Abstract
Background
Osteoarthritis of the knee affects up to 10% of the elderly population. The condition is frequently treated by intra-articular injection of hyaluronic acid. We performed a systematic review and meta-analysis of randomized controlled trials to assess the effectiveness of this treatment.
Methods
We searched MEDLINE, EMBASE, CINAHL, BIOSIS and the Cochrane Controlled Trial Register from inception until April 2004 using a combination of search terms for knee osteoarthritis and hyaluronic acid and a filter for randomized controlled trials. We extracted data on pain at rest, pain during or immediately after movement, joint function and adverse events.
Results
Twenty-two trials that reported usable quantitative information on any of the predefined end points were identified and included in the systematic review. Even though pain at rest may be improved by hyaluronic acid, the data available from these studies did not allow an appropriate assessment of this end point. Patients who received the intervention experienced a reduction in pain during movement: the mean difference on a 100-mm visual analogue scale was –3.8 mm (95% confidence interval [CI] –9.1 to 1.4 mm) after 2–6 weeks, –4.3 mm (95% CI –7.6 to –0.9 mm) after 10–14 weeks and –7.1 mm (95% CI –11.8 to –2.4 mm) after 22–30 weeks. However, this effect was not compatible with a clinically meaningful difference (expected to be about 15 mm on the visual analogue scale). Furthermore, the effect was exaggerated by trials not reporting an intention-to-treat analysis. No improvement in knee function was observed at any time point. Even so, the effect of hyaluronic acid on knee function was more favourable when allocation was not concealed. Adverse events occurred slightly more often among patients who received the intervention (relative risk 1.08, 95% CI 1.01 to 1.15). Only 4 trials explicitly reported allocation concealment, had blinded outcome assessment and presented intention-to-treat data.
Interpretation
According to the currently available evidence, intra-articular hyaluronic acid has not been proven clinically effective and may be associated with a greater risk of adverse events. Large trials with clinically relevant and uniform end points are necessary to clarify the benefit–risk ratio.
Osteoarthritis affects about 10% of the population over 55 years of age. Of those, one-quarter are severely disabled.1 The condition is characterized by degeneration of the articular cartilage and subsequent subchondral bone changes. The underlying mechanisms remain unknown, but the glycosaminoglycan–proteoglycan matrix may play a major role.2
Hyaluronic acid, a glycosaminoglycan, is widely used for the treatment of osteoarthritis of the knee. A survey of 2 general practices in the United Kingdom showed that about 15% of patients with osteoarthritis received intra-articular treatment with glucosamine sulfates.3 The costs of such treatment are significant. At present, 1 syringe of hyaluronic acid costs at least Can$130 (US$110). The treatment of knee osteoarthritis is covered by the US Medicare program but not by provincial formularies in Canada. In Austria (which has 8 million inhabitants) more than 10 million euros (approximately US$12 million or Can$15 million) is spent by social insurance programs annually for hyaluronic acid preparations (excluding the cost of application).
Hyaluronic acid has beneficial effects in vitro.4 Because of its viscoelastic quality, it may replace synovial fluid. Furthermore, it may reduce the perception of pain. Beneficial molecular and cellular effects have also been reported.2,4 Hyaluronic acid is frequently applied by intra-articular injection, but the evidence concerning its clinical relevance is conflicting. The European League against Rheumatism (EULAR) recommends the intra-articular application of hyaluronic acid as “category 2” evidence (at least 1 controlled study without randomization).5 The American College of Rheumatology recommends intra-articular hyaluron therapy for patients with no response to nonpharmacologic therapy and simple analgesics.6 In contrast, other specialists have concluded that “hyaluronate sodium is not efficacious” in the treatment of osteoarthritis.7 The first state-of-the-art systematic review and meta-analysis was published recently,8 and its authors concluded “that intra-articular hyaluronic acid, at best, has a small effect.”
We performed a systematic review and meta-analysis of the effect of intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee. In contrast to 2 previous meta-analyses on this subject,8,9 we used a different approach to data synthesis and interpretation: instead of analyzing a composite effect size over time, we allocated trial data, when possible, to 3 outcome groups that we assumed would be relevant for patients with osteoarthritis. We specifically looked at pain at rest, pain during exercise and joint function as distinct outcomes, measured repeatedly over time. In addition, we assessed adverse events and the impact of both trial quality and molecular mass of the product. This analysis allows us to provide important additional insight into the effects of intra-articular administration of hyaluronic acid for the treatment of osteoarthritis of the knee.
Methods
We identified all randomized controlled trials comparing various preparations of hyaluronic acid with placebo in patients with osteoarthritis. We searched MEDLINE, EMBASE, CINAHL, BIOSIS and the Cochrane Controlled Trial Register from inception until April 2004. The search string was [(osteoarthritis) or (degenerative arthritis) or (gonarthrosis) or (knee near arthralgia) or (knee near pain) or (patella near pain) or (patellofemoral near pain) or (patello near femoral pain) or (retropatellar near pain) or (femoropatellar near pain) or (femoro-patellar near pain)] and [(hyaluronan) or (hyaluronate) or (viscosupplementation) or (visco near supplementation) or (hyaluronic acid)]. For the MEDLINE and EMBASE searches we used validated search terms to identify randomized controlled trials (see Appendix 1).10,11 We predefined a variety of clinical outcomes: pain at rest, pain during or immediately after movement, joint function and adverse events. We also predefined time points of assessment in broad categories: 2–6 weeks, 10–14 weeks, 22–30 weeks and 44–60 weeks.
Two reviewers (J.A. and P.M.) independently abstracted data from each trial and entered the data on a predefined form. We compared the results and resolved disagreement by discussion among 3 reviewers (J.A., P.M., M.M.).
We determined whether concealment of allocation to treatment was reported, the degree of blinding (doctor blinded to the intervention, patient blinded to the intervention, assessor of the end point blinded to the intervention) and whether an intention-to-treat analysis was reported.
We extracted estimates of the effect of the intervention and its variance from the figures in the article, if these values were not explicitly reported in the text or tables. If a trial did not report measures of variability, we calculated them from p values or confidence intervals. For one trial12 we took the median to be representative of the mean and converted the interquartile range into a standard deviation by dividing it by 1.35.13 With one exception, all trials with data that could be extracted for quantitative analysis reported on loss to follow-up.
We used random-effects models to pool the data. Continuous outcomes were combined by either weighted mean differences or standardized mean differences, as appropriate.13 We used summary risk ratios to combine adverse events. We calculated 95% confidence intervals (CIs) for all point estimates.
We used Cochrane's Q for heterogeneity, together with the resulting degrees of freedom (df), to calculate the proportion of variation due to unexplained heterogeneity: I2 = (Q – df)/Q.14 A value of less than 20% is consistent with little variability between studies, and 20% to 50% can be considered to represent a moderately large degree of variation. Regression methods were used to assess the presence of publication bias.15
We used multivariate meta-regression analysis to assess whether an effect had been influenced by allocation concealment (blinding of randomization, yes versus no or unclear), blinded outcome assessment (blinded treating physician, patient and outcome assessor, explicitly reported versus not explicitly reported or unclear) and intention-to-treat analysis (explicitly reported versus not explicitly reported or unclear). We repeated the analyses for only those trials that fulfilled all 3 criteria.
We assessed the impact of the molecular mass of the hyaluronic acid on efficacy. We used molecular mass as an ordinal category and then collapsed categories of molecular mass into 2 categories (≤ 900 kDa and > 900 kDa) and repeated the analysis.
Results
The electronic search of databases resulted in 1159 hits, and we retrieved 42 publications for closer inspection (Fig. 1). Of these, 24 studies were potentially eligible.12,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38 The clinical and methodologic characteristics of the trials that reported at least one of the end points of interest are presented in Table 1 and Table 2.
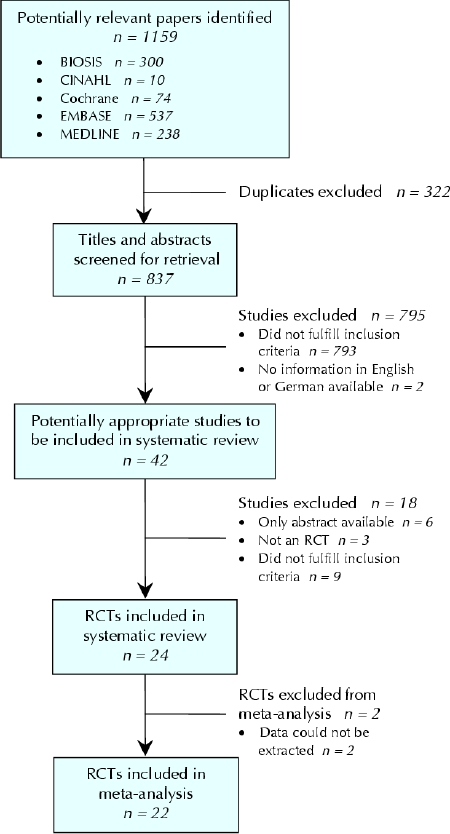
Fig. 1: Flow of articles through the systematic review. RCT = randomized controlled trial.
Table 1
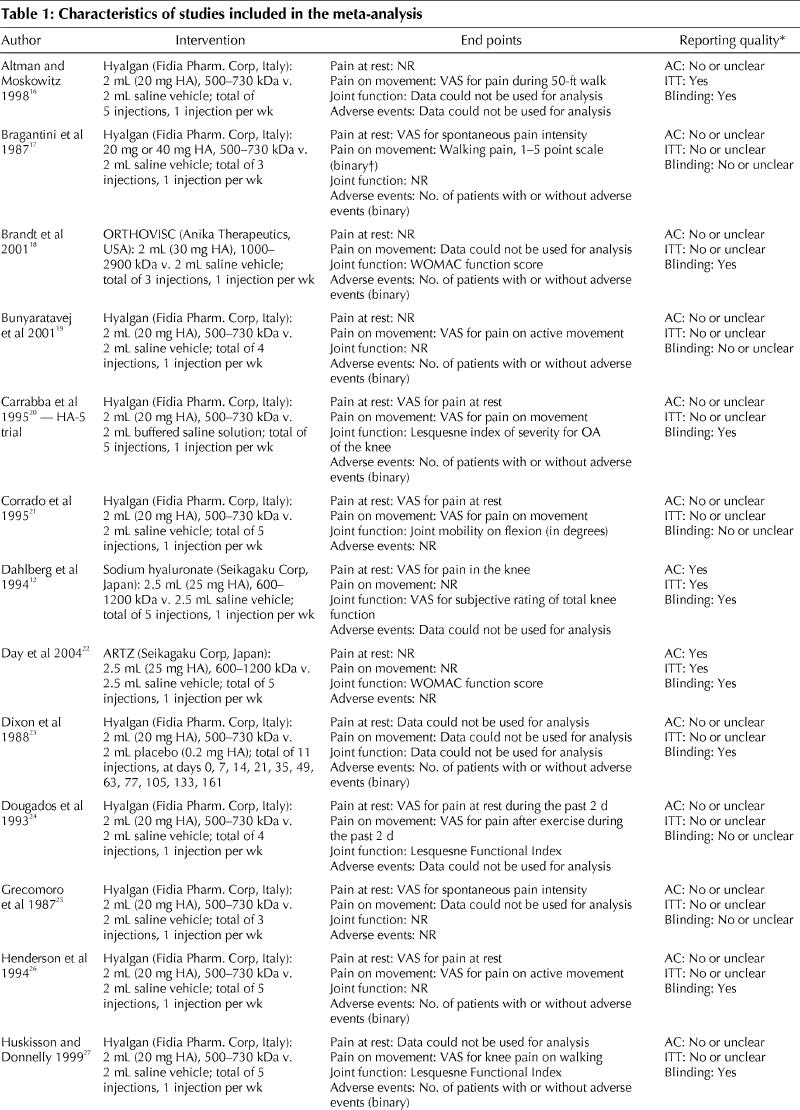
Table 1 continued
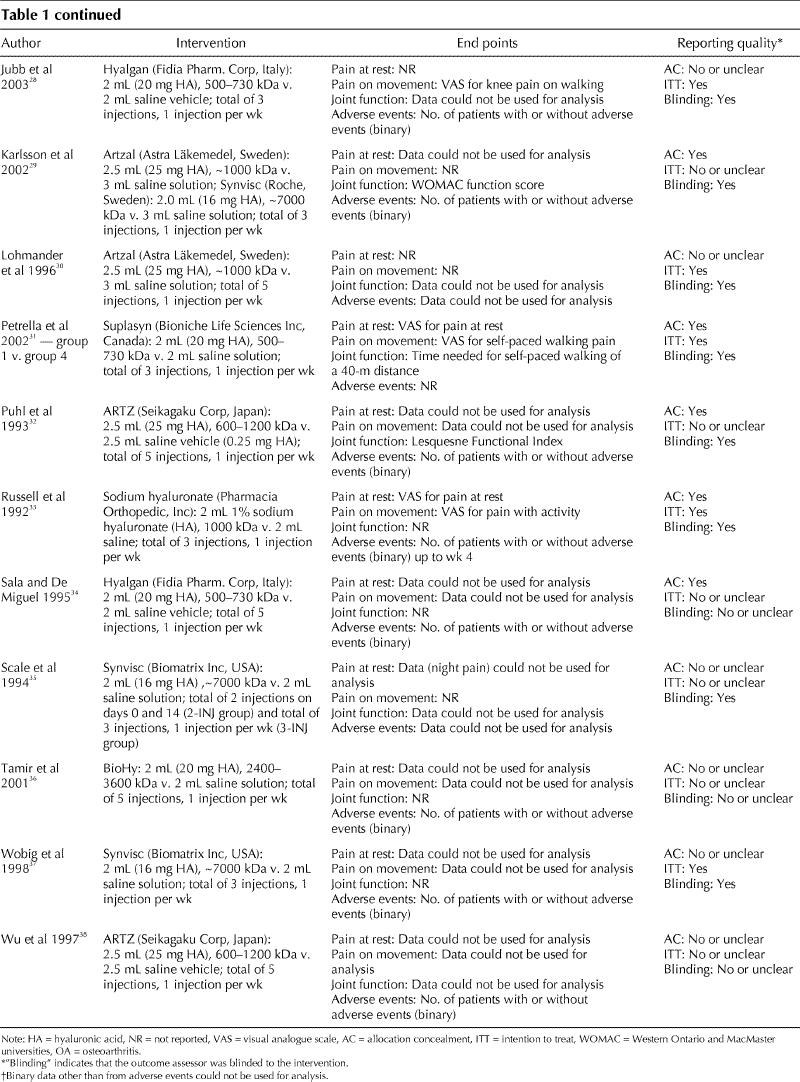
Table 2
Trial quality
Overall, the quality of the reported trials was unsatisfactory. Only 4 trials reported concealment of allocation and blinding of the outcome observer, and presented data from an intention-to-treat analysis.12,22,31,33 Seven trials reported allocation concealment.12,22,29,31,32,33,34 Eight trials reported an intention-to-treat analysis, but only 6 of these presented data that could be extracted from the intention-to-treat analysis.12,16,28,30,31,37 Sixteen trials reported that the outcome observer was blinded to the intervention.12,16,18,20,22,23,26,27,28,29,30,31,32,33,35,37
Two trials did not present any usable quantitative information.30,35 Therefore, only 22 trials were included in the analysis of at least 1 of the predefined efficacy end points (Fig. 1).
Pain at rest
Eight trials (with a total of 10 comparisons) reported reduction of pain at rest for the treatment group (n = 231) relative to the control group (n = 237) at 2–6 weeks.12,17,20,21,24,25,26,31 Unexplained statistical heterogeneity was excessive (I2 = 94%), and we could not identify a particular trial causing this excess variability (Fig. 2). Pooling in the face of such a high degree of heterogeneity of unknown cause is not advisable. If the data were pooled, the mean difference in the visual analogue scale was in favour of hyaluronic acid (–8.7 mm, 95% CI –17.2 to –0.2 mm, p = 0.046) (Table 3). For trials in which allocation concealment was unclear or there was no intention-to-treat analysis, the effect was overestimated by 15.6 mm (95% CI –3.2 to 34.4, p = 0.11). For trials in which outcome assessment was not blinded, the effect was also overestimated, by 13.6 mm (95% CI –0.6 to 27.7, p = 0.06).
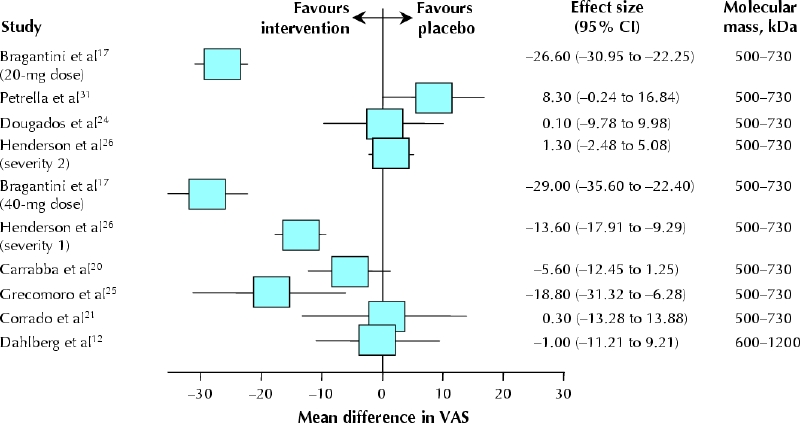
Fig. 2: Effectiveness of hyaluronic acid compared with placebo for pain at rest at 2–6 weeks. Data are presented as the study means (boxes) and 95% confidence intervals (CIs, horizontal lines). There is no summary effect, and the data are not weighted (because of excessive heterogeneity). Bragantini and associates17 reported on 2 strata separately (20-mg and 40-mg doses), as did Henderson and colleagues26 (severity groups 1 and 2). The trials are ranked according to the molecular mass of the hyaluronic acid preparation. VAS = visual analogue scale.
Table 3
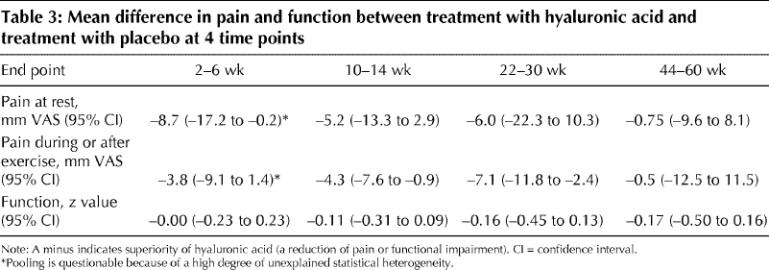
Two high-quality trials12,33 assessed pain at rest at 10–14 and 22–30 weeks, and 2 trials (1 of which was of high quality)12,24 at 44–60 weeks; there were no significant effects at these time points (Table 3).
Pain during or immediately after exercise
Nine trials (with a total of 10 comparisons) reported pain reduction in the treatment group (n = 559) relative to the control group (n = 582) at 2–6 weeks.16,19,20,21,24,26,27,28,31 The weighted mean difference was –3.8 mm on the visual analogue scale (95% CI –9.1 to 1.4 mm, p = 0.15) (Table 3). Again, there was an excessive degree of unexplained statistical heterogeneity (I2 = 81%). One trial had a qualitative interaction: among patients with less severe osteoarthritis (Kellgren and Lawrence grades I and II39), those who received hyaluronic acid had better pain reduction than those who received placebo; however, among patients with more advanced disease (grades III and IV), pain increased with hyaluronic acid.26 When this trial was excluded, the effect remained largely unchanged (weighted mean difference –4.2 mm), although the precision was higher (95% CI –7.5 to –0.8 mm, p = 0.015), and heterogeneity was acceptable (I 2 = 20%).
At 10–14 weeks, 5 comparisons were available (435 intervention patients, 442 control patients).16,19,27,28,33 The weighted mean difference between the treatment and control groups was –4.3 mm (95% CI –7.6 to –0.9 mm, p = 0.013) (Fig. 3A). There was no unexplained heterogeneity (I2 = 0%).
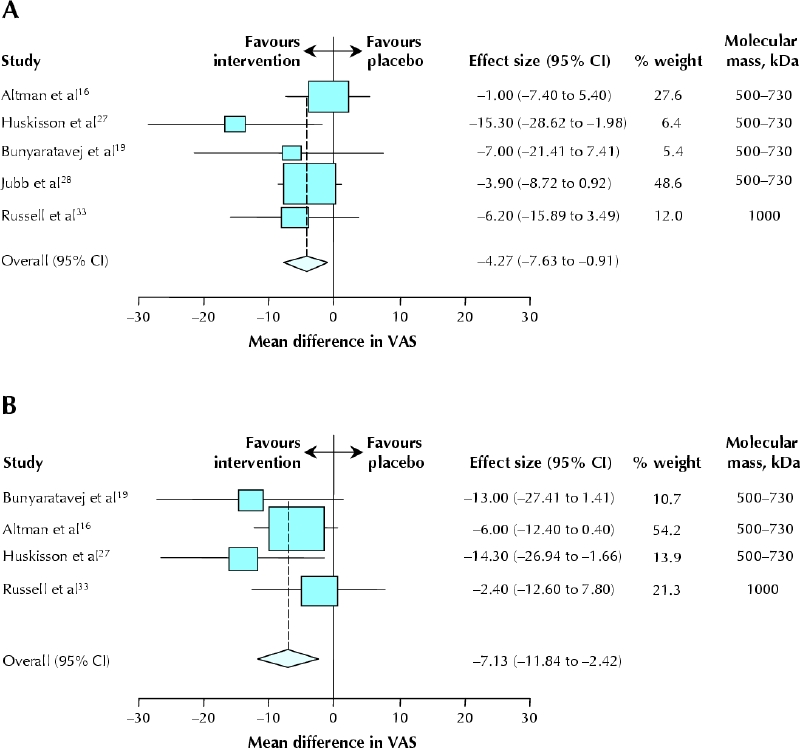
Fig. 3: Effectiveness of hyaluronic acid compared with placebo for pain after exercise. A: At 10–14 weeks. B: At 22–30 weeks. Data are presented as weighted mean difference for each study (boxes), 95% CIs (horizontal lines) and summary weighted mean difference with 95% CI (diamond). The trials are ranked according to the molecular mass of the hyaluronic acid preparation.
At 22–30 weeks, 4 comparisons were available (227 intervention patients, 236 control patients).16,19,27,33 The weighted mean difference between the treatment and control groups was –7.1 mm (95% CI –11.8 to –2.4 mm, p = 0.003) (Fig. 3B). There was no unexplained heterogeneity (I2 = 0%).
Only one trial followed patients until 44–60 weeks (47 intervention patients, 48 control patients),24 and it showed no effect (Table 3).
Trial quality had no undue influence on the effect size at any point, but only one trial33 was of high quality.
Joint function
Nine trials reported a measure of joint function at 2– 6 weeks (489 intervention patients, 505 control patients).12,18,20,21,22,24,27,31,32 Because different measurement systems were used in these trials, we calculated standardized effects. The standardized weighted mean difference between the groups at 2–6 weeks was 0.00 (95% CI –0.23 to 0.23, p = 0.99) (Fig. 4A). There was a high degree of unexplained statistical heterogeneity (I2 = 66%). Even though there was no statistically significant pooled effect, unclear or absent allocation concealment led to considerable inflation of the effect (by 2.6 points on the z score, 95% CI 1.2 to 3.9, p < 0.001). Other measures of quality did not influence the effect size.
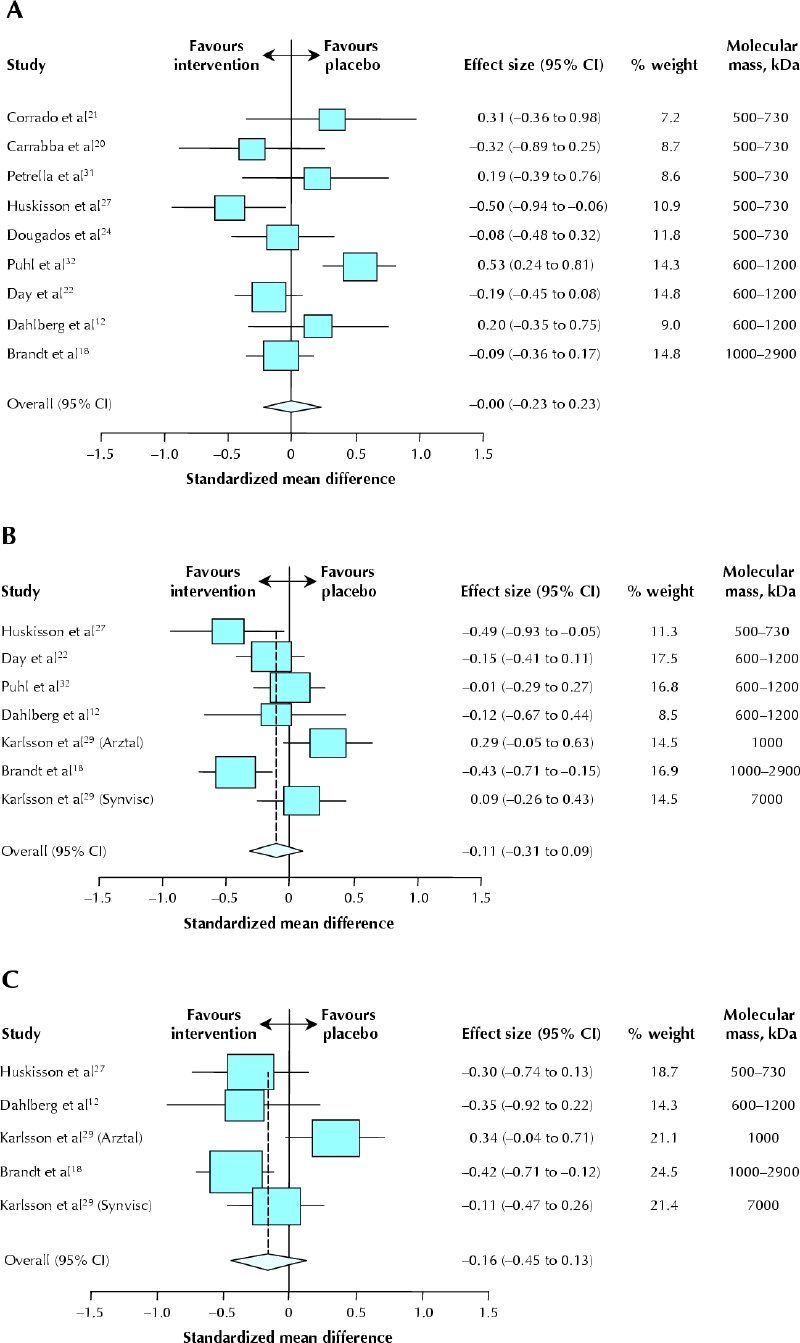
Fig. 4: Effectiveness of hyaluronic acid compared with placebo for joint function. A: At 2–6 weeks. B: At 10–14 weeks. C: At 22–30 weeks. Data are presented as standardized, weighted study mean differences (boxes), 95% CIs (horizontal lines) and summary standardized, weighted mean difference with 95% CI (diamond). Karlsson and collaborators29 reported on 2 strata separately (by brand of hyaluronic acid preparation: Arztal and Synvisc). The trials are ranked according to the molecular mass of the hyaluronic acid preparation.
Six trials (with a total of 7 comparisons) reported data on function at 10–14 weeks (533 intervention patients, 490 control patients).12,18,22,27,29,32 The standardized weighted mean difference between the groups was –0.11 (95% CI –0.31 to 0.09, p = 0.28) (Fig. 4B). Heterogeneity was considerable (I2 = 59%), but there were no obvious differences in study or patient characteristics that would explain this high degree of heterogeneity. Unclear or absent allocation concealment led to considerable inflation of the summary effect (by 3.0 points on the z score, 95% CI 1.1 to 4.9, p < 0.001). Other measures of quality did not influence the effect size.
Data from 22–30 weeks were available for 4 trials (with a total of 5 comparisons) (295 intervention patients, 247 control patients).12,18,27,29 The standardized weighted mean difference did not differ between treatment and control groups (p = 0.27) (Table 3 and Fig. 4C). Heterogeneity was considerable (I2 = 62%).
Two trials followed patients until 44–60 weeks (73 intervention patients, 70 control patients),12,24 and there were no differences in the end points of interest (p = 0.30) (Table 3).
Adverse events
Fifteen trials (with a total of 17 comparisons; 1033 intervention patients, 986 control patients) reported on adverse events in a way that allowed data extraction.17,18,19,20,23,26,27,28,29,32,33,34,36,37,38 The degree of detail and accuracy varied among these trials. Adverse events, mostly of minor clinical relevance (such as transient pain at the injection site), occurred more frequently among patients who received the intervention (summary relative risk 1.08, 95% CI 1.01 to 1.15, p = 0.021). There was no unexplained heterogeneity (I2 = 0%). Bias in the funnel plot analysis (mean bias 0.6, 95% CI 0.2 to 1.1, p = 0.01) could not be explained by lack of blinding in the meta-regression (ratio of risk ratios 0.77, 95% CI 0.49 to 1.21, p = 0.27), which suggests selective over-reporting or over-publication of trials reporting adverse events in patients treated with hyaluronic acid.
Impact of molecular mass
The effect size is ordered in Fig. 2, Fig. 3 and Fig. 4 according to molecular mass, but no clear association is evident. This lack of association was confirmed by the meta-regression analyses.
Sensitivity analysis
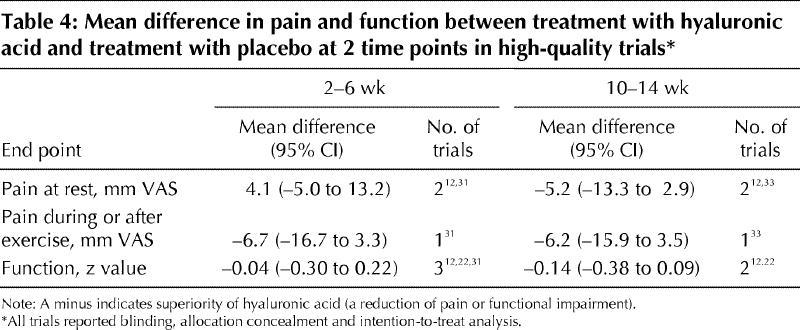
We repeated the analyses for pain at rest, pain after movement and joint function at 2–6 weeks and 10–14 weeks for only those trials that reported allocation concealment, blinded outcome assessment and intention-to-treat analysis. There was no significant effect in favour of the intervention (Table 4).
Table 4
Interpretation
The methodologic quality of most of the trials was poor. Treatment with intra-articular hyaluronic acid did not have a proven beneficial effect on osteoarthritis pain at rest. Pain during or after movement was slightly lower relative to placebo, but this effect is of borderline clinical relevance at best. Moreover, the effect appears to have been inflated by trials of low methodologic quality. Intra-articular hyaluronic acid did not lead to improvement in joint function but may have been associated with a higher rate of side effects than placebo.
Patients with chronic rheumatoid arthritis rated pain as “somewhat better” at a mean difference of 8 mm on a visual analogue scale and as “much better” at a 15-mm difference.40 The summary estimates obtained in this meta-analysis fell short of being the difference defined as “somewhat better,” and the confidence intervals sometimes included the range defined as “much better”; however, the latter were also compatible with increased pain. This finding was augmented by the sensitivity analyses, where the confidence intervals barely exceeded 15 mm (Table 4).
In several instances, only a few trials were available for a given end point at a particular time. A more significant problem, however, was the low methodologic reporting quality of the trials. Low-quality trials, particularly those not reporting allocation concealment and those not reporting blinding, are known to favour interventions.15,41,42,43,44 Our data are compatible with these findings.
Another problem is the wide range of reported end points. We tried to compose clinically useful and comparable outcome categories for the trials. Within our selection algorithm we planned to categorize all reported end points (a total of 125) into 12 categories and to discard end points that could not be allocated. We analyzed only 4 of these 12 categories (the most relevant from a clinical perspective). The remaining 8 categories were global pain, pain on touch, joint effusion, joint stiffness, quality of life, pain medication, overall assessment by patient and overall assessment by observer.
Only 2 papers were excluded because there was no abstract available in English or German. From their titles, it was doubtful that these were randomized controlled trials, but we could not confirm study type. Including non-English studies and unpublished studies in a meta-analysis may even introduce bias.43,45 It is difficult to determine the trade-off between detecting all of the available evidence and introducing bias through inclusion of inferior studies identified by a comprehensive search.45
We applied multiple statistical tests, and a type 1 error — detecting an effect where none existed — is possible. This possibility should be borne in mind when interpreting the data.
Finally, there were only a limited number of trials for each end point and time point, which led to wide confidence intervals. Even so, the limits did not exceed a clinically meaningful difference in many instances. We could not detect publication bias (data not shown) except perhaps with regard to adverse event reporting. This does not necessarily exclude the possibility of such bias, but if there was any selection or publication bias, it would probably have exaggerated the effect.
We are aware of 3 relevant systematic reviews.8,9,46 One of these was an update of the EULAR recommendations for management of knee osteoarthritis.46 The literature search for that review was systematic, but it covered only 2 databases (MEDLINE and EMBASE). A summary quality score was used, and the median score was 20 out of 28. This high score is surprising, considering that the standard of reporting for the 3 most important items was poor.41 Perhaps the high scores were the result of summing individual items. The use of such scores, however, is not advisable.47 The task force that prepared the EULAR update46 did not perform a quantitative summary but counted the number of positive trials, an approach that may be misleading.48 The authors' conclusion that “there is evidence to support the efficacy of HA [hyaluronic acid]” is not, in our opinion, well supported by the information presented.
The second systematic review and meta-analysis8 covered the same search period as ours. Lo and associates8 chose a hierarchy of relevant end points and selected the highest-ranking end point in each trial. The time of assessment was 2–3 months, but if data for this time point were not available, data were extracted on pain at 1–4 months after the first intra-articular injection. This creative approach may lump together end points that are only weakly related. The authors also extracted data on change from baseline, choosing not to rely on the assumption that the groups were comparable before treatment began. Indeed, for 2 trials24,26 the groups were not comparable at baseline. Nevertheless, trials are usually designed for simple between-group comparisons. Using such a change score might reduce the power or precision of an individual study.49
The trials included in the analysis of Lo and associates8 differed slightly from those in our analysis: we identified and included 4 published studies17,19,25,38 not used by Lo and associates, whereas those authors included 3 studies that we did not (2 published only as abstracts50,51 and 1 in which the patient's other knee served as the control [i.e., observations may not be independent]52). Lo and associates concluded that at best there is a small effect. Unsurprisingly, their findings were similar to the results of our meta-analysis.
The third systematic review and meta-analysis9 was published recently, but the search included only studies published up to 2001. Wang and colleagues9 used 3 end points to calculate “efficacy scores,” standardizing for different pain measures and summing efficacy scores over time. This approach transforms scores to a palatable size but makes clinical inferences very difficult. It is questionable if combining data from trials of highly variable length is reasonable. We found 7 additional studies,12,19,22,28,29,31,33 including 2 published before 2002 and 1 published before 2001. Wang and colleagues stated that hyaluronic acid led to significant improvements in pain and functional outcomes with few adverse events. Even though some of their reported results were of statistical significance, they were certainly not of clinical relevance.
Experimental studies and animal studies suggest that the molecular mass of hyaluronic acid may affect pain and the underlying inflammatory mechanisms in osteoarthritis.2,53 Lo and associates8 observed that studies using the highest-molecular-weight hyaluronic acid had much greater effect sizes but also exhibited crucial heterogeneity, but another trial found no significant difference in clinical efficacy between 2 hyaluronic acid preparations.29 We observed no association between molecular mass and effect of hyaluronic acid by informal methods (ranking the effects) or formal methods for indirect comparisons (meta-regression analysis).
The currently available evidence suggests that intra-articular hyaluronic acid is not clinically effective and may be associated with increased risk of adverse events. Therefore, this type of therapy should not be used for the treatment of painful osteoarthritis (except in clinical trials) until a large long-term trial with clinically relevant and uniform end points has clarified the benefit–risk ratio. Using predefined clinically important differences could further help in the assessment of its value for patients with knee osteoarthritis. Such an approach is of particular importance when considering the public health impact of the disease and its treatment.
Supplementary Material
Acknowledgments
We are grateful to Walter Grunt and Maximilan Gstöttner (Oberösterreichische Gebietskrankenkasse, Linz, Austria) for providing Austrian expenditure data for hyaluronic acid in 2001. We also thank Melanie Carr for editorial assistance.
Appendix 1.
Footnotes
An abridged version of this article appeared in the Apr. 12, 2005, issue of CMAJ and is available online at www.cmaj.ca/cgi/content/full/172/8/177/DC1
This article has been peer reviewed.
Contributors: All authors were involved in the conception and design of the article. Jasmin Arrich and Philipp Mad were responsible for data acquisition. Jasmin Arrich, Philipp Mad and Marcus Müllner were responsible for data interpretation. Marcus Müllner was responsible for the statistical analysis and wrote the first draft of the article. Franz Piribauer, Daniela Schmid, Klaus Klaushofer and Marcus Müllner revised the article. All authors approved the final version for publication.
The study was partly funded by the Hauptverband der Österreichischen Sozialversicherungsträger (Main Association of Austrian Social Security Institutions).
Competing interests: None declared.
Correspondence to: Dr. Marcus Müllner, Universität Klinik für Notfallmedizin, Medizin Universität Wien, Währinger Gürtel 18-20/6D, Allgemeines Krankenhaus Wien, A-1090, Austria; fax +43 (1) 40400 2512; marcus.muellner@meduniwien.ac.at
References
- 1.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91-7. [DOI] [PMC free article] [PubMed]
- 2.Lajeunesse D, Delalandre A, Martel-Pelletier J, Pelletier JP. Hyaluronic acid reverses the abnormal synthetic activity of human osteoarthritic subchondral bone osteoblasts. Bone 2003;33:703-10. [DOI] [PubMed]
- 3.Jordan KM, Sawyer S, Coakley P, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology (Oxford) 2004;43:381-4. [DOI] [PubMed]
- 4.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther 2003; 5:54-67. [DOI] [PMC free article] [PubMed]
- 5.Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000; 59: 936-44. [DOI] [PMC free article] [PubMed]
- 6.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000;43:1905-15. [DOI] [PubMed]
- 7.Felson DT, Anderson JJ. Hyaluronate sodium injections for osteoarthritis: hope, hype, and hard truths. Arch Intern Med 2002;162:245-7. [DOI] [PubMed]
- 8.Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA 2003;290:3115-21. [DOI] [PubMed]
- 9.Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2004;86-A:538-45. [DOI] [PubMed]
- 10.Dickersin K, Larson K. Establishing and maintaining an international register of RCTs. In: Clarke M, Oxman A, editors. Cochrane Collaboration handbook. In: The Cochrane Library; Issue 2, 1996 [updated Sept 1997]. Oxford: Update Software. Appendix 5c: Optimal search strategy for RCTs.
- 11.Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE [abstract]. In: 4th International Cochrane Colloquium; 1996 Oct 20-24; Adelaide (Australia). p. A28.
- 12.Dahlberg L, Lohmander LS, Ryd L. Intraarticular injections of hyaluronan in patients with cartilage abnormalities and knee pain. A one-year double-blind, placebo-controlled study. Arthritis Rheum 1994;37:521-8. [DOI] [PubMed]
- 13.Deeks JJ, Higgins JPT, Altman DG, editors. Section 8: Analysing and presenting results. In: Alderson P, Green S, Higgins J, editors. Cochrane reviewers' handbook 4.2.2. Chichester (UK): John Wiley & Sons; [updated Mar 2004]. Available: www.cochrane.org/resources/handbook/hbook.htm (accessed 2005 Feb 11).
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed]
- 15.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323:101-5. [DOI] [PMC free article] [PubMed]
- 16.Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol 1998;25:2203-12. [PubMed]
- 17.Bragantini A, Cassini M, De B, Perbellini A. Controlled single-blind trial of intra-articularly injected hyaluronic acid (Hyalgan®) in osteoarthritis of the knee. Clin Trials J 1987;24:333-40.
- 18.Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. ORTHOVISC Study Group. Clin Orthop 2001;(385):130-43. [DOI] [PubMed]
- 19.Bunyaratavej N, Chan KM, Subramanian N. Treatment of painful osteoarthritis of the knee with hyaluronic acid. Results of a multicenter Asian study. J Med Assoc Thai 2001;84(Suppl 2):S576-81. [PubMed]
- 20.Carrabba M, Paresce E, Angelini M, Re KA, Torchiana EEM, Perbellini A. The safety and efficacy of different dose schedules of hyaluronic acid in the treatment of painful osteoarthritis of the knee with joint effusion. Eur J Rheumatol Inflamm 1995;15:25-31.
- 21.Corrado EM, Peluso GF, Gigliotti S, De DC, Palmieri D, Savoia M, et al. The effects of intra-articular administration of hyaluronic acid on osteoarthritis of the knee: a clinical study with immunological and biochemical evaluations. Eur J Rheumatol Inflamm 1995;15:47-56.
- 22.Day R, Brooks P, Conaghan PG, Petersen M. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J Rheumatol 2004;31:775-82. [PubMed]
- 23.Dixon AS, Jacoby RK, Berry H, Hamilton EB. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin 1988;11:205-13. [DOI] [PubMed]
- 24.Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. OsteoarthritisCartilage 1993;1:97-103. [DOI] [PubMed]
- 25.Grecomoro G, Martorana U, Di Marco C. Intra-articular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmatherapeutica 1987;5:137-41. [PubMed]
- 26.Henderson EB, Smith EC, Pegley F, Blake DR. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: a randomised single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann Rheum Dis 1994;53:529-34. [DOI] [PMC free article] [PubMed]
- 27.Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology (Oxford) 1999;38:602-7. [DOI] [PubMed]
- 28.Jubb RW, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract 2003;57:467-74. [PubMed]
- 29.Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology (Oxford) 2002;41:1240-8. [DOI] [PubMed]
- 30.Lohmander LS, Dalen N, Englund G, Hamalainen M, Jensen EM, Karlsson K, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group. Ann Rheum Dis 1996;55:424-31. [DOI] [PMC free article] [PubMed]
- 31.Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee. Arch Intern Med 2002;162:292-8. [DOI] [PubMed]
- 32.Puhl W, Bernau A, Greiling H, Köpcke W, Pförringer W, Steck KJ, et al. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage 1993;1:233-41. [DOI] [PubMed]
- 33.Russell IJ, Michalek JE, Lawrence VA, Lessard JA, Briggs BT, May GS. A randomized, placebo (PL) and no-intervention (NI) controlled, trial of intra-articular (IA) 1 percent sodium hyaluronate (HA) in the treatment of knee osteoarthritis (OA). Arthritis Rheum 1992;35:S132-9.
- 34.Sala SF, De Miguel RE. Intra-articular hyaluronic acid in the treatment of osteoarthritis of the knee: a short term study. Eur J Rheumatol Inflamm 1995; 15: 33-8.
- 35.Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Ther Res Clin Exp 1994; 55: 220-32.
- 36.Tamir E, Robinson D, Koren R, Agar G, Halperin N. Intra-articular hyaluronan injections for the treatment of osteoarthritis. Clin Exp Rheumatol 2001;19:265-70. [PubMed]
- 37.Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther 1998;20:410-23. [DOI] [PubMed]
- 38.Wu JJ, Shih LY, Hsu HC, Chen TH. The double-blind test of sodium hyaluronate (ARTZ) on osteoarthritis knee. Zhonghua Yi Xue Za Zhi (Taipei) 1997;59:99-106. [PubMed]
- 39.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis 1957;16:499-502. [DOI] [PMC free article] [PubMed]
- 40.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. J Rheumatol 1993;20:557-60. [PubMed]
- 41.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed]
- 42.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708. [DOI] [PubMed]
- 43.Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges LV, et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet 1997;350:834-43. [DOI] [PubMed]
- 44.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed]
- 45.Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003;7:1-76. [PubMed]
- 46.Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145-55. [DOI] [PMC free article] [PubMed]
- 47.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054-60. [DOI] [PubMed]
- 48.Bushman BJ. Vote counting procedures in meta-analysis. In: Cooper H, Russel HL, editors. The handbook of research synthesis. New York: Sage Foundation; 1994: p. 194-213.
- 49.Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123-4. [DOI] [PMC free article] [PubMed]
- 50.Cohen MA, Shiroky JB, Bellachey ML, Neville C, Esdalle JM. Double-blind randomized trial of intra-articular (I/A) hyaluronate in the treatment of osteoarthritis of the knee [abstract]. Arthritis Rheum 1994;37:R31.
- 51.Pham T, Le Hananff A, Ravaud P. Lack of symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, when compared to diacerein and placebo in a one year controlled study in symptomatic knee osteoarthritis [abstract]. Arthritis Rheum 2003;48(Suppl):9. [DOI] [PMC free article] [PubMed]
- 52.Creamer P, Sharif M, George E, Meadows K, Cushnaghan J, Shinmei M, et al. Intra-articular hyaluronic acid in osteoarthritis of the knee: an investigation into mechanisms of action. Osteoarthritis Cartilage 1994;2:133-40. [DOI] [PubMed]
- 53.Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum 2004;50:314-26. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


