Abstract
Background and aim
A recent sham-controlled trial showed that external trigeminal nerve stimulation (eTNS) is effective in episodic migraine (MO) prevention. However, its mechanism of action remains unknown. We performed 18-fluorodeoxyglucose positron emission tomography (FDG-PET) to evaluate brain metabolic changes before and after eTNS in episodic migraineurs.
Methods
Twenty-eight individuals were recruited: 14 with MO and 20 healthy volunteers (HVs). HVs underwent a single FDG-PET, whereas patients were scanned at baseline, directly after a first prolonged session of eTNS (Cefaly®) and after three months of treatment (uncontrolled study).
Results
The frequency of migraine attacks significantly decreased in compliant patients (N = 10). Baseline FDG-PET revealed a significant hypometabolism in fronto-temporal areas, especially in the orbitofrontal (OFC) and rostral anterior cingulate cortices (rACC) in MO patients. This hypometabolism was reduced after three months of eTNS treatment.
Conclusion
Our study shows that metabolic activity of OFC and rACC, which are pivotal areas in central pain and behaviour control, is decreased in migraine. This hypometabolism is reduced after three months of eTNS. eTNS might thus exert its beneficial effects via slow neuromodulation of central pain-controlling areas, a mechanism also previously reported in chronic migraine and cluster headache after percutaneous occipital nerve stimulation. However, this finding needs to be confirmed by further studies using a sham condition.
Keywords: Migraine, orbitofrontal cortex, treatment, external trigeminal nerve stimulation, imaging, brain metabolism
Introduction
Migraine is a widespread, disabling neurological disorder characterised by recurrent attacks of moderate to severe head pain associated with either digestive signs and/or sensoriphobia (1). Drugs currently prescribed for migraine prevention are not disease specific and can have many intolerable side effects, causing a high rate of discontinuation (2). Non-pharmacological approaches are thus being developed as an alternative to medications, and recently various non-invasive neurostimulation therapies have joined the antimigraine armamentarium (see Magis (3) for review). Among them, external trigeminal nerve stimulation (eTNS) was found effective in episodic migraine (MO) prevention. In the randomised, sham-controlled trial PREMICE, which included 67 patients with MO, eTNS with the Cefaly® device in daily 20-minute sessions for three months significantly decreased monthly migraine days (–29.7%, +4.9% in the sham group), and the 50% responder rate was greater in the verum (38.1%) than in the sham group (12.1%) (4). The beneficial effect of eTNS in low-frequency migraine prevention was also suggested by a small, open study in 24 drug-naive migraineurs (5). A prospective registry involving 2313 patients showed that eTNS is a well-tolerated and safe therapy with mild adverse events reported by only 4.3% of the patients (6).
The precise mechanisms of action of eTNS in migraine are currently unknown and all proposed theories are speculative. The purpose of the present study was to identify eTNS-induced short- and middle-term modifications within the central nervous system using 18-fluorodeoxyglucose positron emission tomography (FDG-PET). There are few publications on functional imaging in cranial nerve stimulation. Willoch et al. (7) have investigated brain changes after invasive stimulation of the trigeminal ganglion in patients with trigeminopathic pain using H215O PET. After stimulations lasting 30 to 50 minutes, but not after 60-second stimulations, they observed a significant cerebral blood flow increase in rostral parts of the anterior cingulate cortex (rACC), and neighbouring orbitofrontal (OFC) and medial frontal cortices, thus suggesting a potential involvement of these brain areas in electrostimulation-induced analgesia. In a previous clinical trial, we used FDG-PET to study the central effects of percutaneous occipital nerve stimulation (pONS) in 10 patients with refractory chronic cluster headache (8). We found that long-term pONS modulated the metabolism in the so-called ‘salience neuromatrix’ (including the ACC) with a significant hypermetabolism in the perigenual ACC in patients improved by at least 50%. Using H215O PET, Matharu et al. analysed the influence of pONS in patients with chronic migraine and found cerebral blood flow changes in the dorsal rostral pons, ACC, cuneus and pulvinar (9). Finally, Kovacs et al. performed 3 Tesla magnetic resonance imaging (3T-functional (f)MRI) in a single healthy volunteer (HV) during pONS and found activation in the hypothalamus, thalamus, OFC, prefrontal cortex and periaqueductal grey (10).
These studies thus support an involvement of the ACC and medial frontal regions in the neuromodulation of cephalic pain. Whether it is causal or collateral is not fully understood. In this study we hypothesised that eTNS would induce similar changes within the central nervous system.
Methods
Population
Twenty-eight individuals participated in the study: 14 patients with episodic migraine without aura (MO, International Classification of Headache Disorders, third edition beta (ICHD3 beta) criteria (1)) having between four and 14 days of migraine/month (three males, 11 females, mean age: 39 ± 14 years) and 20 HVs of similar sex and age distribution (five males, 15 females, mean age: 36 ± 11 years). Patients were recruited by headache-specialised neurologists (DM and JS) and came from the outpatient clinic of the University Department of Neurology, CHR Liège, Belgium. HVs were recruited through an announcement on the university and hospital websites. They were interviewed face to face before the recordings and filled in the extended French version of the ID-Migraine questionnaire (11) to rule out any current or past history of recurrent headaches. They were excluded if they had a family history of headaches, chronic pain, psychiatric or current systemic disorders and if they were taking medications regularly. MO patients had no other headache disorder, no psychiatric or somatic disorder, nor regular drug treatment except for the contraceptive pill. They were not allowed to have prophylactic treatment since at least two months before inclusion or to use opioid derivatives as acute therapy, but were allowed to take painkillers and/or triptans for migraine attacks. Moreover, none of the participants underwent PET imaging and/or used eTNS before.
We conducted the study in accordance with the Declaration of Helsinki, version 2013. Written informed consent was obtained from all participants and the local Ethics Committee approved the study.
Procedure
18-FDG-PET
The PET acquisitions were made in the Nuclear Medicine Department of the CHU Sart-Tilman, Liège, Belgium using a Gemini TF PET/computed tomography (CT) scanner (Philips®, Eindhoven, The Netherlands). Resting cerebral metabolism was studied 30 minutes after intravenous injection of 150 MBq FDG. Blood glucose level was measured and was lower than 150 mg/dl in all individuals. Participants were injected and scanned in a dark room with minimal environmental noise. Images were reconstructed using an iterative list mode time-of-flight algorithm. Corrections for attenuation, dead-time, random and scatter events were applied.
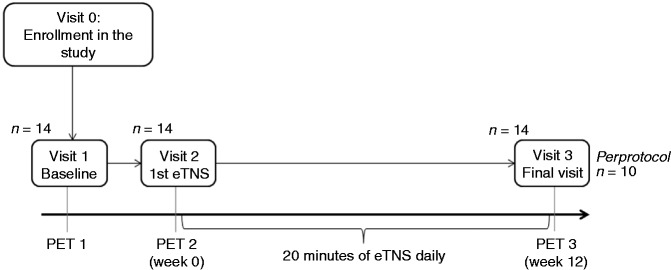
HVs had only one PET scan (PET1), whereas MO underwent three PET scans: at baseline (before any stimulation, PET1), immediately after a session of eTNS (PET2), and after three months of daily eTNS therapy (PET3, see Figure 1 flowchart). PET2 could not be performed the same day as PET1 for technical and safety reasons. Just before PET2 acquisition, MO patients received in the Nuclear Medicine Department a first prolonged eTNS session that lasted one hour and started immediately after the 18-FDG isotope injection, during the incubation period of 18-FDG, using a pre-programmed Cefaly® device placed by the same investigator (VDP) in order to ensure an adequate stimulation. The PET2 acquisition was performed immediately after the end of the first eTNS session. The one-hour duration of this session was set after a discussion with the nuclear medicine specialist. We hypothesised that this duration would be potent enough to induce brain metabolic changes detectable by the FDG-PET scan technique. Finally, PET3 was conducted at the end of the 12-week eTNS prophylactic therapy.
Figure 1.
Study design.
PET: positron emission tomography; eTNS: external Trigeminal Nerve Stimulation.
External trigeminal nerve stimulation (eTNS)
eTNS was delivered using the portable Cefaly® device (Cefaly Technology®, Grâce-Hollogne, Belgium). Patients were stimulated for the first time in the hospital before PET2 (see above) and thus trained to use the device properly. Subsequently, they received a Cefaly® device and were asked to apply eTNS at home daily for 20 minutes for three months as preventive treatment (its use as acute therapy was not recommended). Neurostimulation was administered with a 30 mm × 94 mm self-adhesive electrode placed on the forehead and covering the supratrochlear and supraorbital nerves bilaterally (first trigeminal division). The Cefaly® device provided to patients had a single stimulation program (contrary to the commercially available Cefaly® device, which has three programs with different stimulation parameters). It generated biphasic rectangular impulses with an electrical mean equal to zero and the following characteristics: pulse width 250 µs, frequency 60 Hz, and maximal intensity 16 mA. Built-in electronic software allowed recording time of use per patient and hence verifying compliance at the end of the study.
Eligible patients were asked to fill in headache diaries for four successive months: one month of baseline and three months with eTNS treatment. They recorded headache occurrence, intensity (on a three-point scale: 1-mild to 3-severe), presence of nausea/vomiting, phonophobia and/or photophobia and intake of acute migraine drugs (analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), triptans).
Data analysis
Clinical data provided by the migraine diaries were analysed using non-parametric tests (Wilcoxon paired test or Friedman analysis of variance (ANOVA), Statistica® version 8.0, StatSoft, France).
PET acquisitions were analysed using Statistical Parametric Mapping (SPM8, Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.4.0 (MathWorks Inc, Sherborn, MA, USA). Images were spatially normalised into a standard stereotactic space using an MNI PET template (Montreal Neurological Institute) and smoothed using an 8 mm full-width-half-maximum (FWHM) isotropic kernel. We performed global normalisation by applying proportional scaling. Significance level of resulting SPM maps was set at a p < 0.001 uncorrected, with an extended threshold of 20 voxels or a p < 0.05 using a family-wise error (FWE) correction for multiple comparisons at a cluster level. The FDG-PET analyses were performed by two investigators blinded to diagnosis (KD and AT).
The first analysis identified brain regions that were significantly hypo- or hypermetabolic in MO (n = 11) at baseline compared to HVs (n = 20) using a two-sample t-test. Three MO patients had been removed from this analysis because they had a migraine attack the day of PET acquisition, while the remaining 11 patients were pain free for at least 48 hours. Age and gender were entered as confounding covariates in the design matrix. We subsequently repeated the analysis by excluding two patients who had a history of acute medication-overuse headache (MOH) up to six months before PET1. Thus, the latter analysis was performed in patients who were interictal and free from acute medication overuse.
We then performed t-tests to compare brain metabolism in MO before and after three months of treatment, as compared to HVs. For this analysis, we included only patients who used eTNS at least one-third of the recommended time (i.e. 10 patients). We chose the 30% compliance threshold on an empirical basis, having experienced from clinical practice and previous trials (4,6) that patients may report a therapeutic benefit with non-daily use of the device. As before, we excluded from the analysis three patients who were in an ictal phase the day of the PET, which left seven analysed MO patients.
Additionally, we performed a parametric analysis to model the neuromodulatory effect of eTNS. The contrast modelling the effect of each variable within the design matrix was set according to the expected modulation of brain metabolism (i.e. PET1 patients: –2; PET2 patients: –1; PET3 patients: +1; PET1 controls: +2).
Finally, in a separate analysis without HVs, we compared metabolism between PET1 and PET2, PET1 and PET3, PET2 and PET3. In order to have reasonable power, we included all 10 compliant MO patients in this analysis and controlled for attack-related modifications by adding the factor ‘attack’ as a covariate in the design matrix.
The WFU PickAtlas 2.5.2 (Wake Forest University, NC, USA) was used as an anatomical reference.
Results
Clinical outcome
The clinical characteristics and outcomes of MO patients are summarised in Table 1. There were no serious adverse events, neither during eTNS therapy, nor during PET acquisitions. One patient dropped out because she could not tolerate the paraesthesia due to the stimulation. One patient did not return the device and the diaries. The analysis of the number and duration of eTNS at the end of the study showed that 10 patients (71%) had performed at least 30% of the 90 recommended sessions. On ‘per protocol’ analysis these patients had a significant decrease of monthly attack frequency during treatment (Friedman ANOVA, n = 10; p = 0.02), as well as between baseline and the third month of treatment (4.2 ± 1.1 to 2.6 ± 1.2; n = 10; p = 0.03). Five out of these 10 patients (50%) had at least a 50% reduction of monthly migraine attacks and were considered as responders. The number of migraine days failed, however, to be significantly lowered by eTNS (7.3 ± 3.6 at baseline to 5.4 ± 4.4 after three months, n = 10; p = 0.28), although five out of 10 patients had at least a 50% reduction of monthly migraine days. There was a significant decrease of mean headache intensity during the first and second month of eTNS therapy (Wilcoxon test, n = 10; p = 0.05 and 0.01) and a trend for such a decrease over the three-month treatment period (Friedman ANOVA, n = 10; p = 0.07). There was no significant decrease of acute medication intake (9.4 to 7.2, n = 9; p = 0.27).
Table 1.
Characteristics of patients and clinical outcome.
| Compliance |
Monthly migraine attack frequency |
||||
|---|---|---|---|---|---|
| Patients | Sex | Age | Number of sessions | Before treatment | After treatment |
| 1 | M | 36 | U | 6 | 2 |
| 2 | F | 45 | >90 | 3 | 2 |
| 3 | M | 32 | >90 | 4 | 2 |
| 4 | M | 69 | >90 | 4 | 3 |
| 5 | F | 45 | 31 | 4 | 2 |
| 6 | F | 46 | MV | MV | MV |
| 7 | F | 49 | >90 | 2 | 2 |
| 8 | F | 59 | Drop out | – | – |
| 9 | F | 37 | 71 | 4 | 4 |
| 10 | F | 26 | 20 | MV | MV |
| 11 | F | 22 | 46 | 5 | 2 |
| 12 | F | 18 | 5 | 1 | 0 |
| 13 | F | 40 | 35 | 5 | 5 |
| 14 | F | 26 | 87 | 3 | 2 |
| > 30% of compliance (n = 10) | |||||
| Number of attacks/ month | MD | SA | |||
| Before treatment | 4.2 ± 1.1 | 7.3 ± 3.7 | 3 ± 2 | ||
| First month of treatment | 2.6 ± 1.3 | 4.8 ± 2.7 | 1.4 ± 1.6 | ||
| Second month of treatment | 2.8 ± 1.3 | 4.7 ± 2.3 | 1 ± 0.9 | ||
| Third month of treatment | 2.6 ± 1.2 | 5.4 ± 4.4 | 2 ± 1.8 | ||
MV: missing value; M: male; F: female; U: unknown; eTNS: external trigeminal nerve stimulation; PET: positron emission tomography; MD: Migraine Days; SA: Severe Attacks.
Patient 1 used the device more than 30% of the time but sent the device back long after the end of the trial. Thus, her compliance during the three months could not be assessed as she continued to use eTNS daily and the number of recorded sessions was >150.
Patients in grey had a migraine attack during PET1. Patient 14 had an attack during PET2 only, and all patients were pain free during PET3.
FDG-PET results
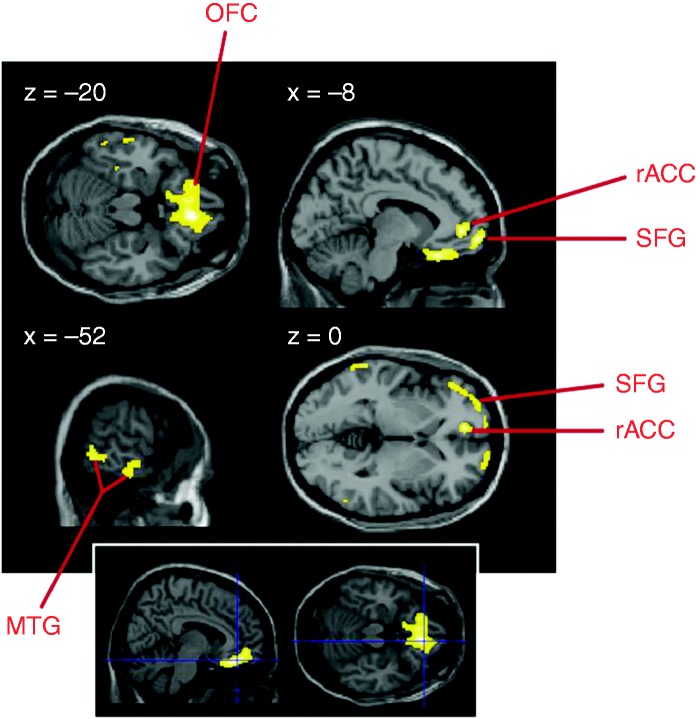
Baseline analysis (PET1) revealed that in MO patients (N = 11) fronto-temporal regions were hypometabolic compared to HVs (threshold: p < 0.001 uncorrected, 20 voxels), especially the OFC and rACC (Table 2), while motor areas were hypermetabolic. Only the OFC hypometabolism remained significant after correction for multiple comparisons (pFWEcluster < 0.001, Figure 2). Removing the data of the two patients with a history of MOH between three and six months before the first PET scan did not change the results.
Table 2.
Statistical results and localisation of peak voxels for the comparison patients < controls.
| Anatomical region | MNI coordinates | Cluster size | Z score | p value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| OFC | 8 | 36 | –20 | 2205 | 4.55a | <0.0001 |
| 15 | 25 | 24 | 4.35 | <0.0001 | ||
| L. med frontal gyrus | –8 | 52 | –10 | 4.33 | <0.0001 | |
| R. mid temporal | 58 | –58 | 4 | 37 | 4.27 | <0.0001 |
| L. rACC | –8 | 48 | –2 | 109 | 4.23 | <0.0001 |
| R. sup frontal | 20 | 66 | –4 | 146 | 4.01 | <0.0001 |
| L. inf temporal | –38 | –30 | –24 | 72 | 3.96 | <0.0001 |
| L. mid/inf temporal | –44 | –24 | –28 | 3.30 | 0.0005 | |
| –64 | –42 | 0 | 157 | 3.91 | <0.0001 | |
| –52 | –52 | –6 | 3.88 | <0.0001 | ||
| –60 | –12 | –14 | 174 | 3.84 | <0.0001 | |
| –58 | –40 | –24 | 3.57 | 0.0002 | ||
| –58 | –30 | –24 | 3.47 | 0.0003 | ||
| R. fusiform gyrus | 44 | –26 | –28 | 42 | 3.67 | 0.0002 |
| 52 | –32 | –25 | 3.17 | 0.0007 | ||
L: left; R: right; OFC: orbitofrontal cortex; rACC: rostral anterior cingulate cortex; MNI: Montreal Neurological Institute. apFWE (corrected for multiple comparisons) < 0.001.
Figure 2.
Hypometabolic areas in patients with episodic migraine compared to healthy individuals (p < 0.001 uncorrected, 20 voxels; bottom inset: p < 0.001 corrected for multiple comparisons). Results are displayed on sections of a normalised brain MRI template. Remaining hypometabolic voxels are: rACC: rostral anterior cingulate cortex; MRI: magnetic resonance imaging; MTG: middle temporal gyrus; OFC: orbitofrontal cortex; SFG: superior frontal gyrus.
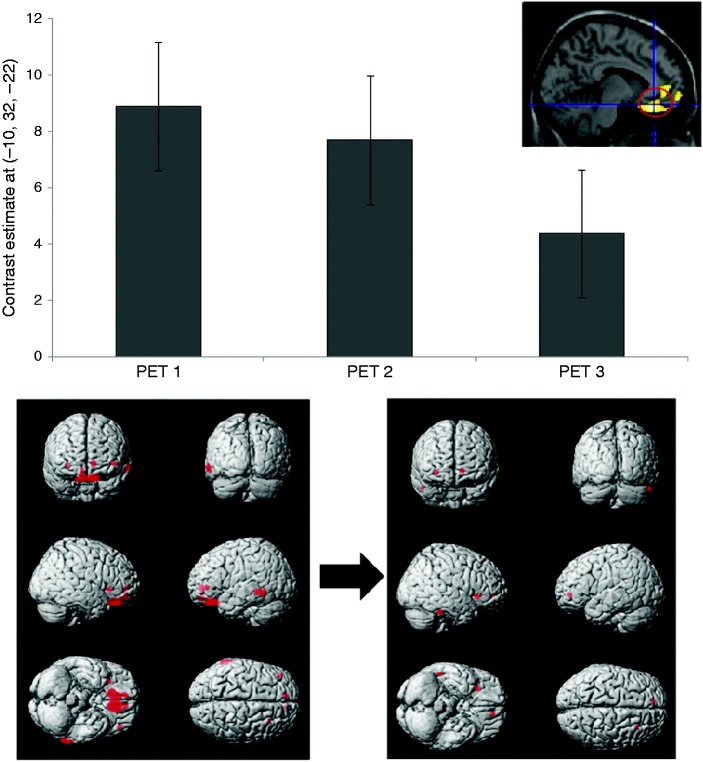
In compliant patients who were headache free the day of the scan (n = 7), three months of eTNS therapy was associated with reduced fronto-temporal hypometabolism when compared to HVs (Figure 3). Furthermore, the parametric analysis indicated a gradual normalisation of OFC/rACC metabolism (Figure 3).
Figure 3.
Top: Effect size (normalised values) at the peak voxel (i.e. orbitofrontal cortex, OFC) for the parametric analysis in seven patients who were headache free the day of the scan (controlled for age and gender), representing the increases in metabolism between healthy volunteers and the subgroup of compliant patients for the three FDG-PET acquisitions. Bottom: Hypometabolic areas in the seven MO patients, before (left) and after (right) a three-month treatment with eTNS (p < 0.001, >20 voxels). Note that fronto-temporal regions, especially OFC/rACC, are less hypometabolic after eTNS treatment.
FDG-PET: 18-fluorodeoxyglucose positron emission tomography; eTNS: external trigeminal nerve stimulation; rACC: rostral anterior cingulate cortex.
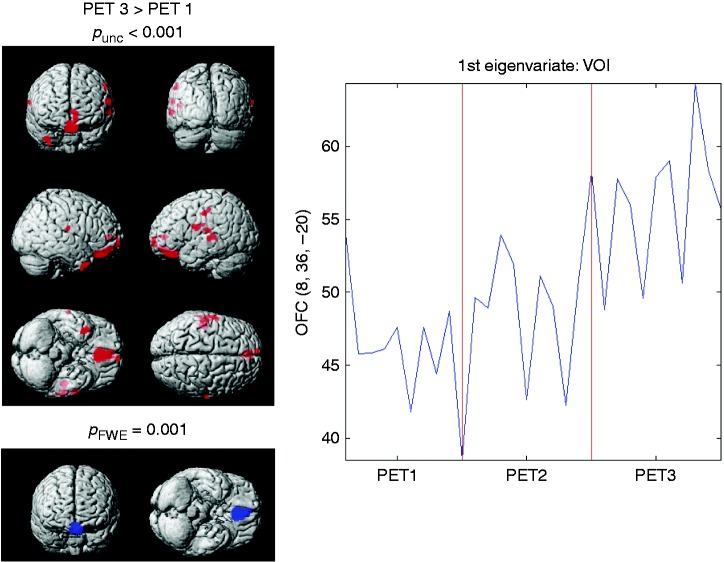
Additional analyses assessing differences within the migraine group across the three PET scans revealed that a single one-hour session of eTNS (PET2 vs PET1) was not sufficient to significantly change brain glucose uptake at this threshold. By contrast, metabolism in fronto-temporal regions significantly increased after eTNS treatment, i.e. between PET3 and PET1, especially in the OFC (pFWEcluster = 0.001) (Figure 4).
Figure 4.
FDG uptake for PET 3 vs PET 1 in compliant MO patients (n = 10). Data were controlled for attack-related modifications. Left: Fronto-temporal metabolism is increased after three months of eTNS treatment (in red: p < 0.001 uncorrected, 20 voxels). The OFC remains significant after correction for multiple comparisons (in blue). Right: First eigenvariate extraction around 10 mm from the peak voxel in the OFC (coordinates obtained from the comparison between PET1 and HVs).
FDG: 18-fluorodeoxyglucose; PET positron emission tomography; MO: migraine without aura; OFC: orbitofrontal cortex; HVs: healthy volunteers.
There was no significant correlation between acute medication intake and OFC metabolism at baseline. A direct comparison of brain metabolism between responders (n = 5) and non-responders (n = 5) to eTNS therapy failed to show a significant difference, probably because of the small sample size.
Finally, we found no metabolic changes in subcortical regions, nor in the brainstem or cerebellum.
Discussion
In this study, we aimed to evaluate the short- and long-term effects of eTNS with the Cefaly® device on brain metabolism using FDG-PET. To our knowledge, this is the first study on the central effects of non-invasive cranial nerve stimulation in headache. We found that glucose metabolism was decreased in specific brain regions of migraine patients at baseline and increased after a three-month eTNS treatment. These changes were associated with an overall clinical improvement after eTNS, i.e. a decrease of migraine attack frequency and intensity.
At baseline before eTNS we found a pronounced resting hypometabolism of the OFC and rACC (i.e. a decrease of glucose uptake) in our sample of MO patients. The rACC is known to belong to the endogenous opioid pain control circuit (12), whereas the OFC is involved in cognitive aspects of pain modulation. Previous imaging studies in migraine have identified a similar OFC hypometabolism, but mainly in chronic migraine patients with MOH. Using FDG-PET in 16 MOH patients (13), we have described an OFC hypometabolism that was even more pronounced three weeks after drug withdrawal, especially in patients over-consuming combination analgesics. Such a dysfunction of a brain area involved in compulsive behaviour has also been reported after withdrawal in substance abusers and alcoholics. In a recent MRI study, Riederer et al. (14) showed with voxel-based morphometry that persistent decrease of grey matter OFC correlated with poor response to drug withdrawal. Our patients’ sample comprised four patients with a past history of MOH, two of them between three and six months before the study, two others several years before. Since it was shown in an fMRI study of MOH patients that pain-induced hypoactivation of the lateral pain system normalises completely six months after drug withdrawal (15), we decided to reanalyse PET1 data excluding those two patients with a recent history of MOH. This did not change the finding of a significant hypometabolism in rACC and OFC compared to controls, suggesting that it is not due to the inclusion of patients with a history of MOH, but genuinely associated with MO. This is further supported by the fact that the OFC hypometabolism was not correlated with the amount of acute medication intake.
Cerebral metabolism and function are known to change significantly during a migraine attack (16) including during premonitory symptoms and in prefrontal cortices (17). We have therefore excluded from the analyses patients who had an attack during the day of the PET acquisition. Though unlikely, patients who had premonitory symptoms 24 hours before the headache may have been included. They would not have biased the results towards a frontal hypometabolism, as in the H215O PET study by Maniyar et al. (17) the premonitory phase was associated with increased activity in the OFC rather than hypoactivity. We found no metabolic changes in thalamus or brainstem. This could be partly related to the methodology, as the templates we used for PET acquisition and analysis were mainly designed to study the cerebral cortex.
One may wonder why this frontal hypometabolism has not been emphasised in MO before. A possible explanation could be that most previous studies have used H215O PET or other functional neuroimaging techniques like fMRI or MR spectroscopy during sensory activation or nitroglycerin-triggered attacks. Interictal resting FDG-PET studies are scarce in MO. FDG-PET reflects brain glucose metabolism and is supposed to detect longer-lasting neurono-glial changes. It was used, however, by Kim et al. (18) in another study of episodic migraineurs. These authors report several areas of hypometabolism including prefrontal cortices. Although they do not report specifically such hypometabolism in the OFC, they do so for the perigenual ACC and the temporal cortex as found in our study. They report a negative correlation with disease duration and with lifetime headache frequency. Besides age, headache frequency, intake of acute medications, quality of life impact or cultural imprints in pain perception, a possible reason for some of the differences between the study by Kim et al. (18) and our study could be that our patients had a higher attack frequency (on average 11 migraine days/month) as compared to their patients, who had at the most five attacks/month (60 per year).
Interestingly, a similar OFC hypometabolism using FDG-PET was found in cluster headache and interpreted as reflecting impairment of top-down anti-nociceptive pathways (19). Moreover, in an event-related fMRI study repetitive trigemino-nociceptive stimulation decreased activity of rACC and the prefrontal cortex over time in migraine patients, as opposed to HVs, in whom it increased, suggesting an alteration of the pain inhibitory circuitry in migraine (20). Systematic reviews of morphological brain imaging studies in patients with various migraine types highlight grey matter loss in the frontal lobe proportional to attack frequency or/and disease duration (21), in the posterior insular-opercular regions, the prefrontal cortex, and the ACC (22). Brain connectivity assessed by fMRI in MO patients is overall impaired in regions involved in pain and cognitive processing including the OFC (see Schwedt et al. (23) for a review).
As a second result, our study shows that the above-described baseline resting state OFC/rACC hypometabolism is attenuated after three months of eTNS treatment in MO patients using the Cefaly® device at least 30% of the recommended time. This was paralleled by a significant decrease in attack frequency. The change in OFC/rACC metabolism could be due to the eTNS itself, to clinical improvement or to intrinsic variability of the method. The latter is unlikely, since several studies, for instance the one by Maquet et al. (24) have found good overall test-retest stability and low intra-subject variability in glucose metabolism assessed with FDG-PET (see review in Schaefer et al. (25)). The fact that we found no metabolic differences between eTNS responders and non-responders does at first sight not favour a proper effect of the neurostimulation. However, this must be taken with caution, as our sample size was probably too small to detect significant changes. In our previous study of MOH patients, contrary to the present finding, OFC hypometabolism worsened after drug withdrawal, despite clear improvement of the headache (13). Since mean acute medication intake did not change between baseline and the third month of eTNS therapy, one can rule out a role of medication use in the present study.
The change in OFC/rACC metabolism and the progressive reduction of migraine attack frequency with eTNS might suggest that the treatment exerts a slow central neuromodulatory effect – like other peripheral nerve stimulations (26). For logistical and technical reasons, the PET recordings could not be performed during an eTNS session, unlike in some other studies of pONS (9) or deep brain stimulation (27). As mentioned in the introduction, functional neuroimaging studies in chronic cluster headache (8) and chronic migraine patients (9) have shown that pONS is able to increase to increase metabolism in central areas belonging to descending pain control networks, including the ACC, but leaves unchanged disease-specific structures like, respectively, the hypothalamus (8) or the dorsal pons (9). By the same token, long electrostimulation of the trigeminal ganglion in patients with trigeminal neuropathic pain increased regional blood flow in the ACC, OFC and medial frontal cortices, which was correlated with pain relief (7). Finally, opioid and placebo analgesia are also associated with increased activity of the OFC and rACC, suggesting a common underlying mechanism (12). We cannot rule out a placebo effect or a positive expectation phenomenon influencing OFC/rACC metabolism, but, with the above-mentioned methodological reservation, this is unlikely given the lack of difference between treatment responders and non-responders. Moreover, our previous randomised, controlled trial (RCT) has demonstrated that eTNS with Cefaly® is superior to sham stimulation in MO prevention (4).
Our study has several shortcomings. Because of the small number of evaluable patients, the results must be taken with caution. As discussed, the study design does not allow assessing a direct causal effect of eTNS on brain metabolism since a sham condition is missing. We found sham stimulation for three months would be unethical knowing that there is evidence for eTNS efficacy from an RCT (4). The compliance rate with eTNS therapy was rather low. For preventive drug treatments, adherence varies from 48% to 94% between studies (28). Neurostimulation is more time-consuming (20 minutes daily in our study), which provokes lower compliance. In the PREMICE trial patients had a compliance rate of 62% (4), while participants renting the eTNS Cefaly® device via the internet used it on average 58% of the recommended time (6). In this study we considered patients who performed at least 30% of the sessions as ‘compliant’; this threshold was chosen on an empirical basis and experience from clinical practice showing that patients may benefit from eTNS with non-daily use of the device. However, the minimal time of use to obtain a clinical improvement in migraine is unknown, and may vary between patients. Although the headache diaries allowed monitoring global intake of acute medications for each patient, they did not allow us to determine the precise proportion of drugs taken within each of the pharmacological classes, analgesics, NSAIDs, triptans, nor its possible change after eTNS. It is unlikely, however, that such a change would have influenced brain metabolism.
Conclusions
Our study suggests that OFC and rACC are hypometabolic in MO patients at rest. After a three-month treatment with eTNS, this hypometabolism was reduced and the changes were associated with a significant decrease of migraine attack frequency. It is known that neurostimulation can modulate OFC and rACC activity (7,8). Like cluster (19) and MOH (13), MO seems to be associated with dysfunction of medial frontal cortex areas involved in affective and cognitive dimensions of pain control. Because our study was underpowered and had no sham arm, we are unable to formally attribute the metabolic changes to the non-invasive neurostimulation treatment. Nonetheless, the observed effect is likely similar to that found with invasive neurostimulation of pericranial nerves, such as pONS (8,9). Further trials are needed to confirm these findings.
Key findings
Orbitofrontal and rostral anterior cingulate cortices are significantly hypometabolic in episodic migraineurs at rest compared to healthy controls.
Episodic migraine, like chronic migraine with medication overuse, seems to be associated with a dysfunction of medial frontal areas involved in affective and cognitive dimensions of pain control.
A three-month preventive treatment with external trigeminal nerve stimulation (eTNS) is associated with normalisation of this hypometabolism and significantly reduces migraine attack frequency.
Assuming that the 18-fluorodeoxyglucose positron emission tomography (FDG-PET) changes are causally related to eTNS, the effect on the medial prefrontal cortex seems similar to that reported after invasive pericranial nerve stimulation therapies.
Acknowledgements
Cefaly Technology® provided the eTNS devices used for this study, as well as technical assistance (compliance analysis). The manuscript was reviewed by a qualified English-speaking medical writer.
Author contributions include the following:
– DM conceived and wrote the study protocol, recruited and followed-up MO patients, interpreted the results, reviewed the introduction, methods and results parts of the manuscript, conducted the literature search and wrote the discussion and conclusion.
– KD recruited HVs, compiled the statistics, wrote the first draft of introduction, methods and results, and designed the tables and figures.
– AT helped with neuroimaging processing.
– VDP escorted patients to the Nuclear Medicine Department for the one-hour eTNS session (PET2).
– PG ensured the logistics of the study (appointments, eTNS training and headache diaries).
– RH is head of the Nuclear Medicine Department where PETs were performed, and reviewed the methods.
– SL supervised AT.
– JS conceived and reviewed the study protocol, recruited and followed-up MO patients, and reviewed the final manuscript.
– The final version of the manuscript was sent to all coauthors for approval before submission.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SL and JS are consultants for Cefaly Technology.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Walloon Region, DG06, Direction Générale Opérationnelle de l’Économie, de l’Emploi et de la Recherche (TRADONI convention 1117427), and by the European Union (EUROHEADPAIN, Seventh framework programme, grant agreement number 602633).
References
- 1.Torelli P, Jensen RH, Tavanaiepour D, et al. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 53: 137–146. [DOI] [PubMed] [Google Scholar]
- 2.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: Results from the second International Burden of Migraine Study (IBMS-II). Headache 2013; 53: 644–655. [DOI] [PubMed] [Google Scholar]
- 3.Magis D. Neuromodulation in migraine: State of the art and perspectives. Expert Rev Med Devices 2015; 12: 329–339. [DOI] [PubMed] [Google Scholar]
- 4.Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology 2013; 80: 697–704. [DOI] [PubMed] [Google Scholar]
- 5.Russo A, Tessitore A, Conte F, et al. Transcutaneous supraorbital neurostimulation in “de novo” patients with migraine without aura: The first Italian experience. J Headache Pain 2015; 16: 69–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magis D, Sava S, D’Elia TS, et al. Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly® device in headache treatment: A survey of 2,313 headache sufferers in the general population. J Headache Pain 2013; 14: 95–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willoch F, Gamringer U, Medele R, et al. Analgesia by electrostimulation of the trigeminal ganglion in patients with trigeminopathic pain: A PET activation study. Pain 2003; 103: 119–130. [DOI] [PubMed] [Google Scholar]
- 8.Magis D, Bruno MA, Fumal A, et al. Central modulation in cluster headache patients treated with occipital nerve stimulation: An FDG-PET study. BMC Neurol 2011; 11: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matharu MS, Bartsch T, Ward N, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: A PET study. Brain 2004; 127: 220–230. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs S, Peeters R, De Ridder D, et al. Central effects of occipital nerve electrical stimulation studied by functional magnetic resonance imaging. Neuromodulation 2011; 14: 46–47. [DOI] [PubMed] [Google Scholar]
- 11.Streel S, Donneau AF, Dardenne N, et al. Validation of an extended French version of ID Migraine as a migraine-screening tool. Cephalalgia 2015; 35: 437–442. [DOI] [PubMed] [Google Scholar]
- 12.Petrovic P, Kalso E, Petersson KM, et al. Placebo and opioid analgesia – imaging a shared neuronal network. Science 2002; 295: 1737–1740. [DOI] [PubMed] [Google Scholar]
- 13.Fumal A, Laureys S, Di Clemente L, et al. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 2006; 129: 543–550. [DOI] [PubMed] [Google Scholar]
- 14.Riederer F, Gantenbein AR, Marti M, et al. Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: Possible influence of orbitofrontal cortex. J Neurosci 2013; 33: 15343–15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraro S, Grazzi L, Mandelli ML, et al. Pain processing in medication overuse headache: A functional magnetic resonance imaging (fMRI) study. Pain Med 2012; 13: 255–262. [DOI] [PubMed] [Google Scholar]
- 16.Sprenger T, Borsook D. Migraine changes the brain: Neuroimaging makes its mark. 2012; 25: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 2014; 137: 232–241. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Kim S, Suh SI, et al. Interictal metabolic changes in episodic migraine: A voxel-based FDG-PET study. Cephalalgia 2010; 30: 53–61. [DOI] [PubMed] [Google Scholar]
- 19.Sprenger T, Ruether KV, Boecker H, et al. Altered metabolism in frontal brain circuits in cluster headache. Cephalalgia 2007; 27: 1033–1042. [DOI] [PubMed] [Google Scholar]
- 20.Aderjan D, Stankewitz A, May A. Neuronal mechanisms during repetitive trigemino-nociceptive stimulation in migraine patients. Pain 2010; 151: 97–103. [DOI] [PubMed] [Google Scholar]
- 21.Bashir A, Lipton RB, Ashina S, et al. Migraine and structural changes in the brain. Neurology 2013; 81: 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai Z, Zhong J, Xiao P, et al. Gray matter correlates of migraine and gender effect: A meta-analysis of voxel-based morphometry studies. Neuroscience 2015; 299: 88–96. [DOI] [PubMed] [Google Scholar]
- 23.Schwedt TJ, Chiang C, Chong CD, et al. Functional MRI of migraine. Lancet Neurol 2015; 14: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maquet P, Dive D, Salmon E, et al. Reproducibility of cerebral glucose utilization measured by PET and the [18F]-2-fluoro-2-deoxy-d-glucose method in resting, healthy human subjects. Eur J Nucl Med 1990; 16: 267–273. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer S, Abercrombie H, Lindgren K. Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp 2000; 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bari AA, Pouratian N. Brain imaging correlates of peripheral nerve stimulation. Surg Neurol Int 2012; 3 (Suppl 4): 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May A, Leone M, Boecker H, et al. Hypothalamic deep brain stimulation in positron emission tomography. J Neurosci 2006; 26: 3589–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsey RR, Ryan JL, Hershey AD, et al. Treatment adherence in patients with headache: A systematic review. Headache 2014; 54: 795–816. [DOI] [PMC free article] [PubMed] [Google Scholar]