Abstract
Background
Visceral adipose tissue (VAT) measured by computed tomography (CT) is related to insulin resistance, lipids, and serum inflammatory markers. Our objective was to compare the strength of the associations of visceral adipose tissue (VAT) measured with dual energy x-ray absorptiometry (DXA-VAT) and computed tomography (CT-VAT) with insulin resistance, serum lipids, and serum markers of inflammation.
Methodology
For 1,117 men age 65 and older enrolled in the Study of Osteoporotic Fractures in Men (MrOS), the cross-sectional associations of DXA-VAT and CT-VAT with Homeostasis Model Assessment of insulin resistance (homa2ir), C-reactive protein (CRP), and HDL cholesterol were estimated with regression models, and compared using a Hausmann test.
Results
Adjusted for age and body mass index (BMI), DXA-VAT was moderately associated with homa2ir (effect size 0.38, 95% C.I. 0.28 to 0.47) and modestly associated with HDL cholesterol (DXA effect size −0.29, 95% C.I. −0.38 to −0.21). These associations were significantly greater than for CT-VAT with homa2ir (0.30, 95% C.I. 0.24 to 0.37; p-value for effect size difference 0.03) and CT-VAT with HDL cholesterol (-0.22, 95% C.I. −0.29 to −0.15; p-value for difference 0.005). Neither DXA-VAT nor CT-VAT were associated with CRP after adjustment for age and BMI (DXA-VAT effect size 0.14, 95% C.I. −0.04 to 0.32; CT-VAT effect size 0.08, 95% C.I. −0.08 to 0.25; p-value for difference 0.35).
Conclusion
DXA-VAT has similar or greater associations with insulin resistance, and HDL cholesterol as does CT-VAT in older men, confirming the concurrent validity of DXA-VAT. Investigations of how well DXA measurements of VAT predict incident cardiovascular disease events are warranted.
Keywords: Visceral Adipose Tissue (VAT), DXA-VAT, CT-VAT, Insulin Resistance, Serum Inflammatory Markers
Introduction
Abdominal visceral adipose tissue (VAT) measures from computed tomography (CT) scans have been shown to predict subsequent cardiovascular disease events(1–4), and may be a risk factor for cognitive decline(5, 6). These associations are postulated to be mediated in part through raised inflammatory cytokines and insulin resistance. VAT is infiltrated with macrophages and other inflammatory cells, and its presence is associated with higher levels of serum inflammatory markers(7). Moreover, VAT is associated with raised fasting insulin levels, lower HDL cholesterol, and raised triglycerides (8). CT scans are considered a gold standard measure of VAT,(9) but are expensive and involve radiation exposure associated risks.
VAT can be estimated on dual energy X-ray absorptiometry (DXA) scans obtained for body composition analysis, at lower cost and radiation exposure than CT scans. DXA measures of VAT are highly correlated with CT(10–13) and MRI(13, 14) measures of VAT, and DXA-VAT has been shown to be associated with several cardio-metabolic risk factors (raised systolic and diastolic blood pressure, low HDL cholesterol, high triglycerides, and high fasting glucose).(15) However, no study has compared how well DXA-VAT predicts these and other biologic phenomena compared to a gold standard measure of VAT, such as CT.
Our objectives were to estimate the cross-sectional associations of DXA-VAT with; a) insulin resistance, fasting insulin, and fasting glucose; b) serum inflammatory markers (C-reactive protein [CRP], tumor necrosis factor alpha [TNF-α], and interleukin-6 [IL-6]); and c) serum lipid levels (HDL cholesterol, triglycerides, and LDL-cholesterol); and d) to compare the strength of the associations of these biologic phenomena with DXA-VAT vs. CT-VAT.
Materials and Methods
Between 2000 and 2002, the Osteoporotic Fractures in Men (MrOS) study enrolled 5,994 community-dwelling ambulatory men age 65 years and older at six geographic sites in the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA), as described in previous publications(16).
Measurement of Visceral Adipose Tissue (VAT) with Computed Tomography (CT-VAT)
At the baseline visit, the first 650 enrollees and all non-Caucasian men at each study site had CT scans of the abdomen, using a standardized protocol and cross calibrated across the six study enrollment sites.(17) In 2006, 1300 of these men were randomly selected to have measures of abdominal regional fat depots within a 5 mm slice at the L4-5 intervertebral disc space using a standardized protocol and commercially available software, with each voxel having dimensions of 5 mm × 1 mm × 1mm (AnalyzeDirect, Overland Park, KS). As described in prior publications,(18, 19) adipose tissue (AT) and muscle tissue were recognized by voxels having Hounsfield Units (HU) in the ranges of respectively, -190 to -30 and 0 to 100. The fascial borders of the skeletal muscles were traced manually and segmented out of the image. A closed contour was then drawn in the space created by removal of the skeletal muscle wall. SAT was defined as voxels outside of the contour and with HU in the range for AT (Figure 1). VAT was defined as voxels within the contour and with HU in the AT range (Figure 1). For each depot, volumes (cm3) were computed automatically by the software as the number of voxels multiplied by the voxel volume. Inter- and intra-reader reliability was monitored throughout image processing with intra-class correlations coefficients (ICC), and ICCs were all ≥0.94 for each tissue measure.(18)
Figure 1. Adipose Tissue Compartments on Computed Tomography (brown areas, Panel A)* and on DXA (Panel B)ˆ.

*CT-VAT inside and CT-SAT outside of abdominal wall musculature (marked by red circumference)
ˆDXA-VAT is Total Adipose Tissue overlying and within visceral cavity minus estimated SAT overlying visceral cavity. SAT overlying visceral cavity is estimated from SAT lateral to the abdominal wall musculature.
Measurement of VAT with Dual Energy X-ray Absorptiometry (DXA-VAT)
Whole body scans were obtained on Hologic QDR4500W densitometers for all men at the baseline visit. A Hologic whole body phantom was circulated and measured at the 6 enrollment sites. The variability across clinics was within acceptable limits, and cross-calibration correction factors were not required. To adjust for inter-clinic differences, statistical models include indicator variables for the individual scanners.
In 2016 the baseline whole body DXA scans for the 1300 men who had adipose tissue measurements from CT were re-analyzed centrally with Hologic APEX software version 5.5 to obtain visceral adipose tissue (VAT) measurements, using a standard algorithm.(20) This estimates total adipose tissue within a 5 cm transverse slice, the inferior border of which is placed at the top of the iliac crests (at about the L4 vertebral level) on the 2-dimensional projection of the abdominal- pelvic region. The lateral and medial edges of the abdominal wall musculature are identified (Figure 1, panel B); all of the adipose tissue in the areas outside of the lateral edges is subcutaneous adipose tissue (SAT). VAT is contained in the visceral cavity inside the medial edges of the abdominal wall musculature (Figure 1, panel B), but this area also includes SAT anterior and posterior to the abdominal wall musculature superimposed on the 2-dimensional projection. The amount of SAT in the medial VAT area can be estimated from the SAT lateral to the abdominal wall musculature, and the estimated visceral fat area (cm2) is then calculated as total adipose tissue overlying and within the visceral cavity minus the SAT overlying this area.
Serum glucose metabolism markers and lipid levels
As described in previous publications,(21, 22) fasting insulin was measured by a two site immunoenzymometric assay, with inter- and intra-assay coefficients of variation <10%. The assay was accurate down to a concentration of 2 micro-units per ml. Fasting glucose was measured enzymatically with an inter-assay coefficient of variation was <3%. Homeostasis model assessment of insulin resistance (homa2ir) was calculated using the algorithm of Matthews and colleagues,(23) which calculates homa2ir as a non-linear function of fasting insulin and fasting glucose. Fasting triglycerides, HDL cholesterol, and LDL cholesterol were each directly measured at the Oregon Veterans Administration Medical Center Laboratory.
Serum Inflammatory Markers
In 2010, a random sample subset of 982 men who attended the baseline visit had levels of C-reactive protein (CRP), tumor necrosis alpha (TNF-α), and interleukin-6 (IL-6) measured at the Laboratory for Clinical Biochemistry Research, University of Vermont on sera that had been stored at -80 degrees Celsius since the baseline MrOS visit, as described in detail in a prior publication.(24) A subset (230) of these men had also previously been included in the random sample of 1300 men who had adipose tissue measurements done on abdominal CT. The assay range for CRP was 0.16 – 1100 ug/mL, and the inter-assay CVs range was 1.52 to 3.68%. The assay range for IL-6 was 0.16 – 12.0 pg/mL; expected values in normal, healthy individuals are <10 pg/mL, and the inter-assay CVs range was 6.11 to 8.47%. The assay range for TNF-α was 0.13–2000 pg/mL, with inter-assay CVs ranging from 4.93 to 9.13%.
Statistical Analysis
Linear regression models using Stata 14.0 (Stata Corp, College Station, TX) were run to estimate the associations of both DXA-VAT with CT-VAT with each serum glucose metabolism marker, each serum lipid level, and each serum inflammatory marker. Models were tested for heteroscedasticity, for omitted variable bias with the Ramsey Powers test, and for model mis-specification with Pregibon’s link test.(25) We used log transformed values of DXA-VAT and CT-VAT on the basis of model diagnostics. Parameter coefficients were expressed as effect sizes (per standard deviation change of log [VAT]), so that the strength of associations for DXA-VAT and CT-VAT would be directly comparable. The base models were adjusted only for age and study enrollment site, and then further adjustment included BMI to test for the added value of VAT above and beyond this clinical measure in prediction of the outcome measures.
Transformations of fasting glucose as a continuous variable did not yield acceptable model diagnostics; hence we considered two clinical fasting glucose categories (normal: <100mg/dl and impaired fasting glucose/diabetic: ≥100mg/dl) and used logistic regression in place of linear regression, and tested model fit using the Hosmer-Lemeshow test.(26) The model based discrimination between those with impaired vs normal fasting glucose was assessed by the area under receiving operating characteristics (AUROC) curve.
For each serum glucose metabolism parameter, serum lipid level, and serum inflammatory marker, the null hypothesis that the standardized effect size of association with DXA-VAT was the same as the association with CT-VAT was tested with a modified Hausmann test, using the suest command of Stata.
Fasting insulin resistance, insulin, and glucose might be affected by use of hypoglycemic agents, potentially confounding the relationship between VAT and these outcomes. Therefore we conducted sensitivity analyses for these outcomes excluding men using hypoglycemic agents.
Results
Of the 1300 men randomly selected to have abdominal adipose tissue deposits measured on CT scans, 1296 had useable DXA whole body scans, and of these 1117 also had a valid CT measure of VAT (Figure 2). Of these, 1026 had fasting insulin and glucose values measured, 1042 had lipid levels measured, and 230 had serum inflammatory markers measured with further exclusions detailed for each specific outcome (Figure 2).
Figure 2.
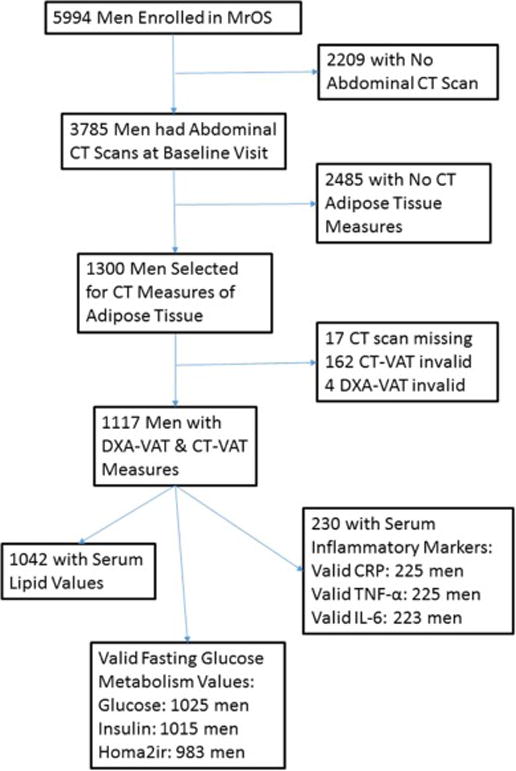
Flow Diagram for Selection of Men Included in Analyses
The majority of the 1117 men with valid measures of both DXA-VAT and CT-VAT were non-Hispanic White (Table 1). More than one fifth of the study sample had BMI > 30 kg/m2 and over half were overweight (BMI between 25 and 29.9 kg/m2). The association between the standardized values of CT-VAT and DXA-VAT (Figure 3, panel A) was non-linear, with a correlation of 0.72. The association log transformed values of CT-VAT and DXA-VAT (Figure 3, panel B) was nearly linear, with a correlation of 0.78.
Table 1.
Characteristics of Study Population (Men with Valid Baseline CT-VAT & DXA-VAT Measures)
| Characteristic | Mean (SD), Median (IQR) or N (Frequency,%) | |
|---|---|---|
| Age, years (n=1117) |
Mean (SD) Median (IQR) |
72.9 (5.4) 72 (68, 77) |
| Body Mass Index (BMI) (n=1116) |
<25 kg/m2 25 to 29.9 kg/m2 ≥ 30 kg/m2 |
321 (28.8%) 631 (56.5%) 164 (14.7%) |
| Race (n=1117) |
Non-Hispanic White: 1007 (90.1%) African-American: 31 (2.8%) 7-=Asian: 31 (3.3%) Hispanic: 27 (2.4%) Other: 15 (1.3%) |
|
| Educational Status (n=1117) |
Less than High School: 58 (5.2%) High School: 163 (14.6%) Some College: 238 (21.3%) College: 219 (19.6%) Some Graduate School: 133 (11.9%) Graduate School 306 (27.4%) |
|
| DXA-VAT Area (cm2), (n=1117) |
Mean (SD) Median (IQR) |
168.8 (58.4) 164.5 (−128.2, 207.9) |
| CT-VAT Volume, cm3 (n=1117) |
Mean (SD) Median (IQR) |
65.1 (26.4) 61.3 (47.2, 78.0) |
|
*Fasting Glucose, mg/dl (n=1025) |
Mean (SD) Median (IQR) |
103.9 (25.0) 99 (92, 108) |
|
**Fasting Insulin, μIU/ml (n=1015) |
Mean (SD) Median, (IQR) |
8.6 (5.8) 7.4 (5.2, 10.4) |
|
***Homa2-ir (n=983) |
Mean (SD) Median (IQR) |
1.4 (0.8) 1.2 (0.9, 1.7) |
|
ˆInterleukin-6, pg/ml (n=223) |
Mean (SD) Median (IQR) |
2.71 (1.55) 2.25 (1.67, 3.35) |
|
†TNF-alpha, pg/ml (n=225) |
Mean (SD) Median (IQR) |
4.47 (2.42) 4.16 (3.14, 5.38) |
|
‡C-reactive protein, mcg/ml (n=225) |
Mean (SD) Median, (IQR) |
2.31 (3.52) 1.32 (0.76, 2.28) |
| High density lipoprotein, mg/dl Mean (SD) (n=1042) | 50.7 (15.4) 48 (40, 58) |
|
| Low density lipoprotein, mg/dl (n=1042) |
Mean (SD) | 114.1 (31.1) 112 (92, 135) |
| Triglycerides mg/dl (n=1042) |
Mean (SD) | 146.0 (97.7) 119 (88,170) |
Excludes 1 man with fasting glucose<40mg/dl,
Excludes 11 men with fasting insulin levels < 2 μIU/ml,
Excludes 40 men with fasting insulin <2.8 μIU/ml, fasting insulin>46.1 μIU/ml, fasting glucose<54 mg/dl or fasting glucose>450 mg/dl
Excludes 6 men with IL-6 values > 6500 pg/ml
Excludes 5 men with TNF-alpha values <0.13 or >2000 pg/ml
Excludes 4 men with CRP values <0.16 mcg/ml and 1 man with CRP value> 100 mcg/ml
Figure 3. Scatterplots of Standardized* DXA-VAT vs CT-VAT and Standardized Log of DXA-VAT vs Log of CT-VAT.
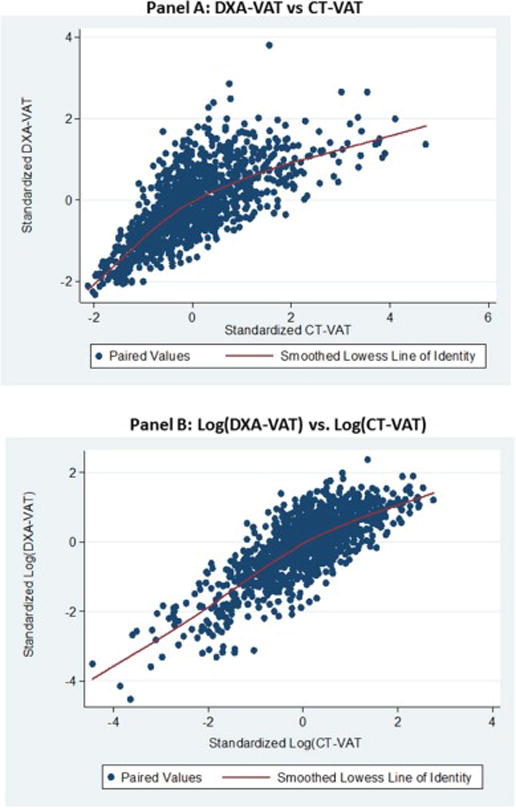
*Mean of zero, standard deviation of 1.0
Homa2ir was more strongly associated with DXA-VAT (effect size 0.50, 95% C.I. 0.43, to 0.56) than with CT-VAT (effect size 0.45, 95% C.I. 0.40 to 0.51, p-value for difference 0.04, table 2). Similarly, fasting insulin was more strongly associated with DXA-VAT (effect size 0.49, 95% C.I. 0.44, to 0.55) than with CT-VAT (effect size 0.45, 95% C.I. 0.40 to 0.50, p-value for difference 0.02, table 2). The incremental proportion of the total variance (R-squared) of fasting insulin and homa2-ir explained by VAT (beyond that explained by age and study enrollment site) was slightly higher when log of DXA-VAT was included in the model compared to CT-VAT. When these models were further adjusted for BMI, the associations between DXA-VAT and CT-VAT with fasting insulin and homa2-ir were mildly attenuated, but still moderate in strength (Table 3). The associations were still slightly stronger for log of DXA-VAT compared to CT-VAT for both fasting insulin and homa2ir (p-value 0.006, 0.03, respectively). The incremental proportions of the variances of fasting insulin and homa2ir explained by VAT (beyond that explained by age, study enrollment site, and BMI) were modest and roughly similar whether VAT was measured by DXA or CT. These results were unchanged when 57 men taking any hypoglycemic medication were excluded (data not shown).
Table 2.
Comparison of Standardized Associations* of DXA-VAT and CT-VAT with Glucose Metabolism Markers, Lipid Levels and Inflammatory Serum Markers, Adjusted for Age and Enrollment Site
| Marker | Log DXA-VAT | Log CT-VAT | Chi-squared (P-Value)† | ||
|---|---|---|---|---|---|
| Parameter Coefficient | Change in R-Squaredˆ | Parameter Coefficient | Change in R-Squaredˆ | ||
| Log Homa2-ir (n=983) |
0.50 (0.43, 0.56) |
0.213 |
0.45 (0.40, 0.51) |
0.171 |
4.38 (0.04) |
| Log Fasting Insulin (n=1015) |
0.49 (0.44, 0.55) |
0.229 |
0.45 (0.40, 0.50) |
0.184 |
5.72 (0.02) |
| Log HDL (n=1042) |
−0.31 (−0.36, −0.25) |
0.091 | −0.28 (−0.34, −0.22) |
0.074 | 2.77 (0.10) |
| Log Triglycerides (n=1042) |
0.35 (0.30,0.40) |
0.117 |
0.36 (0.30, 0.41) |
0.120 | 0.23 (0.63) |
| LDL (n=1042) |
0.04 (−0.01 to 0.10) |
0.002 | 0.03 (−0.03, 0.09) |
0.001 | 0.38 (0.54) |
| TNF-alpha** (n = 225) |
0.17 (0.06, 0.27) |
0.026 |
0.15 (.04, .26) |
0.021 | 0.46 (0.50) |
| Log IL-6 (n=223) |
0.25 (0.11, 0.38) |
0.056 |
0.20 (0.07, 0.32) |
0.037 | 1.41 (0.24) |
| Log CRP (n=225) |
0.24 (0.11, 0.36) |
0.051 |
0.18 (0.05, 0.32) |
0.033 | 1.63 (0.20) |
Number of standard deviations change of VAT per standard deviation change of predictor. Associations significant at <0.05 are in bold
The standardized TNF-alpha variable was created using the square-root of TNF-alpha
Incremental increase in variance of marker explained (R-Squared) when VAT is added to base model with covariates age and study enrollment site
Chi-squared and P-value to test hypothesis that parameter coefficients for DXA-VAT and CT-VAT are the same (modified Hausmann test using suest command of Stata)
Table 3.
Comparison of Standardized Associations* of DXA-VAT and CT-VAT with Glucose Metabolism Markers, Lipid Levels, and Inflammatory Serum Markers, Adjusted for Age, BMI, and Enrollment Site
| Marker | Log DXA-VAT | Log CT-VAT | Chi-sqared (P-Value)† |
||
|---|---|---|---|---|---|
| Parameter Coefficient | Change in R-Squaredˆ | Parameter Coefficient | Change in R- Squaredˆ | ||
| Log Homa2-ir (n=982) |
0.38 (0.28, 0.47) |
0.061 |
0.30 (0.24, 0.37) |
0.056 |
4.67 (0.03) |
| Log Fasting Insulin (n=1014) |
0.37 (0.29, 0.45) |
0.063 |
0.30 (0.24, 0.36) |
0.056 |
7.58 (0.006) |
| Log HDL (n=1041) |
−0.29 (−0.38, −0.21) |
0.040 | −0.22 (−0.29, −0.15) |
0.031 |
7.92 (0.005) |
| Log Triglycerides (n=1041) |
0.35 (0.28, 0.43) |
0.058 |
0.32 (0.25, 0.38) |
0.065 | 2.22 (0.14) |
| LDL (n=1041) |
0.11 (0.02, 0.20) |
0.005 | 0.06 (−0.02, 0.13) |
0.002 | 3.33 0.07 |
| TNF-alpha (n = 225) |
0.13 (−0.04, 0.30) |
0.007 | 0.10 (−0.03, 0.23) |
0.006 | 0.32 (0.57) |
| Log IL-6 (n=223) |
0.11 (−0.07, 0.29) |
0.005 | 0.07 (−0.08, 0.23) |
0.003 | 0.30 (0.58) |
| Log CRP (n=225) |
0.14 (−0.04, 0.32) |
0.009 | 0.08 (−0.08, 0.25) |
0.005 | 0.86 (0.35) |
Number of standard deviations change of log(VAT) per standard deviation change of predictor Associations significant at <0.05 are in bold
The standardized TNF-alpha variable was created using the square-root of TNF-alpha
Incremental increase in variance of marker explained (R-Squared) when VAT is added to age, study enrollment site, and BMI.
Chi-squared and P-value to test hypothesis that parameter coefficients for DXA-VAT and CT-VAT are the same (modified Hausmann test using suest command of Stata)
Each standard deviation increase of DXA-VAT and CT-VAT was associated, respectively, with an odds ratio of 1.71 (95% C.I. 1.49 to 1.96) and of 1.45 (95% C.I. 1.27 to 1.66) of impaired vs. normal fasting glucose. The association of DXA-VAT with fasting glucose was stronger than that of CT-VAT (p-value=0.001), and the model with DXA-VAT discriminated those with high from those with low fasting glucose better than the model with CT-VAT (AUROC 0.64 vs 0.60, p-value for difference <0.001). After further adjustment for BMI, the association of DXA-VAT with high fasting glucose (OR 1.42, 95% C.I. 1.17 to 1.73) was still significantly higher than that for CT-VAT (OR 1.18, 95% C.I. 1.01 to 1.38; p-value 0.01), but the areas under ROC curves for the model with DXA-VAT (0.65) was the same as the model for CT-VAT (0.64, p-value for comparison 0.10). Results were unchanged when these analyses were repeated excluding those on any hypoglycemic medication (data not shown).
The logs of DXA-VAT and CT-VAT were also significantly negatively associated with the log of HDL-cholesterol with similar, moderate effect sizes, after adjustment for age and study enrollment site (Table 2). With additional adjustment for BMI (Table 3), and the associations were attenuated, but remained significant and were greater for DXA-VAT compared to CT-VAT (p-value =0.005). In contrast, the age-adjusted associations of the log of triglycerides with logs of DXA-VAT and CT-VAT were the same with adjustment for age and clinical site (Table 2) and with additional adjustment for BMI (Table 3). The incremental proportions of the variances of logs of HDL cholesterol and triglycerides explained by VAT were modest and similar, whether VAT was measured with CT or DXA. The logs of CT-VAT and DXA-VAT were not significantly associated with LDL cholesterol (Tables 2 and 3).
The logs of both CT-VAT and DXA-VAT had weak but statistically significant age-adjusted associations with TNF-α, log of IL-6, and log of CRP,(Table 2). After additional adjustment for BMI, neither DXA-VAT nor CT-VAT were significantly associated with any serum inflammatory marker (Table 3). The incremental proportions of the variances of TNF-α, log of IL-6, and log of CRP explained by VAT were small and similar, whether VAT was measured with CT or DXA (Tables 2 and 3).
Discussion
These data show that in older men DXA measures of VAT are associated with insulin resistance, fasting glucose, HDL cholesterol, triglycerides, and serum inflammatory markers with equal or greater strength than CT measures of VAT. These associations were moderate for insulin resistance and triglycerides, weaker for HDL cholesterol, and weakest for serum inflammatory markers. Body composition measures, including VAT, can be obtained with DXA at lower cost and far less radiation exposure than CT. Moreover, DXA measures of VAT are fully automated, whereas CT measures require some manual manipulation of the images. Our data support using DXA in future studies of VAT and its associations with other biologic phenomena and clinical health outcomes.
While moderate to severe obesity (BMI ≥ 35 kg/m2) predicts incident cardiovascular disease and mortality(27), there is increasing evidence that VAT may be an even stronger predictor of subsequent cardiovascular disease events. VAT assessed on CT scans is associated with fasting plasma glucose, insulin resistance, triglyceride levels, and HDL cholesterol in both men and women even after adjustment for BMI(28–30), and (in the Framingham study) after further adjustment for waist circumference(28). Insulin resistance and raised inflammatory cytokines are associated with subsequent cardiovascular disease events, and possibly with impaired cognitive function.(31–34)
If these pathogenic mechanisms of vascular disease are more strongly associated with VAT than general measures of adiposity, then direct measures of VAT may be more strongly associated with incident cardiovascular disease events compared with global measures of adipose tissue such as BMI, or measures of central obesity that include SAT (i.e. waist circumference and waist to hip ratio). Abdominal CT-VAT has been reported to be associated with the presence of coronary artery disease on CT angiography and coronary artery calcium score, whereas abdominal SAT and waist-to-hip ratio were not.(35) Similarly, in a Japanese prospective cohort study, baseline CT-VAT was associated with progression of uncalcified coronary artery plaque on follow-up CT coronary angiography after multivariable adjustment, whereas BMI was not.(36) In the Framingham study of middle-aged (40 to 64 years) women and men, each standard deviation increase of CT-VAT, but not SAT, was associated with a hazard ratio of 1.47 for subsequent incident cardiovascular disease events, after multi-variable adjustment for clinical cardiovascular disease risk factors and waist circumference(1). In the Health ABC study of individuals age 70 to 79, CT-VAT was associated with incident myocardial infarction only in women and not in men, but the association with other cardiovascular outcomes (such as stroke) was not reported(2). To be sure, other regional fat depots may be as or more important than VAT in other populations and/or for outcomes other than cardiovascular disease. For example, in the Health ABC study, baseline total fat mass and abdominal CT-SAT were both more strongly associated with subsequent changes in global cognitive function than was CT-VAT.(6)
Since our data show that DXA-VAT is as robustly associated as CT-VAT with some of the metabolic parameters that have been hypothesized to mediate the associations of VAT with incident cardiovascular disease events, investigations of the direct associations of DXA-VAT with cardiovascular outcomes are clearly warranted. This is particularly true for individuals age 65 to 75; since a DXA test is already recommended at least once for women age 65 and older and for many men age 70 and older, the incremental cost of obtaining measures of VAT on these individuals would be small. To be sure, establishing that DXA-VAT predicts adverse health outcomes in these individuals would be only the first step required to establish that measurement of DXA-VAT has clinical utility. DXA-VAT would need to be shown to improve prediction of these events compared to global measures of obesity such as BMI sufficiently to alter clinical decision-making for a significant proportion of the target population being screened.
Our study has significant limitations. The correlation between DXA-VAT and CT-VAT was not as strong as reported in some smaller studies (>0.90).(10, 11) The reason for this is not known, but it may in part be because those studies used multi-slice CT or a large single CT slice (10 mm). Our single 5 mm CT slice, at the L4-L5 disc level, was often below the iliac crest and therefore below the area of the visceral cavity measured by DXA. Our study was limited to older Caucasian men, and our results may not be generalizable to women, younger men, or members of other ethnic groups. The sub-sample of men who were randomly selected for both CT measures of VAT and inflammatory cytokines was small. The associations of DXA-VAT with these inflammatory markers may be statistically significant in larger studies.
In conclusion, DXA measurements of VAT, compared to CT measurements of VAT, are at least as strongly associated with insulin resistance, low HDL cholesterol and triglycerides, and serum inflammatory markers, all of which are hypothesized to mediate the associations of VAT with subsequent adverse cardiovascular disease outcomes. Investigations of how well DXA measurements of VAT predict incident cardiovascular disease events are clearly warranted.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by the following National Institutes of Health: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
Fasting glucose and insulin measures were obtained with grant support from the American Diabetes Association (grant number 1-04-JF-46, Strotmeyer ES). CT abdominal body composition image processing was supported by the National Institutes of Health primarily through grant R01 HL084183, and with funds from NIH grants P60 AR05473101, R21 DK066224, and UL1 RR024140 and from the Oregon Medical Research Foundation. Measurement of VAT with DXA was supported with an unrestricted grant from Hologic, Inc, (Schwartz AV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicklas BJ, Penninx BW, Cesari M, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160(8):741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 3.Bruce SA. The association between central fat distribution and recurrent cardiovascular disease events in female survivors of nonfatal myocardial infarction. Journal of Cardiovascular Nursing. 2015;30(2):E15–22. doi: 10.1097/JCN.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto T, Morimoto S, Ikenoue T, Furumatsu Y, Ichihara A. Visceral fat level is an independent risk factor for cardiovascular mortality in hemodialysis patients. American Journal of Nephrology. 2014;39(2):122–129. doi: 10.1159/000358335. [DOI] [PubMed] [Google Scholar]
- 5.Papachristou E, Ramsay SE, Lennon LT, et al. The relationships between body composition characteristics and cognitive functioning in a population-based sample of older British men. BMC Geriatrics. 2015;15(1) doi: 10.1186/s12877-015-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanaya AM, Lindquist K, Harris TB, et al. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Archives of Neurology. 2009;66(3):329–335. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 8.Bi X, Seabolt L, Shibao C, et al. DXA-measured visceral adipose tissue predicts impaired glucose tolerance and metabolic syndrome in obese Caucasian and African-American women. European Journal of Clinical Nutrition. 2015;69(3):329–336. doi: 10.1038/ejcn.2014.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. British Journal of Radiology. 2012;85(1009):1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat.[Erratum appears in Obesity (Silver Spring). 2012 Jul;20(7):1544] Obesity. 2012;20(6):1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20(5):1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H, Yan H, Rao S, et al. Quantification of visceral adipose tissue using lunar dual-energy X-ray absorptiometry in Asian Chinese. Obesity. 2013;21(10):2112–2117. doi: 10.1002/oby.20325. [DOI] [PubMed] [Google Scholar]
- 13.Cheung AS, de Rooy C, Hoermann R, et al. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int J Obes (Lond) 2016;40(8):1325–1328. doi: 10.1038/ijo.2016.50. [DOI] [PubMed] [Google Scholar]
- 14.Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes. 2016;6(7):e221. doi: 10.1038/nutd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katzmarzyk PT, Greenway FL, Heymsfield SB, Bouchard C. Clinical utility and reproducibility of visceral adipose tissue measurements derived from dual-energy X-ray absorptiometry in White and African American adults. Obesity. 2013;21(11):2221–2224. doi: 10.1002/oby.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Marshall LM, Lang TF, Lambert LC, et al. Dimensions and volumetric BMD of the proximal femur and their relation to age among older U.S. men. J Bone Miner Res. 2006;21(8):1197–1206. doi: 10.1359/jbmr.060506. [DOI] [PubMed] [Google Scholar]
- 18.Miljkovic I, Cauley JA, Wang PY, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring) 2013;21(10):2118–2125. doi: 10.1002/oby.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheu Y, Marshall LM, Holton KF, et al. Abdominal body composition measured by quantitative computed tomography and risk of non-spine fractures: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int. 2013;24(8):2231–2241. doi: 10.1007/s00198-013-2322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TL K, KE W, CR R. In: Estimating visceral fat by dual-energy X-ray absorptiometry. Office USP, editor. United States: 2010. [Google Scholar]
- 21.Lee CG, Boyko EJ, Strotmeyer ES, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59(7):1217–1224. doi: 10.1111/j.1532-5415.2011.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napoli N, Strotmeyer ES, Ensrud KE, et al. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014;57(10):2057–2065. doi: 10.1007/s00125-014-3289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of beta-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care. 2013;36(8):2324–2330. doi: 10.2337/dc12-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauley JA, Barbour KE, Harrison SL, et al. Inflammatory Markers and the Risk of Hip and Vertebral Fractures in Men: the Osteoporotic Fractures in Men (MrOS) J Bone Miner Res. 2016 doi: 10.1002/jbmr.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pregibon D. Goodness of link tests for generalized linear models. Applied Statistics. 1980;29:15–24. [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 2000. [Google Scholar]
- 27.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 29.Silveira LS, Monteiro PA, Antunes Bde M, et al. Intra-abdominal fat is related to metabolic syndrome and non-alcoholic fat liver disease in obese youth. BMC Pediatrics. 2013;13:115. doi: 10.1186/1471-2431-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. American Journal of Clinical Nutrition. 2013;97(3):480–486. doi: 10.3945/ajcn.112.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nettiksimmons J, Ayonayon H, Harris T, et al. Development and validation of risk index for cognitive decline using blood-derived markers. Neurology. 2015;84(7):696–702. doi: 10.1212/WNL.0000000000001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metti AL, Aizenstein H, Yaffe K, et al. Trajectories of peripheral interleukin-6, structure of the hippocampus, and cognitive impairment over 14 years in older adults. Neurobiology of Aging. 2015;36(11):3038–3044. doi: 10.1016/j.neurobiolaging.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letra L, Santana I, Seica R. Obesity as a risk factor for Alzheimer’s disease: the role of adipocytokines. Metabolic Brain Disease. 2014;29(3):563–568. doi: 10.1007/s11011-014-9501-z. [DOI] [PubMed] [Google Scholar]
- 34.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 35.Marques MD, Santos RD, Parga JR, et al. Relation between visceral fat and coronary artery disease evaluated by multidetector computed tomography. Atherosclerosis. 2010;209(2):481–486. doi: 10.1016/j.atherosclerosis.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Imai A, Komatsu S, Ohara T, et al. Visceral abdominal fat accumulation predicts the progression of noncalcified coronary plaque. Atherosclerosis. 2012;222(2):524–529. doi: 10.1016/j.atherosclerosis.2012.03.018. [DOI] [PubMed] [Google Scholar]


