Abstract
Cervical cancer is the fourth leading cause of cancer-related death in women worldwide, with 90% of cases occurring in low- and middle-income countries (LMICs). There has been a global effort to increase access to affordable screening in these settings; however, a corresponding increase in availability of effective and inexpensive treatment modalities for ablating or excising precancerous lesions is also needed to decrease mortality. This article reviews the current landscape of available and developing technologies for treatment of cervical precancer in LMICs. At present, the standard treatment of most precancerous lesions in LMICs is gas-based cryotherapy. This low-cost, effective technology is an expedient treatment in many areas; however, obtaining and transporting gas is often difficult, and unwieldy gas tanks are not conducive to mobile health campaigns. There are several promising ablative technologies in development that are gasless or require less gas than conventional cryotherapy. Although further evaluation of the efficacy and cost-effectiveness is needed, several of these technologies are safe and can now be implemented in LMICs. Nonsurgical therapies, such as therapeutic vaccines, antivirals, and topical applications, are also promising, but most remain in early-stage trials. The establishment of evidence-based standardized protocols for available treatments and the development and introduction of novel technologies are necessary steps in overcoming barriers to treatment in LMICs and decreasing the global burden of cervical cancer. Guidance from WHO on emerging treatment technologies is also needed.
INTRODUCTION
In most high-income countries, the incidence and mortality of cervical cancer have decreased by 75% over the past 50 years due to the development of screening tests and the ability to detect and treat patients with lesions that have not progressed to invasive cancer.1 Yet, cervical cancer remains the third most common cancer in women worldwide, with nearly 90% of new cases diagnosed in low- and middle-income countries (LMICs).2 Low population coverage, poor-quality cytology, incomplete follow-up of screen-positive women, and barriers to effective treatment all contribute to the low success of cervical cancer prevention programs in LMICs. Because cervical cancer predominantly affects women of reproductive age, the societal impact and years of life lost attributable to the disease are substantially greater than for other female cancers.3
Prophylactic vaccines against human papillomavirus (HPV) have been developed and can be expected to prevent 70% to 90% of invasive cancer cases in unexposed women.4-9 Given the large number of women exposed and low rates of vaccine uptake in LMICs, screening and treatment will be needed for the foreseeable future.10
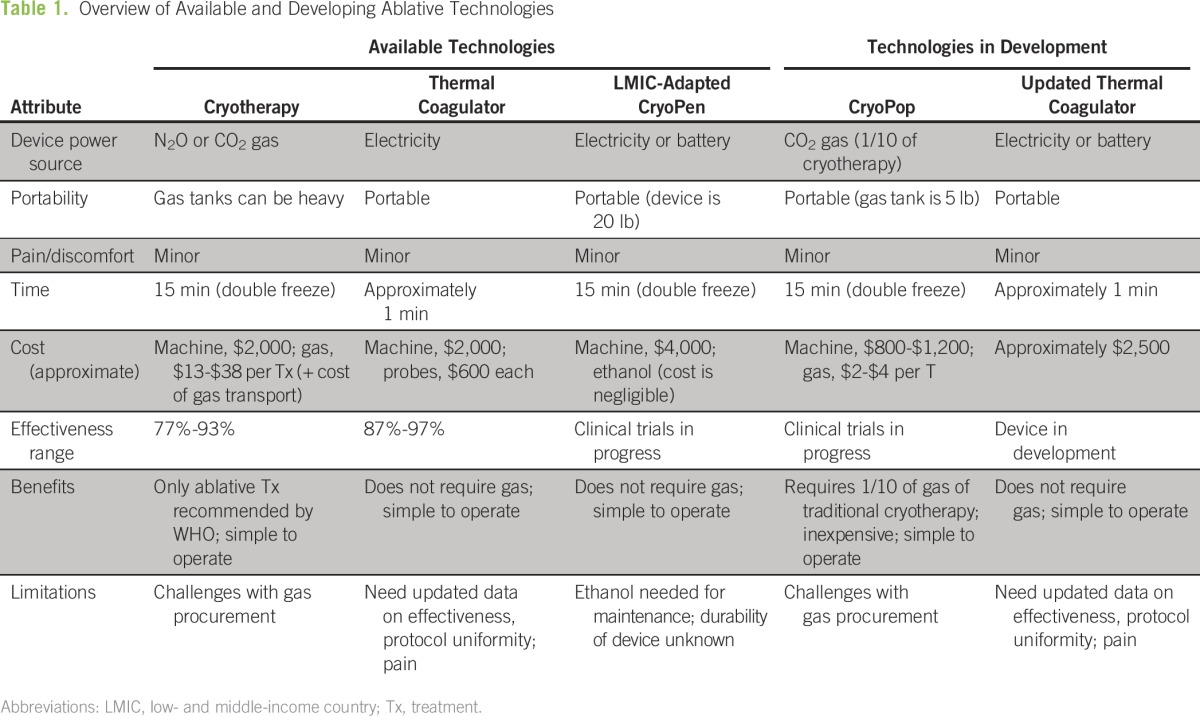
The WHO endorses screening with cytology, visual inspection with acetic acid, or primary high-risk HPV testing. Although many countries have cytology programs, effectiveness is limited by a lack of affordable treatment.11-14 In some countries, up to 80% of women diagnosed with cervical precancer never receive the recommended treatment.15 Current treatment methods are often expensive and difficult to implement at scale. Low-cost, durable, user-friendly treatment options in LMICs are needed. The purpose of this review is to discuss current and emerging technologies (Table 1) for precancer treatment, with a focus on utility in low-resource settings.
Table 1.
– Overview of Available and Developing Ablative Technologies
AVAILABLE TECHNOLOGIES
Loop Electrosurgical Excision Procedure
Brief history of the device.
In the 1900s, loop conization procedures were performed to remove grossly visible tumors. Electrosurgical excision of cervical intraepithelial neoplasia (CIN) with wire loop electrodes, commonly known as loop electrosurgical excision procedure (LEEP), grew in popularity in the last decades of the twentieth century, particularly in high-resource countries. Prendiville et al16 demonstrated in 1986 that a fine wire loop can be used to excise the transformation zone in unanesthetized patients, a significant advance in the management of CIN. LEEP can be performed in outpatient settings under local anesthesia. It is currently the standard of care for high-grade lesions in developed countries.
Description of the device.
The first commercial LEEP machine was introduced by CooperSurgical (Trumbull, CT) in 1991. LEEP equipment includes an electrosurgical unit that generates power, a smoke evacuator with tubing, disposable loop electrodes, and a speculum coated with anticonductive material.
How it works.
LEEP uses wire loop electrodes to remove precancerous lesions and the entire cervical transformation zone, excising and coagulating simultaneously.
Cure rates.
A Cochrane review reported a cure rate of 91% to 98% for CIN grade 3 with LEEP.17
Advantages.
LEEP is considered the standard of care for cervical precancer treatment. It is highly effective and has a low complication rate. In LMICs, LEEP is necessary if any of the following contraindications for cryotherapy are present:
the lesion is not fully visible on the transformation zone or its borders are not distinct
the lesion continues into the endocervix/os
the patient is postmenopausal
the lesion covers more than 75% of the cervix
Tissue collected during LEEP can be biopsied and pathologically examined to ensure that no invasive cancer goes undiagnosed.
Disadvantages/challenges.
LEEP needs to be performed by an experienced clinician, generally an obstetrician/gynecologist. This requirement, in addition to the need for electricity, local anesthesia, and resources for managing rare but serious adverse events such as hemorrhage, limit the use of LEEP as a first-line treatment in LMICs.
Complications.
Complications of LEEP include intraoperative bleeding (3.4%), infection (4.9%), and postoperative bleeding (5.3%).18 Less than 1% of women have cervical stenosis post-LEEP, and in 2% of women, there is inadequate visualization of the squamocolumnar junction.19 Although there was significant heterogeneity, a meta-analysis of studies on fertility and pregnancy outcomes after treatment of cervical precancer revealed that there is no evidence to support that treatment adversely affects these outcomes; the overall pregnancy rate was higher for women who received treatment compared with women who were untreated.20
Sterilization.
LEEP requires an individual disposable probe for each procedure. No sterilization is required.
Costs.
The LEEP device, including the electrosurgical unit and smoke evacuator, generally costs approximately$3,500 and is made by several companies. Disposable probes cost approximately $20 apiece; a box of five probes costs $110. An insulated speculum costs approximately $200.
Approval by regulatory agencies.
The CooperSurgical LEEP device was approved by the Food and Drug Administration (FDA) in 1991 for the treatment of cervical precancer, including CIN 1 to 3.
WHO recommendations.
The WHO recommends LEEP for treatment of CIN 2+ and recognizes the potential benefit of LEEP over cryotherapy. The WHO states that either LEEP or cryotherapy may be used, as available.21
Cryotherapy
Brief history of the device.
Cryotherapy has been used since 3000 BC, when ancient Egyptians used the technique to cure inflammation and as an analgesic. It has been used to treat chronic cervicitis since 1883 and to treat cervical neoplasia since 1964.22
Description of the device.
There are several commercially available cryotherapy devices that are similar in design, consisting of a probe that is attached to a tank of gas that cools the probe. Cryotherapy devices must reach −50°C to be effective.23 Gas-based cryotherapy uses nitrous oxide (N2O), medical-grade carbon dioxide (CO2), or industrial (beverage-grade) CO2. N2O is considered the standard gas for the procedure, because it is colder than CO2 (freezing at −89°C compared with −78°C for CO2) and achieves a greater depth of tissue necrosis than CO2.24
How it works.
The goal of ablative treatment is to freeze or heat the transformation zone, causing cellular necrosis. The transformation zone is the only area in which squamous cervical precancer can arise. To ablate precancer cells, it is important to reach a target depth of necrosis (DON). A DON of 3.5 mm is needed to treat 95% of CIN 1 to 3 lesions, and a depth of 4.8 mm is needed to treat 99% of all patients.25
Cure rates.
According to the Cochrane review, cure rates for CIN 3 following cryotherapy range from 77% to 93%.17 There is conflicting evidence on whether HIV-positive women have lower cure rates than HIV-negative women after treatment with cryotherapy. Although cryotherapy is normally 77% to 93% effective, recent evidence suggests that this is lower in HIV-positive women. Omenge et al26 found that the cure rate was only 37% at 6 months, using N2O.·
Advantages.
Traditional cryotherapy has many advantages. It is effective, relatively low cost, and low maintenance. It is also safe. Major bleeding and infection are less common in cryotherapy compared with cold-knife conization and LEEP.27
Disadvantages/challenges.
There are many challenges with the use of gas-based cryotherapy in low-resource settings, including difficulty in procuring high-quality gas. Industrial CO2 is readily available, but impurities in the gas may lead to inconsistent temperatures and device blockages.28 A trained technician is required to remove some blockages, but simple techniques—either wiping the nozzle with alcohol or placing the nozzle in water while the gas is on—can remove most clogs. The potentially high cost of obtaining and replenishing gas can be a barrier for use in LMICs. The cumbersome equipment can also prove problematic in field settings. Cryotherapy is typically performed using a tank weighing 50 to 70 lb, with 20 lb of compressed gas, that can treat two to 20 patients.29 A smaller tank can be used, but fewer patients can be treated. In addition, continual use reduces the pressure at the cryoprobe tip,29 which can prevent the device from reaching its lowest temperature.
Complications.
Complication rates are generally low. In a review by the WHO,30 risks of spontaneous abortion and infertility were not higher than that of the general population.
Sterilization.
Cryotherapy instruments generally have tips that are removable and can be autoclaved or sterilized using high-level disinfection (HLD) between patients. HLD recommended by the WHO uses chlorine bleach solution and can be completed in 20 to 30 min.31 The probe, which enters the vaginal canal but does not come into contact with mucous membranes, can be disinfected with alcohol between patients.
Costs.
The most widely used cryotherapy devices have an estimated cost between $1,700 and $2,000, with additional tips costing approximately $200 each. These prices may increase significantly because of distribution costs and taxes, specific to each country. Cheaper devices ($250) are available, but decreased cost may be associated with decreased quality. In addition to the cost of the machine, the required gas and related costs can range from $13 to $38 per treatment.32 To perform high-throughput cryotherapy, multiple tips must be available, which can increase cost.
Approval by regulatory agencies.
The Wallach LLl00 (Wallach Surgical, Trumbell, CT) received FDA approval for treatment of CIN 1 to 3 in 1981. Similar cryotherapy devices have received FDA approval by demonstrating equivalency to this device.
WHO recommendations.
Use of CO2 over N2O is recommended in settings where both gases are available, because N2O is not as widely available in LMICs and CO2 is significantly cheaper.21 The WHO recommends a double-freeze technique—a 3-min freeze followed by a 5-min thaw and a 3-min freeze—which is more effective than the single-freeze technique.21 The Cochrane review also recommended the double-freeze approach.17 Cremer et al24 found that the DON resulting from a single freeze using N2O was noninferior to DON resulting from a double freeze using N2O; necrosis with CO2-based cryotherapy was 1-mm less deep. Most devices come with various tip sizes and shapes, including flat and conical shapes ranging from 16 to 21 mm in diameter. A small diameter tip with a flat shape may not adequately cover the lesion, resulting in treatment failures. WHO guidelines suggest that a tip should be 19 ± 2 mm in diameter.29
WHO recommendations: Special cases to consider.
Pregnant patients.
Only women with invasive cervical cancer should be treated during pregnancy, and this should be at a referral facility. Pregnant women with preinvasive lesions should be asked to return 6 to 12 weeks postpartum for evaluation.33
HIV-positive women.
HIV-positive patients have an increased risk of cervical precancers, and these progress faster to invasive cancer than in HIV-negative women. Therefore, HIV-positive women need to be screened more frequently, and their lesions should be treated immediately. Follow-up evaluations of HIV-positive women should be performed within 12 months of treatment.33 In addition, HIV-positive women need to be screened earlier than do HIV-negative women. These women should be screened within 1 year of the onset of sexual activity, but starting no later than 21 years of age.34
Postmenopausal women.
Postmenopausal women should be treated using an excisional, rather than an ablative, technique if the transformation zone is not visible.33
Repeat patients.
Patients who are treated for precancerous lesions should be reevaluated after 12 months. Patients with CIN 1 and CIN 2 lesions who are free of lesions at 12 months can return to the normal screening timeline. Patients with CIN 3 lesions should be screened yearly for 3 years, even if they are lesion free at 12 months. If patients have lesions at 12 months, they should be referred for treatment with LEEP or cold-knife conization.33
Thermocoagulation (Cold Coagulation)
Brief history.
Thermocoagulation was used for decades to treat noninvasive cervical conditions primarily in the United Kingdom, but its application diminished after the introduction of excision procedures in the 1980s.
Description of the device.
The current commercial device, the WiSAP Cold-Coagulator (WiSAP, Brunnthal, Germany) is a simple box with a temperature dial and probes attached by cables. The device runs on either electricity or an automotive battery.
WiSAP is in the process of overhauling the thermocoagulator for the first time since 1966, with a focus on increasing ease of use and optimizing the device for low-resource settings with probe tips that require only HLD.
How it works.
Thermocoagulation uses heat to destroy cervical tissue: the superficial epithelium sloughs off after treatment, and the underlying stroma and glandular crypts are destroyed by desiccation.35 Expert interviews reveal no consensus on treatment regimens, with preference for both single-burn and multiple-burn techniques and with treatment durations ranging from 30 to 60 seconds.
Cure rates.
In 2014, Dolman et al36 published a meta-analysis on thermocoagulation, which is the most comprehensive review of the therapy (13 studies, with 4,569 patients). They concluded that it had cure rates similar to other ablative therapies, that it was safe and effective, and that it held particular promise for low-resource settings.36 They estimated an overall cure rate of 94% for CIN 1 to 3.36
A study of 1,628 women with CIN 3 treated with overlapping, 20-second, 100°C applications found a primary success rate of 95% at 1 year.37 A study of 725 women with CIN 1, 2, and 3 treated at 120°C for 30 to 40 seconds found that 87.2% of women had normal cytology after treatment and long-term negative follow-up.38 More rigorous trials need to be completed to standardize treatment.
Advantages.
Thermocoagulation is easy to administer, requiring only the small device and electricity. The updated model includes only one tip with a larger diameter that will prevent the need for changing tips to accommodate different lesion sizes. The probe tip can be disinfected rather than sterilized.
Disadvantages/challenges.
Reliance on electricity as a power source is a limitation of thermocoagulation. The optimal temperature and application timing have not yet been determined, and consensus is lacking on the DON needed with thermocoagulation. A study of 80 patients found that the mean depth of tissue destruction after a single application of a flat tip ranged from 2.6 mm (100°C for 20 seconds) to 3.5 mm (120°C for 30 seconds).39
Pain during thermocoagulation procedures is also a concern. Studies are needed to establish the optimal treatment parameters and ensure a tolerable patient experience. The meta-analysis by Dolman et al36 noted that at least one study found that 19% of women experienced pain, suggesting analgesia would improve acceptance of the procedure. Although DON increases with higher temperature and longer duration of exposure,39,40 and larger (20-mm) tips allow full coverage of lesions, these factors may contribute to increased pain.
Complications.
Dolman et al36 concluded that thermocoagulation treatment has no effect on fertility, noting that 94% of women were pregnant within 2 years of treatment.
Sterilization.
The thermocoagulation device has two components—only the probe (with attached tip) is in contact with the patient. The probe in the original device must be autoclaved after each use, whereas the probe in the updated device requires only HLD.
Costs.
The original WiSAP Cold-Coagulator device costs approximately $3,000: the electrical unit costs approximately $2,000, the instrument cable attaching the probe to the unit costs approximately $370, and one probe costs approximately $700. The updated device customized for use in LMICs is projected to cost approximately $2,500 and offers cost-saving benefits by requiring HLD rather than autoclave sterilization of the probe.
Regulatory approval and indications.
The WiSAP Cold-Coagulator is CE Mark–certified and indicated for CIN 1 to 3.
WHO recommendations.
Thermocoagulation was not included in the WHO 2013 cervical cancer guidelines. However, use of thermocoagulation is gaining popularity in LMICs, where ablative therapy is still common. Many countries in Africa and Asia have active thermocoagulation projects, and ease of use makes acceptability of this device high. There remains a need for formal WHO guidelines and standardization of the procedure.
LMIC-Adapted CryoPen
Brief history.
The CryoPen is a nongas–based ablation method that destroys tissue through applying a cooled single pen core (cryoprobe) to the cervix. CryoPen was originally founded to provide cryotherapy for dermatologic lesions but was modified for gynecologic use. The original gynecologic-adapted CryoPen was best used in a controlled office setting and required changing the cold core during the procedure. A new LMIC-adapted device has been designed and optimized for use in low-resource settings, because the device is more portable, durable, and cost-effective than the standard model.
Description of the device.
The LMIC-adapted CryoPen consists of a cooling device built into a toolbox, with an adjoining probe. The system is portable, is equipped with a handle, weighs 20 lb, and can treat approximately 24 women per 8-hour day.
How it works.
The machine cools by using a Stirling cooler, with helium as the refrigerant. The single pen core (cryoprobe) is inserted into a sheath with a 20-mm tip. The probe remains cold long enough to complete either a single, 5-min freeze or to follow the double-freeze method. It can use either electricity or batteries. Ethanol is needed to prevent the core from freezing to the device.
Cure rates.
The LMIC-adapted CryoPen is currently being developed and studied by Miriam Cremer, MD, supported by National Institutes of Health grant No. 5UH2CA189883. The purpose of the grant is to compare the LMIC-adapted CryoPen with conventional cryotherapy. A pilot study (n = 5) was performed in women undergoing hysterectomy for indications unrelated to cervical pathology. In these women, the average DON was 4.12 mm. A larger DON trial is under way, and a trial is planned to determine cure rates.
Advantages.
The LMIC-adapted CryoPen does not depend on gas and can be operated on electricity or a car battery. It is lighter and more portable than conventional cryotherapy. Midlevel providers are currently using the device in Haiti and have had positive experiences.
Disadvantages/challenges.
Reliance on electricity as a power source is a limitation of the LMIC-adapted CryoPen, although it can be charged with a car battery, if necessary. The device also requires small amounts of ethanol to keep the probe from freezing to the well.
Complications.
Complications have not been studied but are projected to be low and similar to cryotherapy.
Sterilization.
The LMIC-adapted CryoPen probe does not come into contact with the vaginal tissue. The LMIC-adapted CryoPen tips and the sheath can be cleaned using HLD methods.
Costs.
The LMIC-adapted CryoPen model is projected to cost approximately $4,000, which is more expensive than a gas-based system ($2,000). However, both the long-term cost and the cost per patient will be less than that for gas-based cryotherapy because there is no need to procure and distribute gas.
Regulatory approval and indications.
The CryoPen is approved by the FDA to treat CIN 1 to 3.
WHO recommendations.
To date, the WHO has not evaluated the device.
CryoPop
Brief history of the device.
The idea of the CryoPop was conceived in a class at the Center for Bioengineering Innovation and Design at Johns Hopkins University in 2011. Three graduate students in Biomedical Education worked with the nonprofit organization Jhpiego, an affiliate of the Johns Hopkins University, to come up with creative low-cost solutions for precancer treatment.
Description of the device.
CryoPop is an adaptation of cryotherapy technology that is designed to use less CO2 than existing equipment.
How it works.
The CryoPop consists of an ergonomic hand-held device and a detachable applicator with an anodized metal tip. The detachable applicator is connected to the gas tank to collect CO2 in the form of dry ice. It is then removed from the gas tank, clipped onto the CryoPop handle, and the anodized metal tip is placed against the cervix.
Cure rates.
The cure rates are unknown at this time. There is a current clinical trial under way at Johns Hopkins University, with Jean Anderson, MD, as the principal investigator on the study. The current trial (1UH2CA189923-01) is taking place in the Philippines, where the CryoPop is being compared with standard cryotherapy equipment. The current chamber holds enough dry ice for a single freeze, thus requiring a refill to perform a double freeze. In vitro studies using ballistic gelatin have documented tip temperatures comparable to or lower than existing cryotherapy devices.
Advantages.
The device is durable, easily repairable in the field, and inexpensive. It runs on any grade of CO2 gas, including industrial and food grade, and uses only a tenth as much gas per treatment as do existing cryotherapy devices. Midlevel providers found it easy to use during hands-on simulation exercises.
Disadvantages/challenges.
The CryoPop uses significantly less gas than standard cryotherapy equipment; however, the device still requires gas, which can be challenging to obtain in some settings.
Complications.
Complications are likely to be low and similar to conventional cryotherapy.
Sterilization.
The tips will require HLD or autoclaving. The applicator, which enters the vagina but does not come in contact with mucous membranes, can be disinfected with alcohol.
Costs.
The device is expected to cost approximately $800 to $1,200, in addition to lower per-patient costs because of the reduced gas required. Jhpiego expects to get 15 to 20 treatments out of one 5-lb tank of gas or 150 to 200 treatments from a 56-lb tank.
Regulatory approval and indications.
The CryoPop received FDA approval in the Philippines before the clinical trial in January 2016.
WHO recommendations.
To date, the WHO has not reviewed the device.
TREATMENTS OF THE FUTURE
Current treatment methods are invasive and at least somewhat uncomfortable to the patient. They can also be expensive, both in terms of device and commodity costs and health worker time. There are some promising new technologies in development, including therapeutic vaccines, antivirals, and topical applications. WHO is already studying therapeutic vaccines and antivirals, and has a working group between the cervical cancer and immunology teams. The International Agency for Research on Cancer is conducting several studies, as is the National Cancer Institute.
Therapeutic Vaccines
There are several therapeutic vaccines to cure HPV in development, several of which are entering phase III clinical trials. The goal of a therapeutic vaccine would be to elicit an immune response targeting E6 and E7 antigens; thus, these treatments can be thought of as a form of immunotherapy.41 An excellent summary of potential methods for therapeutic vaccines can be found in the article by Khallouf et al,42 “Therapeutic Vaccine Strategies Against Human Papillomavirus.” The most advanced therapeutic vaccine candidates include ADXS11-001 by Advaxis Immunotherapies’ (Princeton, NJ), GTL001 (ProCervix) by Genticel (Labège, France), and VGX-3100 by Inovio Pharmaceuticals (Plymouth Meeting, PA).43-45 The vaccine candidates all focus on cancers caused by HPV 16, or 16/18, which is responsible for approximately 70% of all cancers. Limitations to the vaccine are that it will still require multiple doses and only targets the 16/18 virus, missing 30% of oncogenic HPV types, although there is evidence of cross-reactivity.
Antivirals
Several groups are studying the use of antiviral drugs to target HPV, although these are early studies. Early-stage trials are under way for ranpirnase,46 HTI-1968, and other antivirals.47
Adoptive T-cell therapy is also being studied. In an early trial reported in 2014, two women with advanced invasive cervical cancer experienced complete recovery for 18 and 21 months at the time of reporting. One patient had a partial response, and six continued to progress.48 Critics of antiviral solutions note that so far, none have advanced to phase III clinical trials, and they have not shown significant effectiveness against HPV.
Artesunate
Artesunate is a semisynthetic analog of artemisinin that is used to treat malaria and is thought to have strong antineoplastic activity. A phase I trial of intravaginal artesunate is under way to evaluate its potential to treat precancerous cervical lesions.49
In conclusion, as low-resource settings begin to implement and improve cervical cancer screening programs, there is a greater need for effective and affordable treatment modalities for precancerous lesions. In LMICs, the current primary methods of gas-based cryotherapy and thermocoagulation are simple, effective, and widely accepted; however, there are clear gaps in evidence and consensus on proper implementation of these technologies, despite decades of use. Insufficient resources and infrastructure in LMICs further impede gas-based cryotherapy, because securing reliable gas supply chains is often difficult and expensive. Thus, the continued development of low-cost, effective treatment methods optimized for low-resource settings is necessary. Devices in development that require no or reduced amounts of gas are promising, as are nonsurgical therapies. Standardized, evidence-based protocols for current treatment modalities and the continued development of novel treatment methods are necessary to serve the increasing number of diagnosed women and decrease the worldwide burden of cervical cancer.
ACKNOWLEDGMENT
Supported by National Institutes of Health grant No. 1UH2CA189883-01 for development of the LMIC-adapted CryoPen (M.L.C. and M.M.). M.L.C. and M.M. do not receive financial compensation for their work with the LMIC-adapted CryoPen.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Mauricio Maza
No relationship to disclose
Celina M. Schocken
Stock or Other Ownership: Praakti Health, Omeros
Consulting or Advisory Role: Praakti Health
Katherine L. Bergman
No relationship to disclose
Thomas C. Randall
No relationship to disclose
Miriam L. Cremer
Honoraria: Merck
Speakers' Bureau: Merck
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2. Schottenfeld D, Fraumeni JF Jr (eds): Cancer Epidemiology and Prevention. Oxford, UK, Oxford University Press, 2006 doi: 10.1093/acprof:oso/9780195149616.001.0001. [Google Scholar]
- 3.Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries--key challenges and issues. N Engl J Med. 2007;356:1908–1910. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 4.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 5.Moore KA, II, Mehta V. The growing epidemic of HPV-positive oropharyngeal carcinoma: A clinical review for primary care providers. J Am Board Fam Med. 2015;28:498–503. doi: 10.3122/jabfm.2015.04.140301. [DOI] [PubMed] [Google Scholar]
- 6.Kang YJ, Lewis H, Smith MA, et al. Pre-vaccination type-specific HPV prevalence in confirmed cervical high grade lesions in the Māori and non-Māori populations in New Zealand. BMC Infect Dis. 2015;15:365. doi: 10.1186/s12879-015-1034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomberg M, Dehlendorff C, Sand C, et al. Dose-related differences in effectiveness of human papillomavirus vaccination against genital warts: A nationwide study of 550,000 young girls. Clin Infect Dis. 2015;61:676–682. doi: 10.1093/cid/civ364. [DOI] [PubMed] [Google Scholar]
- 8.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 9.Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: A randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 10.Graham JE, Mishra A. Global challenges of implementing human papillomavirus vaccines. Int J Equity Health. 2011;10:27. doi: 10.1186/1475-9276-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parham GP, Mwanahamuntu MH, Kapambwe S, et al. Population-level scale-up of cervical cancer prevention services in a low-resource setting: Development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS One. 2015;10:e0122169. doi: 10.1371/journal.pone.0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khozaim K, Orang’o E, Christoffersen-Deb A, et al. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Int J Gynaecol Obstet. 2014;124:12–18. doi: 10.1016/j.ijgo.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Kim YM, Lambe FM, Soetikno D, et al. Evaluation of a 5-year cervical cancer prevention project in Indonesia: Opportunities, issues, and challenges. J Obstet Gynaecol Res. 2013;39:1190–1199. doi: 10.1111/jog.12052. [DOI] [PubMed] [Google Scholar]
- 14.Gaffikin L, Blumenthal PD, Emerson M, et al. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: A demonstration project. Lancet. 2003;361:814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- 15.Gage JC, Ferreccio C, Gonzales M, et al. Follow-up care of women with an abnormal cytology in a low-resource setting. Cancer Detect Prev. 2003;27:466–471. doi: 10.1016/j.cdp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Prendiville W, Davies R, Berry PJ. A low voltage diathermy loop for taking cervical biopsies: A qualitative comparison with punch biopsy forceps. Br J Obstet Gynaecol. 1986;93:773–776. [PubMed] [Google Scholar]
- 17.Martin-Hirsch PPL, Paraskevaidis E, Bryant A, et al. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001318–CD001318. doi: 10.1002/14651858.CD001318. [DOI] [PubMed] [Google Scholar]
- 18.Sutthichon P, Kietpeerakool C. Perioperative complications of an outpatient loop electrosurgical excision procedure: A review of 857 consecutive cases. Asian Pac J Cancer Prev. 2009;10:351–354. [PubMed] [Google Scholar]
- 19.Pathfinder International . Loop Electrosurgical Excision Procedure (LEEP) Clinical Standards of Practice. Watertown, MA: Pathfinder International; 2013. [Google Scholar]
- 20.Kyrgiou M, Mitra A, Arbyn M, et al. Fertility and early pregnancy outcomes after treatment for cervical intraepithelial neoplasia: Systematic review and meta-analysis. BMJ. 2014;349:g6192. doi: 10.1136/bmj.g6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: WHO Press; 2011. [PubMed] [Google Scholar]
- 22.Cooper SM, Dawber RP. The history of cryosurgery. J R Soc Med. 2001;94:196–201. doi: 10.1177/014107680109400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 24.Cremer M, Ditzian L, Winkler JL, et al. Comparison of depth of necrosis using cryotherapy by gas and number of freeze cycles. J Low Genit Tract Dis. 2015;19:1–6. doi: 10.1097/LGT.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Karim FW, Fu YS, Reagan JW, et al. Morphometric study of intraepithelial neoplasia of the uterine cervix. Obstet Gynecol. 1982;60:210–214. [PubMed] [Google Scholar]
- 26. Omenge E, Liu T, Itsura P, et al: Visual inspection with acetic acid (VIA) agrees reasonably well with Pap smear and HR HPV typing for follow-up after VIA/cryotherapy in HIV-infected women. www.croiconference.org/sites/default/files/posters/851.pdf.
- 27.WHO WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. 2013. Geneva, Switzerland, World Health Organization. [PubMed]
- 28.Santos CL, Torres J, Sanchez J, et al. Lack of effectiveness of CO2 cryotherapy for treatment of CIN. Int J Gynaecol Obstet. 2004;87:44–45. doi: 10.1016/j.ijgo.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 29. WHO: WHO Technical Specifications: Cryosurgical Equipment for the Treatment of Precancerous Cervical Lesions and Prevention of Cervical Cancer. Geneva, Switzerland, World Health Organization, 2012. [Google Scholar]
- 30. WHO: WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland, World Health Organization, 2011. Int J Gynaecol Obstet 118:97-102, 2012. [DOI] [PubMed]
- 31.Tietjen L, Bossemeyer D, McIntosh N. Infection Prevention Guidelines for Healthcare Facilities With Limited Resources. 2004. Baltimore, MD, Jhpiego. [Google Scholar]
- 32.Campos NG, Tsu V, Jeronimo J. et al. When and how often to screen for cervical cancer in three low- and middle-income countries: A cost effectiveness analysis. Papillomavirus Res. 2015;1:38–58. [Google Scholar]
- 33.WHO . Comprehensive Cervical Cancer Control: A Guide to Essential Practice. Geneva, Switzerland, World Health Organization; 2014. [PubMed] [Google Scholar]
- 34.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 35.Semm K. New apparatus for the “cold-coagulation” of benign cervical lesions. Am J Obstet Gynecol. 1966;95:963–966. doi: 10.1016/0002-9378(66)90546-1. [DOI] [PubMed] [Google Scholar]
- 36.Dolman L, Sauvaget C, Muwonge R, et al. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: A systematic review. BJOG. 2014;121:929–942. doi: 10.1111/1471-0528.12655. [DOI] [PubMed] [Google Scholar]
- 37.Gordon HK, Duncan ID. Effective destruction of cervical intraepithelial neoplasia (CIN) 3 at 100 degrees C using the Semm cold coagulator: 14 years experience. Br J Obstet Gynaecol. 1991;98:14–20. doi: 10.1111/j.1471-0528.1991.tb10304.x. [DOI] [PubMed] [Google Scholar]
- 38.Zawislak A, Price JH, McClelland HR, et al. Efficacy of cervical intraepithelial neoplasia (CIN) treatment by cold coagulation. Ulster Med J. 2003;72:10–15. [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan ID. Cold coagulation. Baillieres Clin Obstet Gynaecol. 1995;9:145–155. doi: 10.1016/s0950-3552(05)80363-3. [DOI] [PubMed] [Google Scholar]
- 40.Haddad N, Hussein I, Blessing K. et al. Tissue destruction following cold coagulation of the cervix. J Gynecol Surg. 1988;4:23–27. [Google Scholar]
- 41.Trimble CL. HPV infection-associated cancers: Next-generation technology for diagnosis and treatment. Cancer Immunol Res. 2014;2:937–942. doi: 10.1158/2326-6066.CIR-14-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khallouf H, Grabowska AK, Riemer AB. Therapeutic vaccine strategies against human papillomavirus. Vaccines (Basel) 2014;2:422–462. doi: 10.3390/vaccines2020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallecha A, French C, Petit R, et al. Lm-LLO-based immunotherapies and HPV-associated disease. J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: A phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 46.Harrison L. Antiviral promising in early HPV trial. http://www.medscape.com/viewarticle/841876.
- 47.Keshevan M. Startup’s antiviral combats HPV, study shows. http://medcitynews.com/2014/08/san- diego-startups-topical-antiviral-drug-combats-hpv-new-study-shows/
- 48. Hinrichs C, Stevanovic S, Draper L: HPV-targeted tumor-infiltrating lymphocytes for cervical cancer. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA3008) [Google Scholar]
- 49. Sidney Kimmel Comprehensive Cancer Center: Intravaginal artesunate for the treatment of HPV+ high grade cervical intraepithelial neoplasia (CIN2/3). https://clinicaltrials.gov/show/NCT02354534.


