Abstract
Purpose
Programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1) targeted therapies have shown promising survival outcomes in several human neoplasms. However, it is unclear whether the expression of PD-L1 can be correlated to any clinical and pathologic variables in patients with cutaneous angiosarcoma (CA). The aim of this study was to evaluate the clinicopathological significance of PD-L1 expression in CA patients.
Materials and Methods
Data from 52 patients with CA were retrospectively reviewed. PD-L1 expression, tumor proliferation determined by Ki-67 index, and immunohistochemical evaluation of tumor-infiltrating lymphocytes, CD4+ and CD8+, were used to determine correlation with clinicopathological variables.
Results
PD-L1 was positively expressed in 40% of all patients. PD-L1 expression was significantly associated with tumor cell proliferation. Multivariate analysis confirmed that high levels of CD8+ tumor-infiltrating lymphocytes were a significant predictor in patients with clinical stage I CA and the positive expression of PD-L1 was an independent prognostic factor in predicting worse outcome.
Conclusion
PD-L1 expression is a novel pathologic marker for predicting worse outcome in patients with CA.
INTRODUCTION
Angiosarcoma is an extremely rare neoplasm that accounts for less than 2% of all soft tissue sarcomas.1 Cutaneous angiosarcoma (CA) is an aggressive neoplasm arising from vascular or lymphatic endothelial cells and has a dismal outcome. The 5-year overall survival rate of CA has been reported to range from 10% to 30%.2,3 Although CA can occur anywhere in the body, it is frequently localized to the head and neck regions. It has been challenging to determine the etiological factors for CA of the face and scalp because it affects a small percentage of the population. In Japan, a combined approach with chemotherapy and radiotherapy has been preferred as the first-line treatment of CA, instead of surgical resection. However, there is no established biomarker to predict the prognosis of CA in affected patients. Therefore, the need for a biomarker to define patients selected for surgery should be warranted.
Studies have reported the importance of tumor-infiltrating lymphocytes (TILs) in determining patient outcomes in angiosarcoma.4-6 Recently, Fujii et al4 demonstrated that high levels of CD8+ TILs are closely correlated with an improved prognosis and a longer disease-free period of distant metastases in patients with CA, suggesting that immunotherapy using TILs could be a novel treatment approach for angiosarcoma.4 Recent clinical trials targeting programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1) have exhibited a promising survival outcome in patients with cancers such as lung cancer and malignant melanoma. PD-L1 belongs to the B7 superfamily that downregulates T-cell activation through the PD-1 receptor and has a negative effect on the immune response.7-9 Although there are several studies on the expression of PD-L1 and its prognostic significance in different human neoplasms, the clinicopathological significance of PD-L1 expression in soft tissue sarcomas is not well understood. Kim et al10 reported that PD-1-positive TILs and PD-L1 expression were significantly correlated with the progression and poor survival outcomes in soft tissue sarcomas. In their study, the positive expression of PD-L1 after surgical resection was closely related to poor prognosis in various soft tissue sarcomas. However, less than 5% of patients in the study had angiosarcoma and 80% of samples from those patients exhibited positive PD-L1 staining. Therefore, prognostic significance of PD-L1 expression in patients with angiosarcoma could not be clearly determined. Shen et al11 found that PD-L1 expression positively correlated with TILs in osteosarcoma and thus could be a promising immunotherapeutic approach to treat the disease. When considering the possible clinical importance of immune checkpoint inhibitors targeting PD-L1/PD-1 in a disease associated with poor outcomes, further studies on PD-L1 expression can help understand its potential immunotherapeutic and prognostic roles in treating CA. On the basis of the clinical and therapeutic evidence that correlates PD-L1 expression to predicting outcomes in other human neoplasms, we conducted the current study to evaluate the clinicopathological significance of PD-L1 expression and TILs in CA.
MATERIALS AND METHODS
Patients
We identified and retrospectively examined 52 consecutive patients who were positively diagnosed with CA at the Gunma University and University of the Ryukyus hospitals between October 1987 and September 2014. We obtained 52 paraffin-embedded tissue samples (from biopsy or surgical resection) and patient medical records from the two hospitals. This study was approved by the institutional review board of Gunma University and the ethical committee for clinical studies at University of the Ryukyus. The approach used for the evaluation and resection of these tumors has been described previously.4
There is no established method to clinically classify tumor stages in CA. Therefore, as described previously, we tentatively classified patients with CA into three stages: stage I for those with cutaneous tumors, stage II for those with lymph node metastases, and stage III for those with distant metastases, on the basis of the clinical evaluation before treatment.4
Immunohistochemical Staining
For PD-L1 and Ki-67, immunohistochemical staining was performed according to the procedures described in previous studies.12,13 Rabbit monoclonal antibodies against PD-L1 (1:100 dilution; Abcam, Cambridge, MA) and Ki-67 (1:40 dilution; Dako, Glostrup, Denmark) were used. The expression of PD-L1 was considered positive when membrane staining was observed. A semiquantitative scoring method was used for PD-L1: 1, 0% to 5%; 2, 5% to 10%; 3, 10% to 25%; and 4, > 25% of cells were positive. Tumors with score > 2 were graded as positive.
A highly cell-rich area of the immunostained tissue sections was evaluated for Ki-67. Approximately 1,000 nuclei were counted on each slide. Proliferative activity was assessed as the percentage of Ki-67-stained nuclei (Ki-67 labeling index) in the sample. The median value of the Ki-67 labeling index was evaluated, and tumor cells with values greater than the median value were defined as cells with high expression. Immunohistochemical staining was done for CD4+ (1:40 dilution; Dako) and CD8+ (1:200 dilution; Abcam) TILs in the tumor specimens. After evaluating the specimens entirely, the number of CD4+ and CD8+ TILs were counted in the selected hot spot in a ×400 magnified field (0.26 mm2 field area). We determined the median number of CD4+ and CD8+ TILs as the cutoff point for CD4+ and CD8+ TIL density. The tissue sections were examined in a blinded fashion by at least two of the authors, using light microscopy.
Statistical Analyses
Probability values of <.05 indicated a statistically significant difference as determined by Fisher's exact test. The correlation between different variables was analyzed using the nonparametric Spearman's rank test. The Kaplan-Meier method was used to estimate survival as a function of time, and survival differences were analyzed by the log-rank test. Overall survival (OS) was determined as the time elapsed from definite diagnosis to death from any cause. Progression-free survival (PFS) was defined as the time elapsed between definite diagnosis and the disease progression or death as a result of disease. Multivariate analyses were performed using a stepwise Cox proportional hazards model to identify independent prognostic factors. Statistical analyses were performed using GraphPad Prism 4 (Graph Pad Software, San Diego, CA) and JMP 8 (SAS Institute, Cary, NC) for Windows.
RESULTS
Patient Demographics
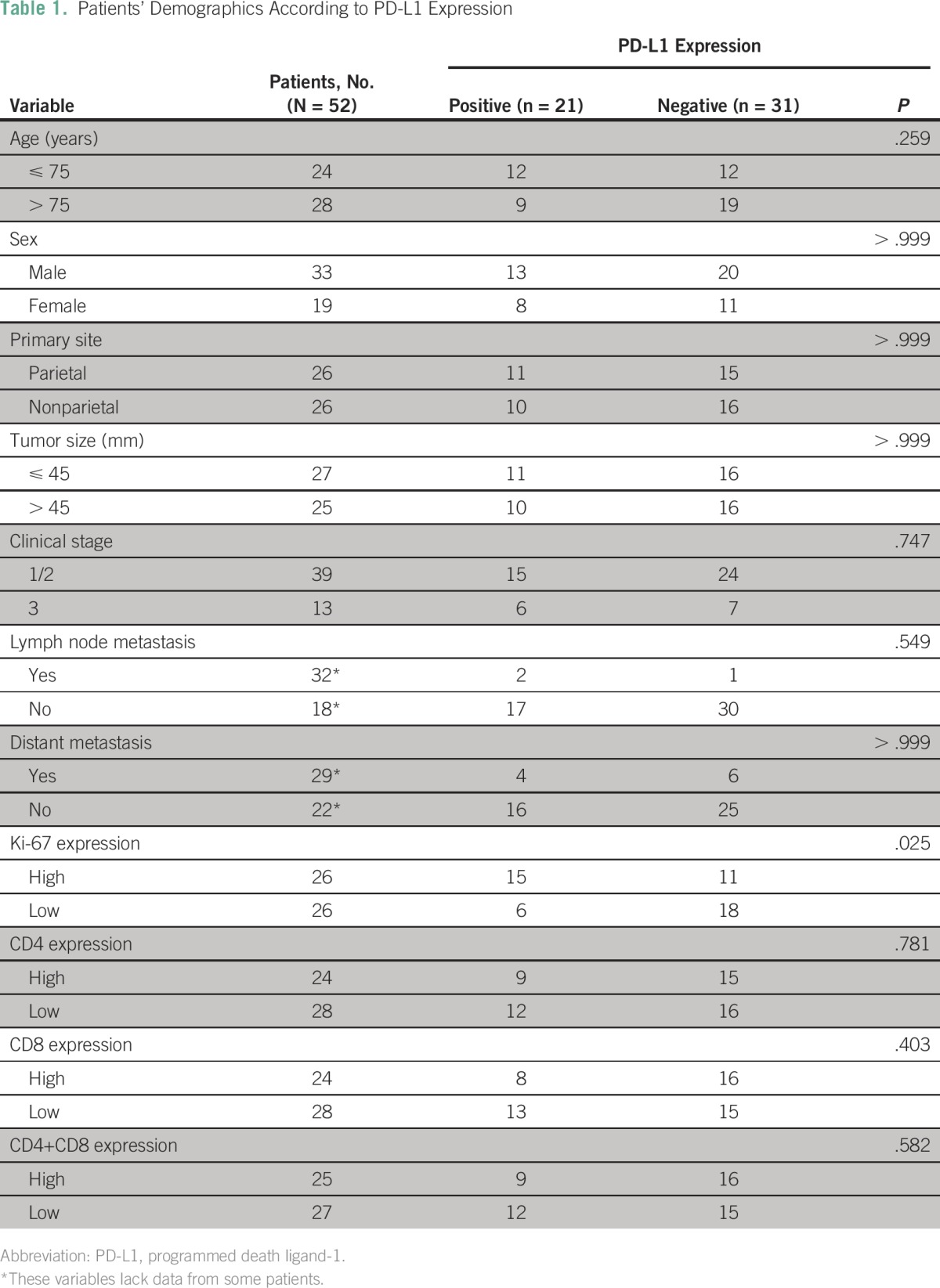
Table 1 summarizes patient demographics according to the expression of PD-L1 in patients with CA. The median age was 76 years (range, 57 to 91 years). The primary site of the lesion was the scalp in 34 patients, face in 13, neck in four, and leg in one. Thirty-nine patients were classified as having stage I disease, two as stage II, and 11 as stage III. Surgical resection had been done in 17 patients, radiotherapy in 45, and systemic chemotherapy in 30. As a chemotherapeutic regimen, 26 patients were treated with taxane agents including docetaxel (n = 25). The median follow-up period was 12.3 months (range: 1.6 to 92.1 months).
Table 1.
Patients’ Demographics According to PD-L1 Expression
Immunohistochemical Analyses
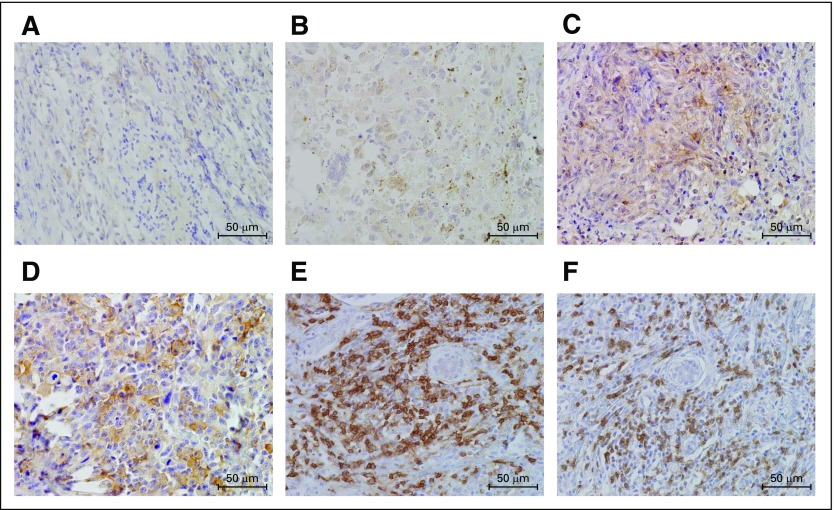
Fifty-two primary lesions of CA were analyzed using immunohistochemical techniques. Figure 1 shows the representative immunohistostained images of PD-L1 expression (on the basis of a scoring scale of 1 to 4) and CD4+ and CD8+ TILs. PD-L1 immunostaining was observed to be localized predominantly on the plasma membrane in the angiosarcoma cells. The rate of positive PD-L1 expression was 40% (in 21 of 52 patients) and the mean ± SD score for PD-L1 was 1.8 ± 0.8. The percentages of PD-L1 immunohistostains scoring 1, 2, 3, and 4 were 60% (31 of 52), 15% (eight of 52), 13% (seven of 52), and 12% (six of 52), respectively. On the basis of the analyses of CA specimens, cutoff values for the Ki-67 labeling index were determined. The median Ki-67 labeling index was 9% (range, 0% to 51%), and a value of 9% was chosen as the cutoff point. High Ki-67 expression was identified in 50% of the patient samples (26 of 52). The median number of CD4+ and CD8+ TILs was 53 (range, 14 to 158) and 41 (range, 0 to 180), respectively. High CD4+ and CD8+ levels were identified in 46% of the patient samples (24 of 52). The expression of PD-L1 was significantly associated with tumor cell proliferation as determined by the Ki-67 labeling index (Table 1).
Fig 1.
Representative immunohistochemical staining of cutaneous angiosarcoma. Immunohistostaining of PD-L1 reveals the membrane-staining pattern of. (A-D) Semiquantitative scoring of PD-L1 staining: (A) 1; (B) 2; (C) 3; (D) 4. The representative images of (E) CD4 and (F) CD8 as primary tumor-infiltrating lymphocytes are shown.
Correlation Between PD-L1 Expression and Different Variables
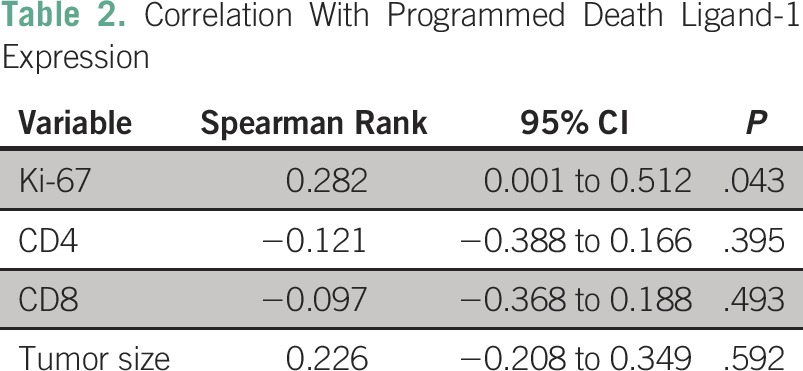
The expression of PD-L1 was significantly correlated with Ki-67 but not CD4+ or CD8+ TILs or tumor size (Table 2).
Table 2.
Correlation With Programmed Death Ligand-1 Expression
Univariate and Multivariate Analyses
The median survival times of OS and PFS for all patients were 448 days and 270 days, respectively. The 1-year survival rates of OS and PFS for all patients were 55% and 37%, respectively. Of 52 patients, 40 developed recurrences after the initial treatment and 41 died eventually.
Table 3 shows the results of the univariate and multivariate analyses for all patients. The expression level of PD-L1 was identified as a significant prognostic factor for OS by univariate analysis. Significant prognostic variables for PFS were found to be sex, clinical stage, and the expression levels of PD-L1 and CD8+. The multivariate analysis confirmed that PD-L1 expression was an independent prognostic factor for predicting a worse OS and PFS. Multivariate analysis also confirmed that clinical stage, sex, and the expression level of CD8+ were independent prognostic variables in PFS.
Table 3.
Univariate and Multivariate Survival Analysis in All Patients

Figure 2A and 2B show the Kaplan-Meier curves for patients with a positive or negative PD-L1 expression for OS and PFS, respectively. For patients with clinical stage I disease, a significant difference in the OS was observed between positive and negative expression of PD-L1 (Fig 2C). There is a significant difference in the OS between high and low numbers of CD8+ TILs (Fig 2D), but not between high and low numbers of CD4+ TILs (Fig 2E).
Fig 2.
Kaplan-Meier curves for the patients with a positive or negative expression of programmed death ligand-1 (PD-L1). A significant difference in the (A) overall survival (OS) and (B) progression-free survival (PFS) was recognized with respect with the expression of PD-L1 in all patients (N = 52). (C) In patients with clinical stage I disease (n = 39), a significant difference in the OS was observed between positive and negative expression of PD-L1 and (D) between high and low numbers of CD8+ TILs, (E) but not between high and low numbers of CD4+ TILs. (F) No statistically significant difference in the OS was observed between high and low expression of Ki-67. (G) OS according to scoring of PD-L1 expression.
Survival Analysis According to Different Clinical Parameters
We performed a survival analysis according to the clinical stages classified earlier. Table 4 shows univariate and multivariate analyses in patients with CA who were classified as having clinical stage I disease (n = 39). Univariate analysis confirmed that the expression of PD-L1 and CD8+ levels was a significant prognostic factor for OS and PFS. The expression of PD-L1 and CD8+ levels was also found to be an independent predictor for worse prognosis, by multivariate analysis. In patients with CA who were classified as having clinical stages II or III disease, no statistically significant differences in the OS (P = .422) and PFS (P = .751) were observed between patients with positive and negative PD-L1 expression.
Table 4.
Univariate and Multivariate Survival Analysis in Patients With Clinical Stage I Disease

Of the 29 patients who initially underwent chemotherapy, 24 were administered docetaxel; five received other regimens. We performed the survival analysis of these 29 patients. No statistically significant difference was found in the OS (P = .095) and PFS (P = .102) between patients with positive (median OS, 263 days; median PFS, 133 days) and negative (median OS, 676 days; median PFS, 423 days) PD-L1 expression.
DISCUSSION
Immune checkpoint inhibitors targeting PD-1 or PD-L1 have been available to treat human neoplasms, including malignant melanoma and lung cancer, in Japan and other countries. Several studies have focused on the prognostic significance of PD-L1 expression in certain cancers.13-18 The positive rate of PD-L1 expression was 50% in breast cancer, 39.2% in pancreatic cancer, 42.2% in gastric cancer, 25% in hepatocellular carcinoma, 43.9% in esophageal cancer, and 66.3% in renal cell carcinoma.13-18 PD-L1 expression may predict worse outcomes in these carcinomas. Another study also identified PD-L1 expression as an independent indicator of poor prognosis in soft tissue sarcoma.10 In 105 patients with soft tissue sarcoma, a positive PD-L1 expression was seen in 65%, and PD-L1 expression was significantly associated with advanced clinical stage, distant metastases, and advanced clinicopathological variables.10 However, that study included only five patients with angiosarcoma, and it was unclear whether the increased expression of PD-L1 could be a significant predictor for poor prognosis in patients with angiosarcoma as well. Recently, D’Angelo et al19 documented the clinicopathological significance of TILs and PD-L1 expression in 50 soft tissue sarcoma specimens: 14 gastrointestinal stromal tumors, five synovial sarcomas, four leiomyosarcomas, three spindle cell sarcomas, three angiosarcomas, and 21 other cancers. They reported that the expression of PD-L1 was not observed in three angiosarcoma specimens without the description of primary site. They concluded that the expression of PD-L1 was low in sarcoma, and there was no association between PD-L1 expression and survival. In contrast, our study revealed that the positive rate of PD-L1 expression in CA was almost similar to that in human epithelial tumors and was an independent prognostic factor for worse outcome. Because PD-1/PD-L1 therapy has been recognized to be effective in patients with advanced cancers,20 it is clinically important to examine the PD-L1 expression in tumor tissues before PD-1/PD-L1 immunotherapy. In fact, 36% of patients with positive PD-L1 tumors responded to anti-PD1 immunotherapy, whereas patients with negative PD-L1 tumors did not.21
In the current study, we found the expression level of PD-L1 to be closely correlated with tumor cell proliferation. A previous study also reported a strong positive link between PD-L1 expression and the cell proliferative Ki-67 marker in patients with breast cancer.22 Fujii et al4 reported that no statistically significant difference was present between the prognosis and Ki-67 labeling index of 30 patients with CA, which corresponds with our results indicating Ki-67 was not identified as a prognostic predictor. Although the Ki-67 index closely correlates with the expression level of PD-L1, it remains unclear why the expression level of Ki-67 was not significantly associated with the prognosis of patients with CA. The small sample size may have biased our results. Further investigation is required to confirm whether the relationship between prognostic significance and Ki-67 labeling index exists in CA.
Angiosarcoma is a rare disease and no effective chemotherapeutic regimen has been established. Surgical resection or radiation therapy has been conventionally recommended. Panel et al23 have described that paclitaxel is an effective and well-tolerated regimen for patients with unresectable angiosarcoma, yielding an overall response of approximately 18% and median survival time of 8 months. Moreover, Nagano et al24 reported that six of nine patients treated with docetaxel revealed good response: complete responses in two patients and partial response in four patients. Thus, docetaxel has been accepted as an effective chemotherapeutic agent against CA. In patients with advanced CA, taxane-based agents have been chosen for treatment. In our study, 26 patients were treated with taxane regimens, including docetaxel (n = 25) and paclitaxel (n = 1). However, we found no significant difference in the prognosis after systemic chemotherapy with respect to the expression level of PD-L1. Further investigation is necessary to understand the relationship between the PD-L1 expression and the clinical effectiveness of chemotherapeutic agents.
One limitation of our study was a small sample size. However, CA is an extremely rare sarcoma among the Japanese and its frequency seems to be different among races.25,26 We could not find a significant difference in OS between patients with positive and negative PD-L1 expression among the 29 patients treated with chemotherapy. Of these 29 patients, 21 had clinical stage I disease and eight had clinical stage III disease. Heterogenous groups of different clinical stages may have biased the results of our survival data. Second, PD-L1 antibodies were not same as those used in previous immunohistochemical studies. We checked several commercially available PD-L1 antibodies to obtain clear staining. Finally, we have not carried out the experimental study using any inhibitors targeting PD-L1. The inhibitors of PD-1/PD-L1 have been widely used for treating lung cancer, malignant melanoma, and other cancers, and these agents show a favorable survival outcome compared with the cytotoxic agents.27,28 Further studies to investigate the antitumor effect of PD-1/PD-L1 in vivo using the animal model of CA would expand our understanding of PD-L1 expression in angiosarcoma.
In conclusion, high expression levels of PD-L1 in CA were identified as a significant predictor for poor prognosis, especially in patients of the clinical stage I disease, and could be an important clinicopathological marker for choice of treatments. Moreover, we confirmed high levels of CD8+ TILs could also be a significant biomarker for favorable outcome, as previously described.4 The inhibition of PD-L1 function could be a novel therapeutic target for CA with high PD-L1 expression.
ACKNOWLEDGMENT
We thank Yuka Matsui (Gunma University), and Ayako Nakamura and Ritsuko Tokumon (University of the Ryukyus) for their technical assistance during the manuscript preparation. We also thank Tomoko Okada (Gunma University) for her help in data collection and technical assistance, and Drs. Ryoko Awazawa, Takuya Miyagi, and Sayaka Yamaguchi (University of the Ryukyus) for their helpful guidance and assistance in data collection.
AUTHOR CONTRIBUTIONS
Conception and design: Akira Shimizu, Kyoichi Kaira, Takayuki Asao, Masahiko Nishiyama, Osamu Ishikawa
Financial support: Takayuki Asao, Masahiko Nishiyama, Kenzo Takahashi
Administrative support: Akira Shimizu, Kyoichi Kaira, Masahito Yasuda, Kenzo Takahashi, Osamu Ishikawa
Provision of study materials or patients: Yuko Okubo, Daisuke Utsumi, Kenzo Takahashi, Osamu Ishikawa
Collection and assembly of data: Akira Shimizu, Kyoichi Kaira, Yuko Okubo, Daisuke Utsumi, Masahito Yasuda, Kenzo Takahashi
Data analysis and interpretation: Akira Shimizu, Kyoichi Kaira, Yuko Okubo, Osamu Ishikawa
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Akira Shimizu
No relationship to disclose
Kyoichi Kaira
No relationship to disclose
Yuko Okubo
No relationship to disclose
Daisuke Utsumi
No relationship to disclose
Masahito Yasuda
No relationship to disclose
Takayuki Asao
No relationship to disclose
Masahiko Nishiyama
Research Funding: Yakult Honsha
Kenzo Takahashi
No relationship to disclose
Osamu Ishikawa
No relationship to disclose
REFERENCES
- 1. Goldblum JR, Folpe AL, Weiss SW: Malignant vascular tumors, in Weiss SW, Goldblum JR (eds), Enzinger and Weiss’s Soft Tissue Tumors. Philadelphia, PA, Mosby Elsevier, 2008, pp 703-732. [Google Scholar]
- 2.Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Clin Oncol. 2006;29:524–528. doi: 10.1097/01.coc.0000227544.01779.52. [DOI] [PubMed] [Google Scholar]
- 3.Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661–667. doi: 10.1002/hed.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii H, Arakawa A, Utsumi D, et al. CD8⁺ tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer. 2014;134:2393–2402. doi: 10.1002/ijc.28581. [DOI] [PubMed] [Google Scholar]
- 5.Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: A study of forty-four cases. Cancer. 1981;48:1907–1921. doi: 10.1002/1097-0142(19811015)48:8<1907::aid-cncr2820480832>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Zietz C, Rumpler U, Stürzl M, et al. Inverse relation of Fas-ligand and tumor-infiltrating lymphocytes in angiosarcoma: Indications of apoptotic tumor counterattack. Am J Pathol. 2001;159:963–970. doi: 10.1016/S0002-9440(10)61772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter L, Fouser LA, Jussif J, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H, Honjo T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim JR, Moon YJ, Kwon KS, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen JK, Cote GM, Choy E, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res. 2014;2:690–698. doi: 10.1158/2326-6066.CIR-13-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaira K, Endo M, Abe M, et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol. 2010;28:3746–3753. doi: 10.1200/JCO.2009.27.4662. [DOI] [PubMed] [Google Scholar]
- 13.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 15.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 16.Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi J, Wada Y, Matsumoto K, et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084–2091. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 19.D’Angelo SP, Shoushtari AN, Agaram NP, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46:357–365. doi: 10.1016/j.humpath.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421–1427. doi: 10.1038/bjc.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 23.Panel N, Bui BH, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: The ANGIOTAX study. J Clin Oncol. 2008;26:269–272. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 24.Nagano T, Yamada Y, Ikeda T, et al. Docetaxel: A therapeutic option in the treatment of cutaneous angiosarcoma: Report of 9 patients. Cancer. 2007;110:648–651. doi: 10.1002/cncr.22822. [DOI] [PubMed] [Google Scholar]
- 25.Luke JJ, Keohan ML. Advances in the systemic treatment of cutaneous sarcomas. Semin Oncol. 2012;39:173–183. doi: 10.1053/j.seminoncol.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sturgis EM, Potter BO. Sarcomas of the head and neck region. Curr Opin Oncol. 2003;15:239–252. doi: 10.1097/00001622-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]