromocytoma and neuroblastoma tumors. Cancer Research. 1989;49:7010–7014. [PubMed] [Google Scholar]
- Pance A, Livesey FJ, Jackson AP. A role for the transcriptional repressor REST in maintaining the phenotype of neurosecretory-deficient PC12 cells. Journal of Neurochemistry. 2006;99:1435–1444. doi: 10.1111/j.1471-4159.2006.04190.x. [DOI] [PubMed] [Google Scholar]
- Parmer RJ, Zinder O. Catecholaminergic pathways, chromaffin cells, and human disease. Annals of the New York Academy of Sciences. 2002;971:497–505. doi: 10.1111/j.1749-6632.2002.tb04514.x. [DOI] [PubMed] [Google Scholar]
- deS Senanayake P, Denker J, Bravo EL, Graham RM. Production, characterization, and expression of neuropeptide Y by human pheochromocytoma. Journal of Clinical Investigation. 1995;96:2503–2509. doi: 10.1172/JCI118310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi R, Andreis PG, Nussdorfer GG. Neuropeptide-Y and Y-receptors in the autocrine-paracrine regulation of adrenal gland under physiological and pathophysiological conditions (Review) International Journal of Molecular Medicine. 2005;15:3–13. [PubMed] [Google Scholar]
- Ullman B, Pernow J, Lundberg JM, Astrom H, Bergfeldt L. Cardiovascular effects and cardiopulmonary plasma gradients following intravenous infusion of neuropeptide Y in humans: negative dromotropic effect on atrioventricular node conduction. Clinical Science. 2002;103:535–542. doi: 10.1042/cs1030535. [DOI] [PubMed] [Google Scholar]
- Vogel TW, Brouwers FM, Lubensky IA, Vortmeyer AO, Weil RJ, Walther MM, Oldfield EH, Linehan WM, Pacak K, Zhuang Z. Differential expression of erythropoietin and its receptor in von Hippel–Lindau-associated and multiple endocrine neoplasia type 2-associated pheochromocytomas. Journal of Clinical Endocrinololgy and Metabolism. 2005;90:3747–3751. doi: 10.1210/jc.2004-1899. [DOI] [PubMed] [Google Scholar]
- Walther MM, Reiter R, Keiser HR, Choyke PL, Venzon D, Hurley K, Gnarra JR, Reynolds JC, Glenn GM, Zbar, et al. Clinical and genetic characterization of pheochromocytoma in von Hippel–Lindau families: comparison with sporadic pheochromocytoma gives into natural history of pheochromocytoma. Journal of Urology. 1999;162:659–664. doi: 10.1097/00005392-199909010-00004. [DOI] [PubMed] [Google Scholar]
- Wolfensberger M, Forssmann WG, Reinecke M. Localization and coexistence of atrial natriuretic peptide (ANP) and neuropeptide Y (NPY) in vertebrate adrenal chromaffin cells immunoreactive to TH, DBH and PNMT. Cell and Tissue Research. 1995;280:267–276. doi: 10.1007/BF00307798. [DOI] [PubMed] [Google Scholar]