Abstract
Purpose
Adherence to international antiemetic prophylaxis guidelines like those of ASCO can result in better control of chemotherapy-induced nausea and vomiting; however, the extent of implementation of such guidelines in India is unknown. Therefore, this survey was planned.
Methods
This study was an anonymized cross-sectional survey approved by the ethics committee. Survey items were generated from the clinical questions given in the ASCO guidelines. The survey was disseminated through personal contacts at an oncology conference and via e-mail to various community oncology centers across India. The B1, B2, and B3 domains included questions regarding the optimal antiemetic prophylaxis for high, moderate, and low-minimal emetogenic regimens.
Results
Sixty-six (62.9%) of 105 responded and 65 centers (98.5%) were aware of the published guidelines. The partial, full, and no implementation scores were 92.5%, 4.5%, and 3.0%, respectively. Full implementation was better for the low-minimal emetogenic regimens (34.8%) than the highly emetogenic regimens (6.1%). The three most frequent reasons for hampered implementation of ASCO guidelines in routine chemotherapy practice cited by centers were a lack of sensitization (26 centers; 39.4%), lack of national guidelines (12 centers; 18.2%), and lack of administrative support (10 centers; 15.2%).
Conclusion
Awareness regarding ASCO antiemetic guidelines is satisfactory in Indian oncology practices; however, there is a need for sensitization of oncologists toward complete implementation of these guidelines in their clinical practice.
INTRODUCTION
Chemotherapy-induced nausea and vomiting (CINV) is one of the most common and most distressing complications of chemotherapy.1 It has a detrimental effect on the quality of life of patients and their functional well-being.2-4 Uncontrolled nausea or vomiting is also associated with frequent hospital and emergency department visits, is resource consuming,5-8 and can lead to impaired compliance with chemotherapy.1,9,10
There has been significant progress in the development of newer antiemetics and the use of combinations of antiemetics. The optimal combination that is required depends on the emetogenic potential of the specific chemotherapy regimen used. Clinical guidelines have been published by professional bodies, such as ASCO and the European Society for Medical Oncology, for the same.11,12 These guidelines have been shown to control the rate and severity of CINV13-16; however, adherence rates internationally are variable (29% to 57.3%).13,14 In India, there are no national guidelines, and oncologists rely most commonly on ASCO clinical updates and the National Comprehensive Cancer Network recommendations, with the extent of implementation largely unknown. To address this gap, the present survey was planned with the primary objective of evaluating the proportion of cancer centers that have fully implemented the ASCO antiemetics clinical practice guideline11 standards in routine chemotherapy practice. Secondary objectives were to determine the proportion of centers that implement these guidelines partially, to determine the standards that are most commonly and least commonly implemented in each category of chemotherapy drugs according to emetogenicity, and to determine the difficulties that are faced in implementing these standards.
METHODS
Survey Instrument
A written survey on the basis of the standards according to the ASCO antiemetics clinical practice guideline was designed.11 We had conducted a similar survey previously and based the present study on the earlier experience.17 Survey items were generated from the clinical questions in the guidelines and in the same order. The first and second clinical questions, which dealt with antiemetic prophylaxis in high and moderate emetogenic chemotherapy regimens, respectively, were further broken down into six subquestions each. These questions enquired about the use of an NK receptor antagonist, 5HT3 antagonist, dexamethasone, and their schedules. The remaining survey questions were based on statements in the guideline. Because our intent was to focus on implementation of the ASCO guidelines in a relatively homogenous population of adults with solid tumors, the survey did not include questions related to pediatric patients, hematologic malignancies, bone marrow transplant centers, patients being treated with radiation, and breakthrough emesis.
The survey included questions regarding institutional antiemetic policy (domain A); optimal antiemetic prophylactic regimen for highly emetogenic antineoplastic drugs (domain B1); optimal antiemetic prophylactic regimen for moderately emetogenic antineoplastic drugs (domain B2); optimal antiemetic prophylactic regimen for low-minimal emetogenic antineoplastic drugs (domain B3); and antiemetic use in special situations (domain C). Response options in the survey included a four-item Likert scale (always, usually, rarely, and never), a binary scale (yes or no), or a multiple-choice format, depending on the type of question. In addition, we inquired about the important factors that prevented the center from fully implementing the standards. This response was in the form of a multiple-choice item along with a free-text option. The survey instrument is shown in the Appendix.
In addition, some questions regarding the nature of oncology practice were also added. These questions addressed the following items: state in which the center was located, setting of practice (urban or rural), teaching status (yes or no), funding source (public or private), and approximate number of patients seen daily.
Survey Distribution
This was an anonymized cross-sectional survey. The survey was designed on Google forms (Google, Mountain View, CA). Oncologists that administer chemotherapy were identified from the ICON (Indian Cooperative Oncology Network) database and invited to participate in this survey. Individual emails with a link to the survey form were sent to recognized cancer center chemotherapy units, and oncologists in these units were requested to complete the survey between October 22, 2015, and January 10, 2016. The invitation for the survey was restricted to a single oncologist from each unit. If a center had a single team, only one of the oncologists was contacted. The center’s team was considered a single unit. If a center had multiple chemotherapy units that functioned independently of each other and had policies independent of each other, then one oncologist from the unit was invited and was considered an independent entity in the survey
In addition, the survey instrument in PDF format was distributed through personal contacts in a national biennial joint conference of the ISMPO (Indian Society of Medical and Pediatric Oncology) and the Indian Society of Oncology that was held from November 6 to 8, 2015, at Hotel Grand Hyatt, Mumbai, India. Only members from units that were not invited (for lack of a valid e-mail address), or who were invited but had not completed the survey online, were given the option of completing the survey at the conference.
Electronic responses were automatically captured in a Google spreadsheet that was linked to the online form, and responses collected on the PDF version were manually entered into the same sheet.
Survey Population
The survey population consisted of adult oncology practices that administer chemotherapy on a regular basis. This included regional cancer centers, dedicated corporate cancer centers, cancer wings of medical colleges, hospitals, and private oncology day care centers. As much as possible, only a single oncologist was contacted from each center. If multiple oncologists from one center participated, they were asked to collaborate and submit a single response.
Ethics
The protocol was approved by the ICON ethics committee. ICON is an autonomous body of ISMPO, with a primary mandate for research.
Sample Size
The exact number of oncology centers in which chemotherapy is administered in the country is unknown. As convenience sampling was used for this survey, and formal sample size calculations were not performed.
Statistical Analysis
To calculate the completeness of the implementation of guidelines, we counted the number of correct responses for each major question domain. The correct responses to these questions were decided before the start of the survey by the investigators (V.P. and K.P.) in accordance with the target guidelines. The correct response for each of the survey instrument questions is documented in the Appendix. A domain standard was considered to be fully implemented if > 90% of the items had correct responses for the given standard. It was considered partially implemented if between 50% and 90% of the items had correct responses for the given standard. When < 50% had correct responses for the given standard, it was considered not implemented. Thus, the formula for calculating the percentage implementation rate for each domain was:
The detailed scoring system and calculation of the percentage implementation rate is shown in the Appendix. A facility was considered to have fully implemented ASCO guidelines if it scored a percent implementation rate that exceeded 90% in the B (B1, B2, and B3 combine) domains. The percentage of 90% was decided by consensus among the investigators.
Descriptive data regarding frequencies of implementation for a given standard as well as the domain are presented. We have calculated the number and proportion of oncology centers that have fully implemented each standard as well as the full domain. Frequency of major reasons for nonimplementation of a given standard are presented. Linear regression analysis was performed to identify factors that predicted low implementation scores in B1, B2, and B3 domains.
RESULTS
Baseline Details of Participating Centers
Sixty-six (62.9%) of 105 centers participated in the survey. Details about these centers are listed in Table 1. The majority of these centers (60; 90.9%) were located in urban areas, were dedicated cancer centers (55; 83.3%), and were teaching institutes (45; 68.2%). The median number of patients seen per physician was 40 (interquartile range, 30 to 50 patients). The average number of patients seen per physician was 54.5 in the government sector, whereas it was 33.8 in the private sector (P = .009). Sixty-five (98.5%) of 66 centers were aware of the presence of international antiemetic guidelines.
Table 1.
Baseline Details of Participating Centers
Implementation of Standards
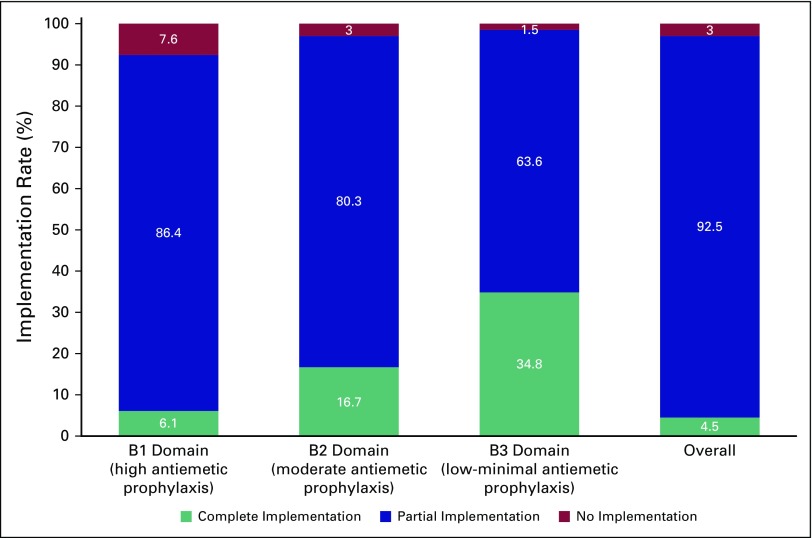
The target of partial, full, and no implementation of standards was seen in 92.5% (95% CI, 83.0% to 97.0%), 4.5% (95% CI, 1.1% to 13.2%), and 3.0% (95% CI, 0.3% to 11.2%) of centers, respectively, as shown in Figure 1. Only two centers had all standards implemented fully, whereas one center had > 90% standards implemented. Full implementation was better for the low-minimal emetogenic regimens (34.8% of centers; 95% CI, 24.5% to 46.9%) than the highly emetogenic regimens (6.1% of centers; 95% CI, 2% to 15.1%).
Fig 1.
Implementation rate of ASCO guidelines in each of the domains. B1, B2, and B3 domains had question dealing with high, moderate, and low-minimal emetogenic agents respectively. Although overall calculation suggested ≥ 90% score in three centers, only two centers had consistent ≥ 90% scores in each domain. These two centers had 100% scores in all domains. The third center had 100% scores in B2 and B3 domains but had a score of 83.33% in the B1 domain.
Details about the implementation of each individual standard in each domain are listed in the Appendix (Appendix Tables A1 to A6). In the B1 domain (high antiemetic prophylaxis), the recommendations with lowest compliance were the use of olanzapine when aprepitant is not used (nine centers; 13.6%), appropriate use of 5HT3 antagonist on days 2 and 3 (13 centers; 19.7%), and use of dexamethasone on days 2 and 3 (44 centers; 66.7%). Similarly, in B2 domain, the appropriate use of 5HT3 and dexamethasone on days 2 and 3 was significantly lacking (Appendix Tables A2 and A3).
Factors Adversely Impacting Implementation
The three most frequently cited reasons for hampered implementation of ASCO guidelines in routine chemotherapy practice were a lack of sensitization (26 centers; 39.4%), lack of national guidelines (12 centers; 18.2%), and lack of administrative support (10 centers; 15.2%). (Appendix Table A7). None of the following factors—place of practice, funding source, presence of dedicated cancer center, and patient load—were independently associated with low implementation scores in B1, B2, or B3 domains (Appendix Tables A8 to A10).
As a post hoc linear regression analysis failed to identify any single predictive factor, a composite regression tree analysis was performed using R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria) for B1 (high antiemetic prophylaxis); B2 (moderate antiemetic prophylaxis); and B3 (low-minimal antiemetic prophylaxis) domains independently with respect to the dependent variables (funding source [government or private]; center [academic or not]; location [urban or rural]; and type of cancer center [dedicated or nondedicated]). The lowest implementation rates were observed in the high antiemetic prophylaxis recommendations (B1 domain) in private rural centers. The lowest implementation rates were observed in the moderate antiemetic prophylaxis recommendations (B2 domain) in noncancer, dedicated government centers. The lowest implementation rates were observed in the low-minimal antiemetic prophylaxis recommendations (B3 domain) in noncancer, dedicated government centers and in private urban centers.
Institutional Antiemetic Policy Details
A written institutional antiemetic policy was present in 27 participating centers (40.9%). Recommendations regarding high and moderate antiemetic prophylaxis were included in > 90% of institutional antiemetic policies. Recommendations regarding management of anticipatory and refractory CINV were present in 16 (59.3%) and 15 (55.6%) centers, respectively. The primary reasons for hampered implementation of an institutional antiemetic policy are listed in Table 2.
Table 2.
Details of Domain A: Institutional Antiemetic Policy
Knowledge and Practice in Special Situations
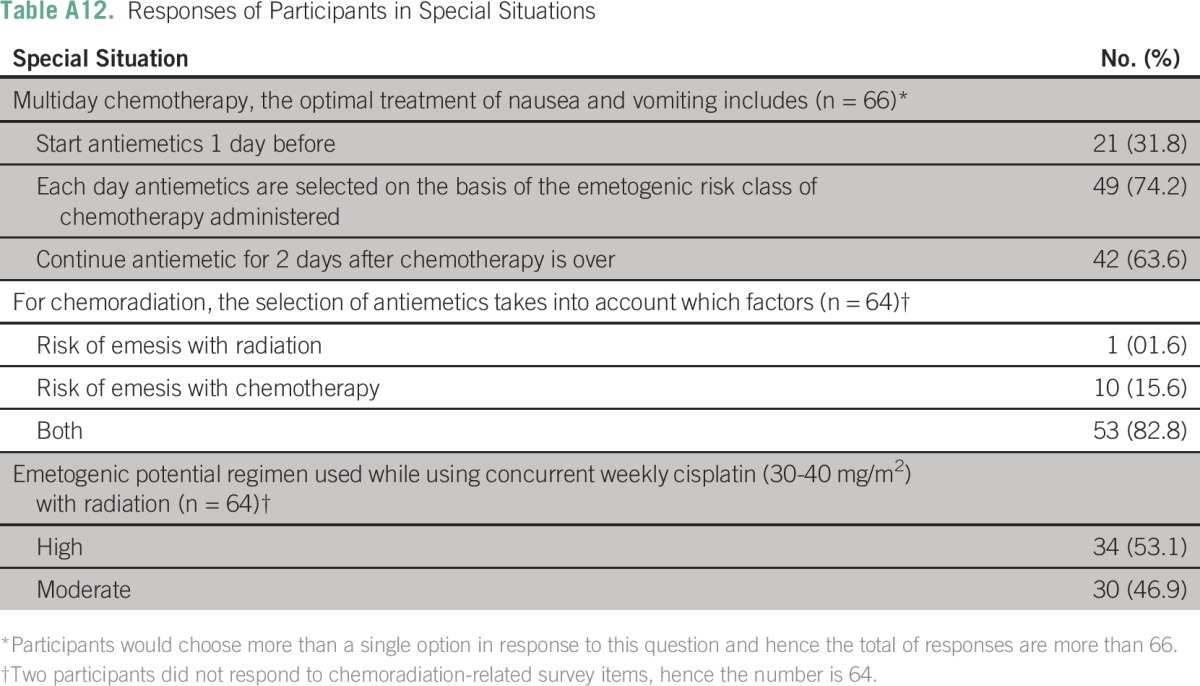
Details of the responses to special situations are listed in Appendix Tables A11 and A12). In situations that pertained to multiday regimens, 31.8% (21) of centers started antiemetics 1 day before the start of chemotherapy, 74.2% (49) of centers selected antiemetic protocol for each day on the basis of the emetogenic risk class of chemotherapy administered, and 63.6% (42) of centers continued antiemetic therapy for 2 days after the chemotherapy was completed. In protocols that pertained to chemoradiation, 82.8% (53) of centers selected antiemetics, taking into account the risk of emesis of both radiation and chemotherapy. Participating centers were divided in their protocols regarding the emetogenic risk of weekly cisplatin (30 to 40 mg/m2) administered concurrently with radiation. Of centers, 53.1% (34) considered it as highly emetogenic and the remaining considered this protocol moderately emetogenic.
DISCUSSION
The profile and distribution of cancer centers in India presents several unique challenges in managing the complications of chemotherapy, including CINV. Although 75% to 80% of the population stays in rural areas, cancer centers are predominantly located in major cities.18 Thus, a majority of patients do not have ready access to medical care, and this adds to the challenge in deciding the appropriate antiemetic regimen. Compounding the issue is the average number of patients seen by individual oncologists, which has been reported to be much higher than in the West.19-22 In fact, this factor was mentioned as one of the factors that hindered appropriate antiemetic prophylaxis (12.3% of centers).
Selection of the optimal antiemetic regimen consists of gauging the emetogenic potential of chemotherapy regimens and then deciding the appropriate antiemetic prophylaxis considering the factors that are unique to each country. Whereas international evidence-based guidelines have been formulated for the selection of appropriate antiemetic prophylaxis, there are minor variations depending on local oncologic practice. In general, treatment guidelines formulated in developed countries are difficult to implement in developing countries.17 Unfortunately, many developing countries, such as India, do not have their own guidelines for antiemetic prophylaxis. Hence, most oncologists in India use international guidelines, such as the ASCO antiemetic guidelines. This is also reflected in the current study, where 98.5% of the responding centers were aware and had knowledge of these guidelines.
Overall, an encouraging finding in our study was the fact that 97.0% of centers had > 50% of ASCO antiemetic clinical guideline standards implemented in routine practice; however, only three centers implemented > 90% of standards and only two centers implemented all standards fully. Guidelines regarding high emetogenic prophylaxis were the least implemented (only four centers; 6.1%). Our survey identified three major areas of concern relating to the ASCO antiemetic guidelines: the absence of olanzapine when aprepitant is not used (86.4%), overuse of 5HT3 antagonist for delayed emesis (80.3%), and absence of dexamethasone for delayed emesis (33.3%). Whereas it may be argued that the ASCO antiemetic guidelines did not offer olanazapine as an option, a reference was made about its role in a scenario precluding aprepitant.11 On this basis, the investigators decided that olanzapine is an essential component of an antiemetic regimen when aprepitant cannot be used. Overuse of 5HT3 antagonist for delayed emesis (71.1% in aprepitant users and 57.1% in nonusers) and inappropriate use of dexamethasone on days 2 and 3 postchemotherapy (42.1% overuse in aprepitant users and 67.0% underuse in aprepitant nonusers) were the major deficiencies in implementation of moderate antiemetic prophylaxis. Guidelines were fully implemented in the low and minimal risk setting in 90.9% and 42.4% centers, respectively. Of centers, 57.6% used antiemetics with agents that had minimal risk of emetogenesis. Overuse of 5HT3 antagonist for delayed emesis prophylaxis and underuse of dexamethasone for the same are the main issues in published work from other developed countries.23-26
Our survey also highlights the variable practices in oncology centers regarding antiemetics for multidrug chemotherapy and antiemetic prophylaxis for concurrent chemoradiation. One of the factors this survey did not touch upon was patient risk factor adjusted antiemetic regimens. It is a known fact that female patients and patients who had previous episodes of intractable vomiting are at a high risk of emesis and that modification in selection of antiemetic regimens might be warranted.13,27,28
As previously noted, the major factors that hindered wider implementation of ASCO antiemetic guidelines is the lack of sensitization, despite a majority of centers being aware of the existence of such guidelines. In this context, lack of sensitization means lack of concern, or apathy, regarding chemotherapy-induced nausea and vomiting. Awareness and knowledge unfortunately do not always translate into action, and emetic prophylaxis seems to be one example. The authors therefore decided to organize a biannual continuous medical education program for practicing oncologists and oncology trainees on antiemetic prophylaxis under the aegis of ICON. The program would stress the recommendations which were minimally implemented as per our survey. We hope to improve the antiemetic prophylaxis for patients who receive chemotherapy in the country.
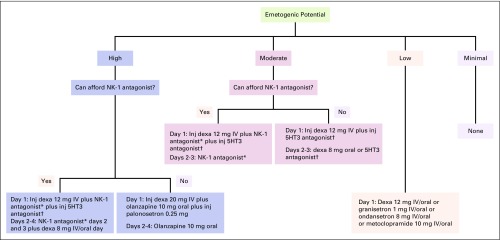
Another factor that impaired implementation of ASCO antiemetic guidelines was lack of national antiemetic prophylaxis guidelines. Presence of national guidelines or better institutional policy mandates physicians adhere to such guidelines or policies. These guidelines and policies are medico-legally and ethically binding. Therefore, it was decided by the authors to provide a simple, single-page algorithm for appropriate selection of antiemetic prophylaxis. Cost of antiemetic regimens was also factored in the algorithm. The algorithm was drafted by the authors (V.P. and K.P.) and was debated by the other members before a final algorithm was drafted (Fig 2). The algorithm is primarily for centers that do not have institutional antiemetic guidelines. As per our survey, 50% (33 centers) of responding centers belong to this category. In addition, the algorithm can be an effective supplement even for those centers where the antiemetic guidelines do not have recommendations for all situations as outlined in the algorithm. We plan to design comprehensive antiemetic guidelines for the Indian subcontinent in partnership with such Indian oncology associations as ICON and ISMPO.
Fig 2.
Evidence-based algorithm for quick selection of appropriate antiemetic regimen. (*) NK-1 antagonist schedule: aprepitant 125 mg day 1 and 80 mg days 2 and 3 orally or fosaprepitant 150 mg IV day 1 only. (†) 5HT3 antagonist: granisetron 1 mg IV/oral or ondansetron 8 mg IV/oral or palonosetron 0.25 mg IV day 1 only. Emetogenic potential: High: AC/EC, carmustine (> 250 mg/m2), cisplatin (any dose), cyclophosphamide (> 1.5 g/m2), dacarbazine, doxorubicin (≥ 60 mg/m2), epirubicin (> 90 mg/m2), ifosfamide (≥ 2 g /m2), mechlorethamine. Moderate (NK-1 antagonist preferred): carboplatin, carmustine (≤ 250 mg/m2), dactinomycin, daunorubicin, doxorubicin (< 60 mg/m2), epirubicin (≤ 90 mg/m2), ifosfamide (< 2 g /m2), irinotecan, methotrexate (≥ 250 mg/m2). Moderate: cyclophosphamide (≤ 1.5 g/m2), IFN-alpha (≥ 10 million U/m2), oxaliplatin, temozolomide. Low: carfilzomib, liposomal doxorubicin, etoposide, eribulin, FU, floxuridine, gemcitabine, INF-alpfa ( > 5 to > 10 million units/m2), ixabepilone, methotrexate (> 50 to < 250 mg/m2), mitomycin, mitoxantrone, pemetrexed, topotecan, taxanes. Minimal: bevacizumab, bleomycin, cetuximab, methotrexate (≤ 50 mg/m2), nivolumab, panitumumab, pertuzumab, ramucirumab, temsirolimus, trastuzumab, vinca alkaloids. dexa, dexamethasone; FU, fluorouracil; IFN, interferon; inj, injection; IV, intravenous; NK-1, neurokinin-1; PO, orally.
In conclusion, awareness regarding the ASCO antiemetic clinical guidelines is satisfactory in Indian oncology practices; however, there is a need for further sensitization of oncologists toward complete implementation of the guidelines in their clinical practice. Developing national guidelines that are specific for India may help in the standardization of antiemetic regimens.
Appendix
Table A1.
Response to B1 Domain of Survey
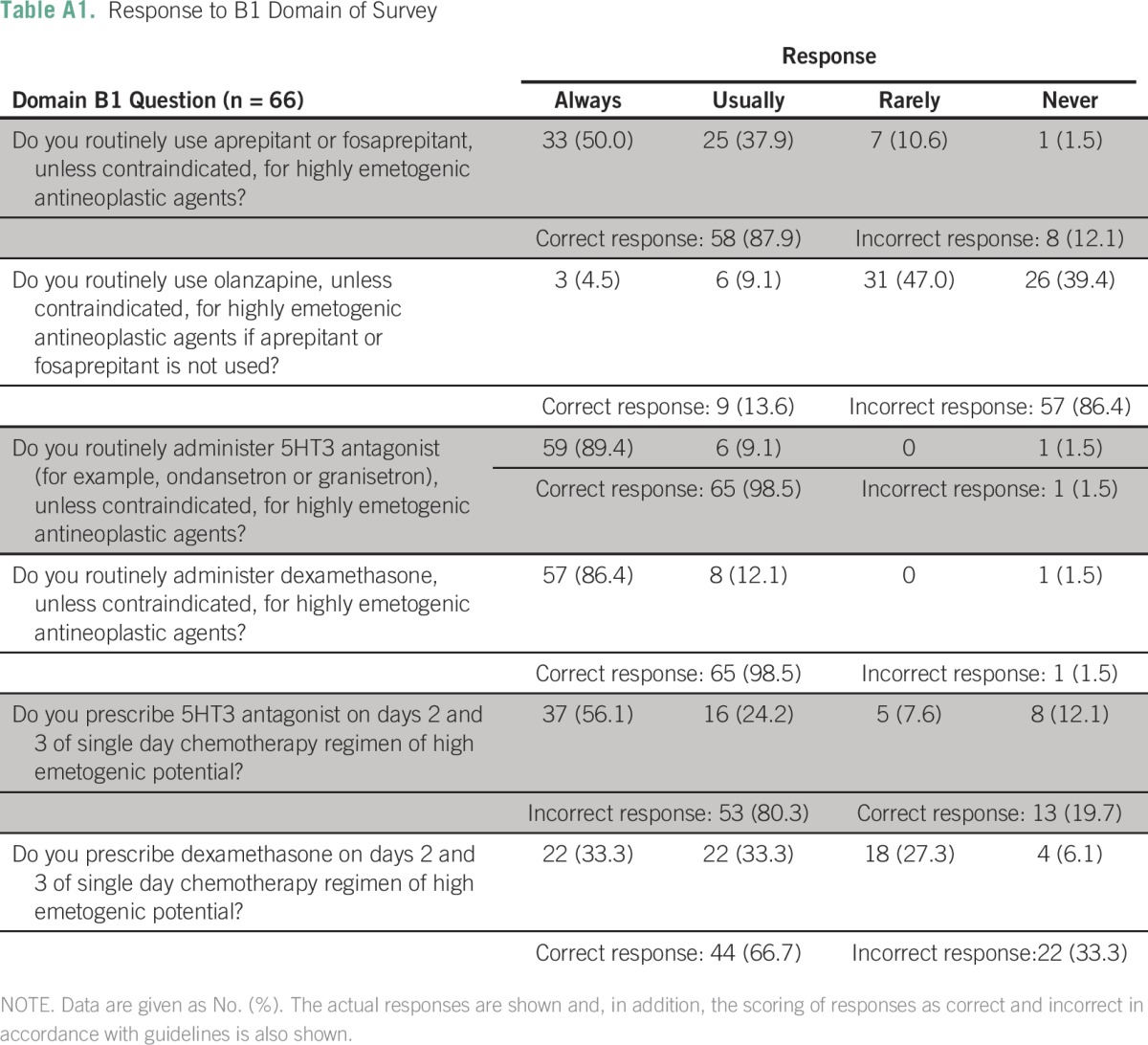
Table A2.
Response to B2 Domain of Survey
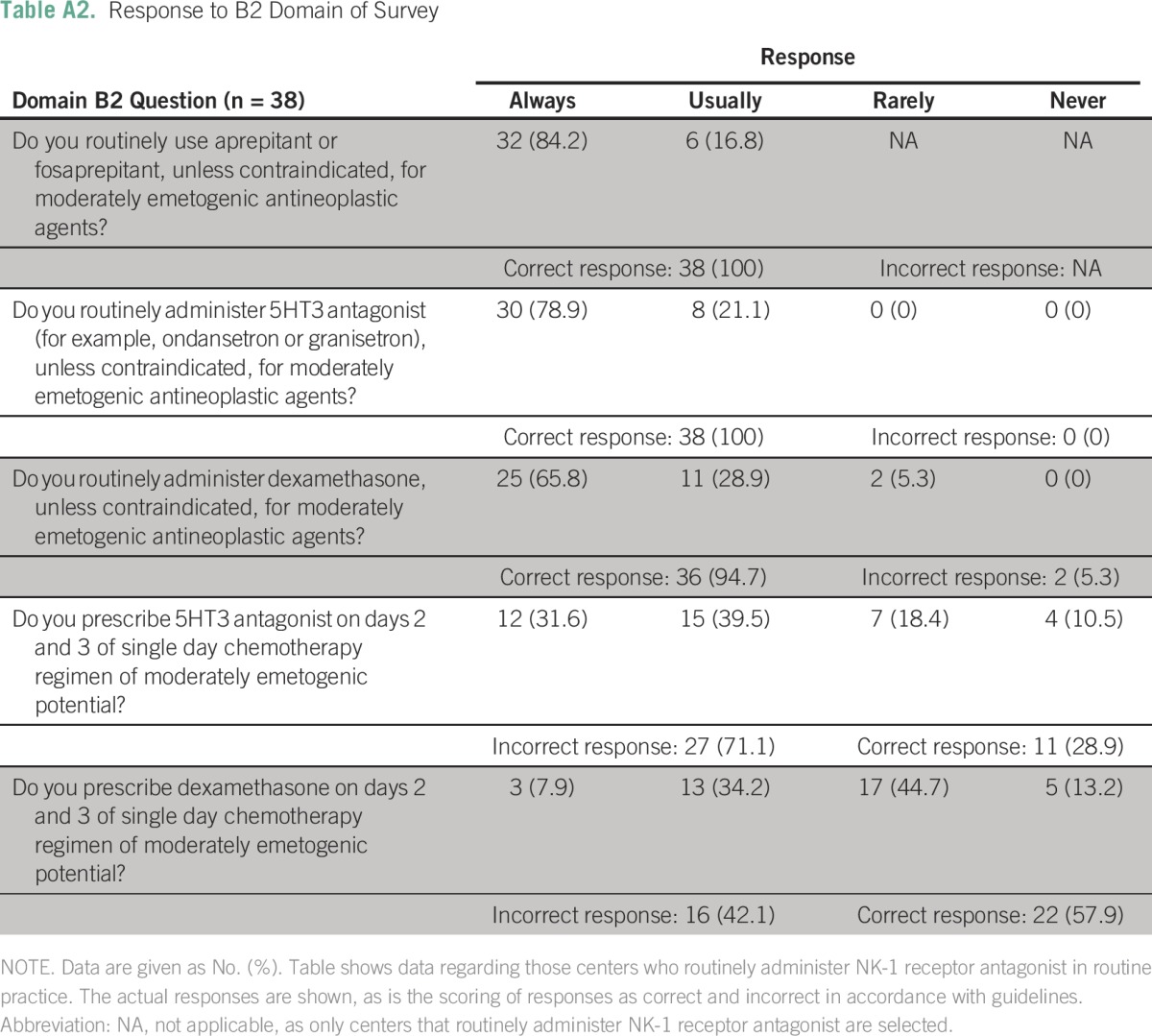
Table A3.
Response to B2 Domain of Survey
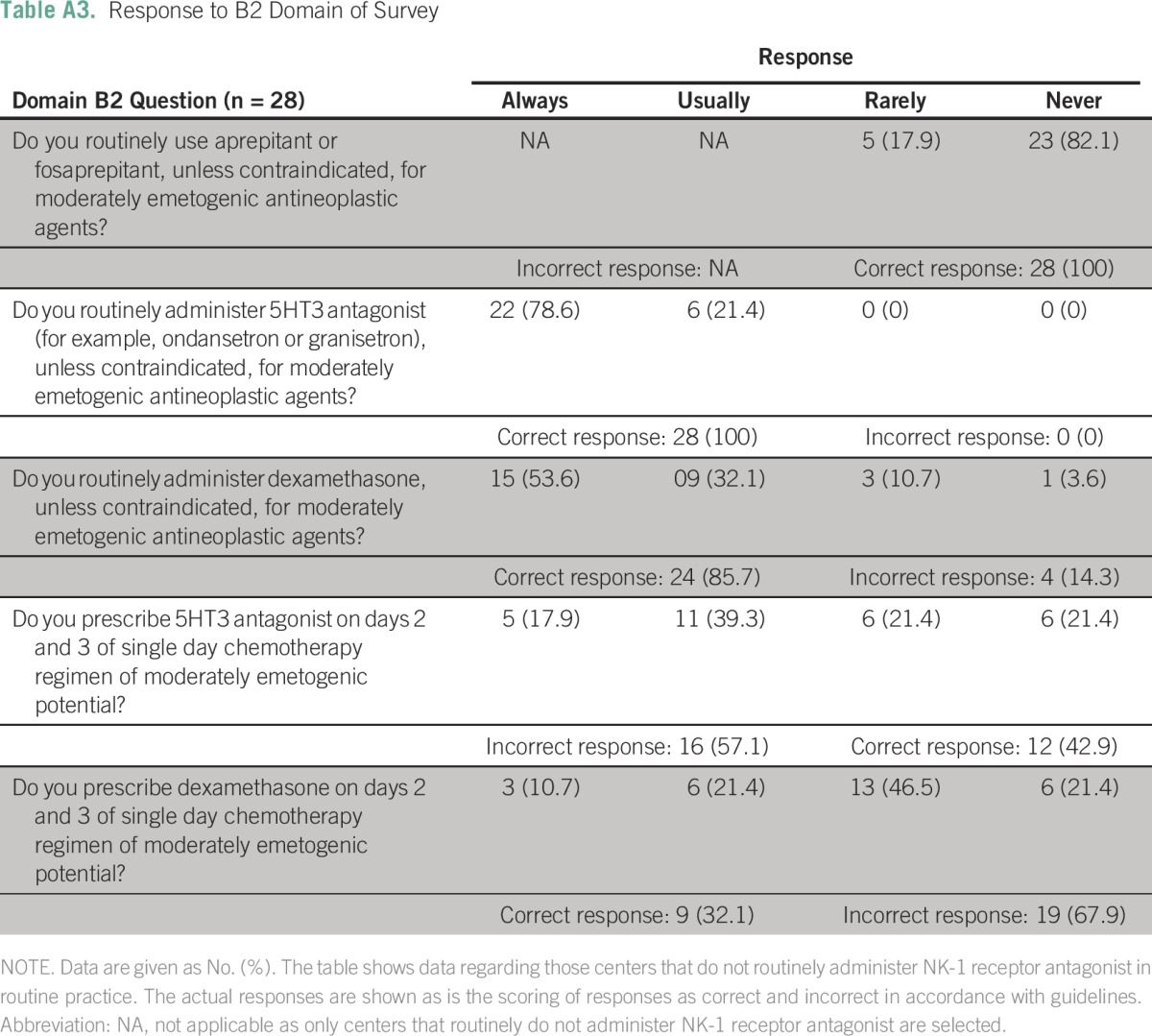
Table A4.
Guideline Implementation Rate in B2 Domain
Table A5.
Choice of 5HT-3 Antagonist in B2 Domain
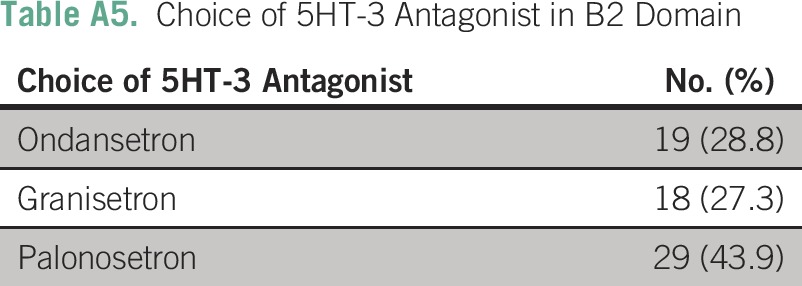
Table A6.
Response to B3 Domain of Survey
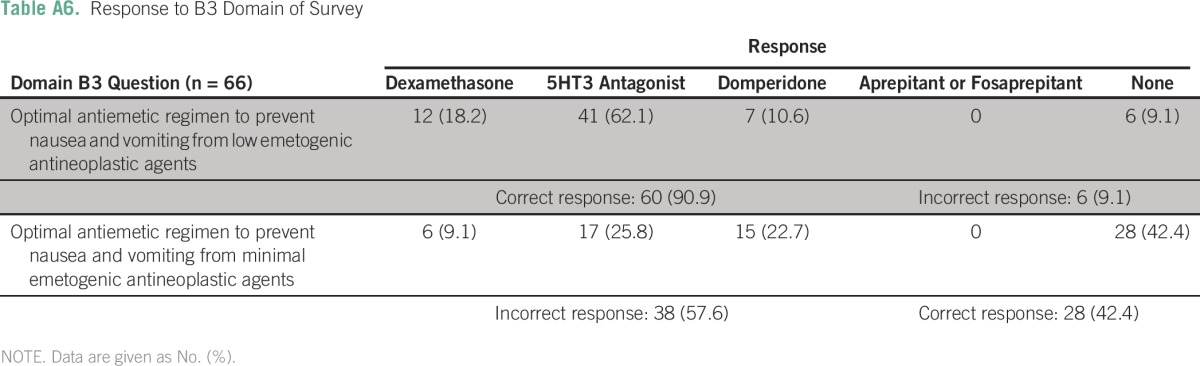
Table A7.
Factors That Hamper Implementation of ASCO Guidelines in Routine Chemotherapy Practice
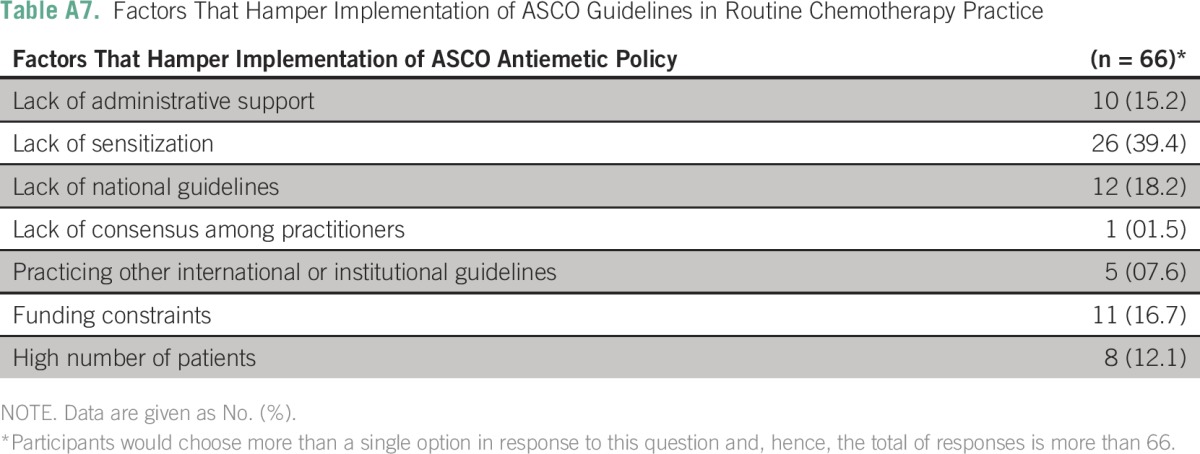
Table A8.
Impact of Various Factors on Center Ability to Implement Standards for High Emetogenic Prophylaxis
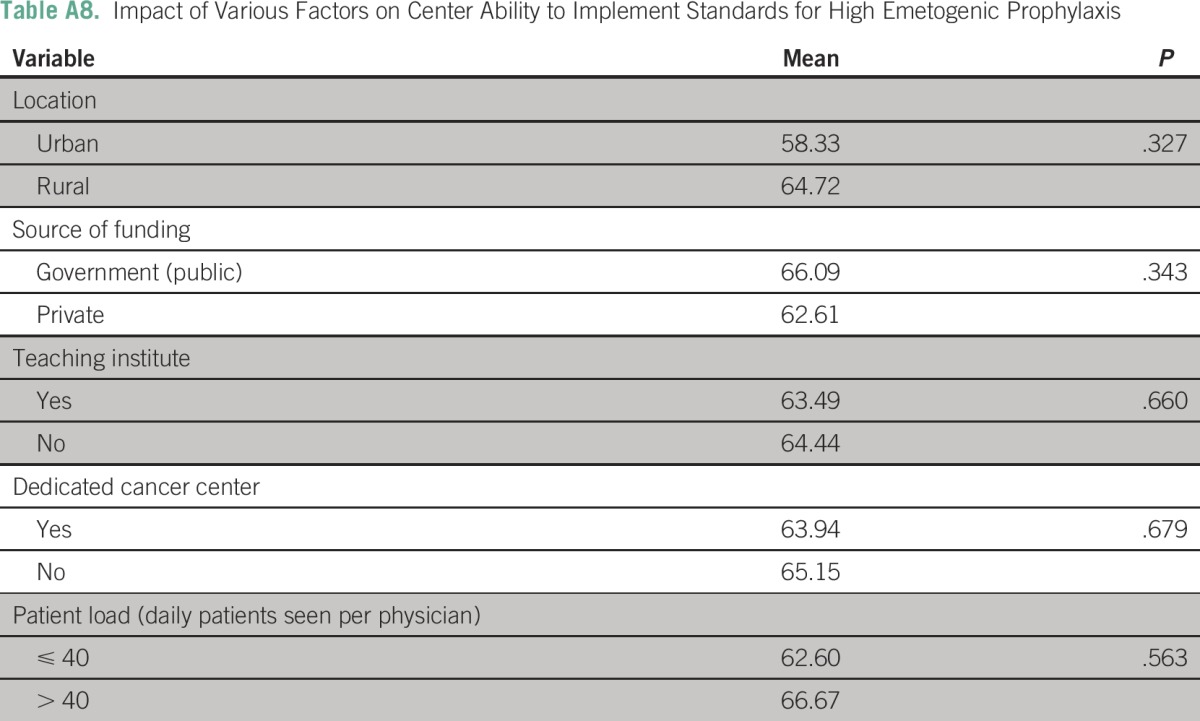
Table A9.
Impact of Various Factors on Center Ability to Implement Standards for Moderate Emetogenic Prophylaxis
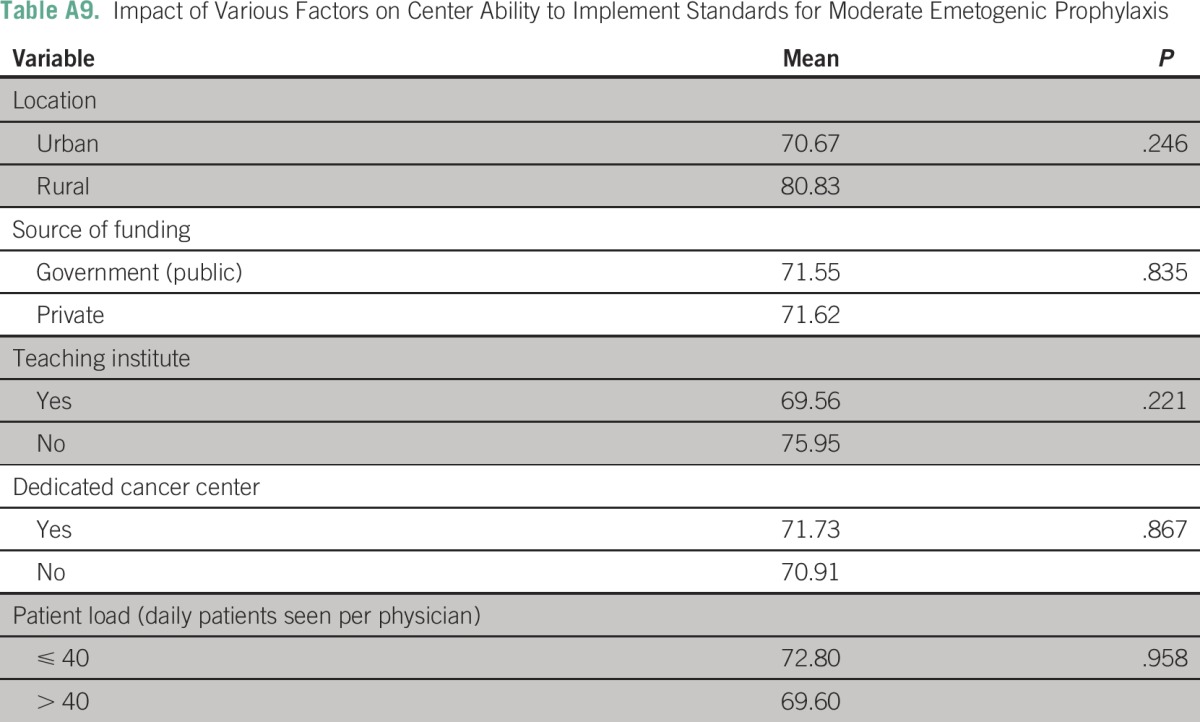
Table A10.
Impact of Various Factors on Center Ability to Implement Standards for Low and Minimal Emetogenic Prophylaxis
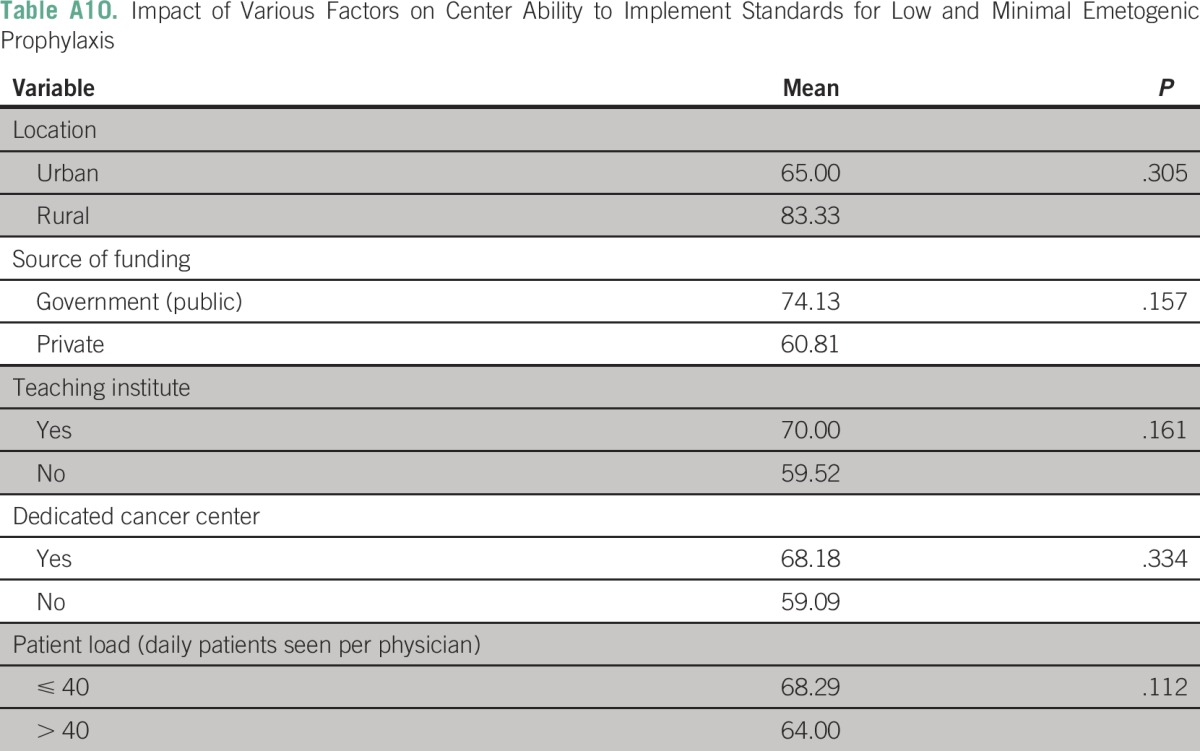
Table A11.
Factors That Prompt a Modification in the Prophylactic Antiemetic Regimen or Its Doses in Chemotherapy Practice
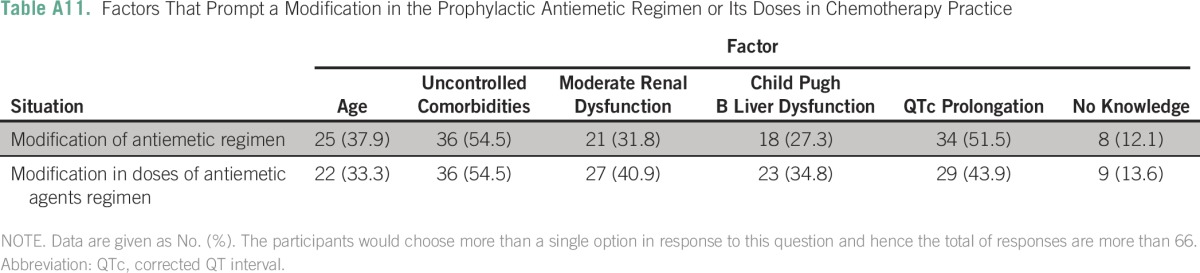
Table A12.
Responses of Participants in Special Situations
AUTHOR CONTRIBUTIONS
Conception and design: Vijay Patil, Kumar Prabhash
Provision of study materials or patients: Vijay Patil, K. Govinda Babu
Collection and assembly of data: Vijay Patil, Vanita Noronha, Sunny Jandyal, Vamshi Muddu, Nilesh Lokeshwar, Sachin Hingmire, Nikhil Ghadyalpatil, Shripad Banavali
Data analysis and interpretation: Vijay Patil, Amit Joshi, Purvish Parikh, Atanu Bhattacharjee, Santam Chakraborty, Anant Ramaswamy, K. Govinda Babu, Kumar Prabhash
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Vijay Patil
No relationship to disclose
Vanita Noronha
No relationship to disclose
Amit Joshi
No relationship to disclose
Purvish Parikh
No relationship to disclose
Atanu Bhattacharjee
No relationship to disclose
Santam Chakraborty
No relationship to disclose
Sunny Jandyal
No relationship to disclose
Vamshi Muddu
No relationship to disclose
Anant Ramaswamy
No relationship to disclose
K. Govinda Babu
No relationship to disclose
Nilesh Lokeshwar
Consulting or Advisory Role: Bristol-Myers Squibb, Eisai, Dr. Reddy's Laboratories
Travel, Accommodations, Expenses: Dr. Reddy's Laboratories
Sachin Hingmire
No relationship to disclose
Nikhil Ghadyalpatil
No relationship to disclose
Shripad Banavali
No relationship to disclose
Kumar Prabhash
No relationship to disclose
REFERENCES
- 1.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 2.Pirri C, Bayliss E, Trotter J, et al. Nausea still the poor relation in antiemetic therapy? The impact on cancer patients’ quality of life and psychological adjustment of nausea, vomiting and appetite loss, individually and concurrently as part of a symptom cluster. Support Care Cancer. 2013;21:735–748. doi: 10.1007/s00520-012-1574-9. [DOI] [PubMed] [Google Scholar]
- 3.Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 4.Perwitasari DA, Atthobari J, Mustofa M, et al. Impact of chemotherapy-induced nausea and vomiting on quality of life in Indonesian patients with gynecologic cancer. Int J Gynecol Cancer. 2012;22:139–145. doi: 10.1097/IGC.0b013e318234f9ee. [DOI] [PubMed] [Google Scholar]
- 5.Moore S, Tumeh J, Wojtanowski S, et al. Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health. 2007;10:23–31. doi: 10.1111/j.1524-4733.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys S, Pellissier J, Jones A. Cost-effectiveness of an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer in the UK. Cancer Manag Res. 2013;5:215–224. doi: 10.2147/CMAR.S44539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ihbe-Heffinger A, Ehlken B, Bernard R, et al. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol. 2004;15:526–536. doi: 10.1093/annonc/mdh110. [DOI] [PubMed] [Google Scholar]
- 8.Aseeri M, Mukhtar A, Al Khansa S, et al. A retrospective review of antiemetic use for chemotherapy-induced nausea and vomiting in pediatric oncology patients at a tertiary care center. J Oncol Pharm Pract. 2013;19:138–144. doi: 10.1177/1078155212457966. [DOI] [PubMed] [Google Scholar]
- 9.Parikh PM, Charak BS, Banavali SD, et al. A prospective, randomized double-blind trial comparing metoclopramide alone with metoclopramide plus dexamethasone in preventing emesis induced by high-dose cisplatin. Cancer. 1988;62:2263–2266. doi: 10.1002/1097-0142(19881115)62:10<2263::aid-cncr2820621032>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Parikh PM, Shah SR, Shah SC, et al. Preliminary experience with use of a selective 5HT3 receptor antagonist (ondansetron) to prevent high dose chemotherapy induced emesis. Indian J Cancer. 1996;33:17–20. [PubMed] [Google Scholar]
- 11. doi: 10.1200/JCO.2010.34.4614. Basch E, Prestrud AA, Hesketh PJ, et al: Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189-4198, 2011 [Erattum: J Clin Oncol 32:2117, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 13.Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER) Ann Oncol. 2012;23:1986–1992. doi: 10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract. 2014;10:68–74. doi: 10.1200/JOP.2012.000816. [DOI] [PubMed] [Google Scholar]
- 15.Caracuel F, Muñoz N, Baños U, et al. Adherence to antiemetic guidelines and control of chemotherapy-induced nausea and vomiting (CINV) in a large hospital. J Oncol Pharm Pract. 2015;21:163–169. doi: 10.1177/1078155214524809. [DOI] [PubMed] [Google Scholar]
- 16.Fujii H, Iihara H, Ishihara M, et al. Improvement of adherence to guidelines for antiemetic medication enhances emetic control in patients with colorectal cancer receiving chemotherapy of moderate emetic risk. Anticancer Res. 2013;33:5549–5556. [PubMed] [Google Scholar]
- 17.Patil VM, Chakraborty S, Bhattacharjee A, et al. Survey of the state of implementation of the American Society of Clinical Oncology/Oncology Nursing Society Safety Standards for chemotherapy administration in India. J Oncol Pract. 2015;11:365–369. doi: 10.1200/JOP.2015.004481. [DOI] [PubMed] [Google Scholar]
- 18.Sirohi B. Cancer care delivery in India at the grassroot level: Improve outcomes. Indian J Med Paediatr Oncol. 2014;35:187–191. doi: 10.4103/0971-5851.142030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akscin J, Barr TR, Towle EL. Benchmarking practice operations: Results from a survey of office-based oncology practices. J Oncol Pract. 2007;3:9. doi: 10.1200/JOP.0712504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towle EL, Barr TR, Senese JL. The National Practice Benchmark for Oncology, 2013 report on 2012 data. J Oncol Pract. 2013;9:20s–38s. doi: 10.1200/JOP.2013.001211. [DOI] [PubMed] [Google Scholar]
- 21.Barr TR, Towle EL. National oncology practice benchmark: An annual assessment of financial and operational parameters-2010 report on 2009 data. J Oncol Pract. 2011;7(suppl 2):2s–15s. doi: 10.1200/JOP.2011.000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noronha V, Tsomo U, Jamshed A, et al. A fresh look at oncology facts on south central Asia and SAARC countries. South Asian J Cancer. 2012;1:1–4. doi: 10.4103/2278-330X.96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Laar ES, Desai JM, Jatoi A. Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): Multinational survey results from 2388 health care providers. Support Care Cancer. 2015;23:151–157. doi: 10.1007/s00520-014-2325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molassiotis A, Brearley SG, Stamataki Z. Use of antiemetics in the management of chemotherapy-related nausea and vomiting in current UK practice. Support Care Cancer. 2011;19:949–956. doi: 10.1007/s00520-010-0909-7. [DOI] [PubMed] [Google Scholar]
- 25.Yu S, Burke TA, Chan A, et al. Antiemetic therapy in Asia Pacific countries for patients receiving moderately and highly emetogenic chemotherapy--A descriptive analysis of practice patterns, antiemetic quality of care, and use of antiemetic guidelines. Support Care Cancer. 2015;23:273–282. doi: 10.1007/s00520-014-2372-3. [DOI] [PubMed] [Google Scholar]
- 26.Almazrou S, Alnaim L. Evaluation of adherence to chemotherapy-induced nausea and vomiting guidelines. An observational study. J Cancer Ther. 2012;3:613–620. [Google Scholar]
- 27.Hesketh PJ, Aapro M, Street JC, et al. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: Analysis of two phase III trials of aprepitant in patients. Support Care Cancer. 2010;18:1171–1177. doi: 10.1007/s00520-009-0737-9. [DOI] [PubMed] [Google Scholar]
- 28.Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: Analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer. 2011;19:807–813. doi: 10.1007/s00520-010-0899-5. [DOI] [PubMed] [Google Scholar]
- 29. Reference deleted.
- 30. Reference deleted.
- 31. Reference deleted.