Abstract
Obesity-related glomerulopathy (ORG) is morphologically defined as focal segmental glomerulosclerosis and glomerulomegaly. Podocyte hypertrophy and reduced density are related to proteinuria which in a portion of patients is in the nephrotic range and evolvs towards renal failure. This article reviews the pathogenetic mechanisms of podocyte injury or dysfunction and lists new possible antiproteinuric strategies based on pharmaceutical targeting of the reported pathogenetic mechanisms. The pathogenetic mechnisms discussed include: renin angiotensin system, plasminogen activation inhibitor-1 (PAI-1), lipid metabolism, adiponectin, macrophages and proinflammatory cytokines, oxidative stress. The proposed antiproteinuric strategies include: AT2 receptor blockers; adipokine complement C19 TNF-related protein-1 blocker; selective PAI-1 inhibitor; farnesoid x receptor activation; increase of circulating adiponectin; selective antiinflammatory drugs; more potent antioxidants (Heme oxigenase, NOX4 inhibitors). However, because ORG is a rare disease, the need for a long term pharmaceutical approach in obese proteinuric patients should be carefully evaluated and limited to the cases with progressive loss of renal function.
Keywords: Obesity-Related Glomerulopathy, Podocyte Injury, Proteinuria Podocytes, Review
2. INTRODUCTION
Obese patients are at greater risk to develop, hyperlipidemia, arterial hypertension, coronary vascular disease, insulin resistance diabetes and sleep apnea.(1). Obesity is defined as body mass index > 30 Kg/mq. The obesity-related glomerulopathy (ORG) is defined morphologically as focal segmental glomerulosclerosis (FSGS) and glomerulomegaly, podocyte hypertrophy and reduced podocyte density, increased mesangial matrix and mesangial-cell proliferation. These are the common histological findings in renal biopsies from obese animals and humans (2,3,4,5,6) in absence of diabetes, hypertension, metabolic syndrome.
Clinical parameters and pathological findings in patients with obesity-related focal segmental glomerulosclerosis compared to patients with idiopathic focal segmental glomerulsoclerosis show predominance of classic perihilar lesions of sclerosis, significant less severe degree of foot process effacement, lower incidence of nephrotic proteinuria and consistent presence of glomerulomegaly (100% compared to only 10%) (7). The kidney of nondiabetic obese patients exhibits glomerular hyperfiltration, increased renal blood flow and renal hypertrophy (8,3). Proteinuria is a recognized complication of obesity, frequently in the nephrotic range, followed by progressive loss of renal function in a substantial proportion of cases (9,10,11).
The incidence of ORG has increased 10-fold over the last years (12) and has been emphasized in numerous publications, reviews and editorials, however the pathophysiological mechanism of obesity-induced glomerulomegaly and glomerular sclerosis is incompletely understood. (13).
It is important to recognize that despite the growing emphasis on obesity as a risk factor for chronic kidney disease, the absolute risk for an obese subject to develop glomerulosclerosis and renal failure is low; even in individuals with massive obesity, little evidence for overt renal disease occurs (14). Thus, the obesity per se seems not be sufficient to result in glomerulosclerosis in most subjects despite the presence of multiple mechanisms that are postulated to promote renal injury. This observation suggests that either the pathogenesis of proteinuria and glomerulosclerosis in obesity depends on mechanisms that are rare in obese subjects or that there are differences in genetic susceptibility to develop glomerulosclerosis despite similar degrees of exposure. Studies in obese animal models failed to elucidate the reasons for a different suceptibility even though these models exhibit a variable propensity to develop glomerulosclerosis (15,16,17). Substantial glomerulosclerosis has also been observed in obese people at autopsy without evidence of antemortem proteinuria (3,18) indicating that glomerulomegaly, proteinuria and glomerulosclerosis may separately occur. However, data show an adverse impact of obesity and the salutary effect of weight loss on proteinuria and progression of concomitant chronic kidney disease (10,19). Despite these observations, the incidence of ORG has increased in last years. Kambham et al reviewed 6818 renal biopsies from 1986 to 2000 and noted a progressive, 10-fold increase in biopsy frequency of ORG from 0.2% to 2.0% (7). The ORG is a risk factor for end-stage renal disease. Hsu et al. performed a cohort study of 320,252 adults who volunteered for health check up between 1964 and 1985. A total of 1471 cases of end-stage renal disease appeared and a higher body mass index was a risk factor for progression to end-stage kidney disease after adjusting for other variables.(20)
ORG is now described as a secondary form of FSGS that is a proteinuric disease. Visceral podocyte contributes to the formation of glomerular crescents in FSGS (21,22). Decreased podocyte density and number were observed in patients with obesity-related glomerulopathy and the changes in podocyte correlated with degree of proteinuria and renal function in obese patients (3,4,5,6,23). We review the role of the podocyte dysfunction in ORG. Furthermore we intend to provide a rationale for a pharmacologic action on definite components of the podocyte injury in order to prevent development and progression of renal damage.
3. MECHANISMS OF PODOCYTE INJURY OR DYSFUCTION
3.1. Angiotensin II
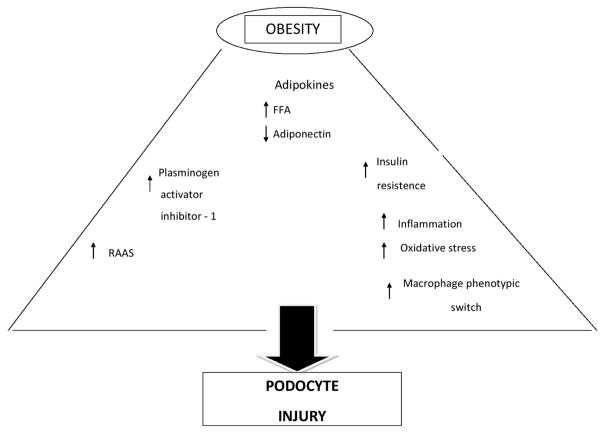
Figure 1 shows the principal mechanisms of podocyte injury or disfunction in ORG. Circulating levels of Angiotensin (Ang) II increase with increasing BMI (24) because adipocytes and adipose-infiltrating macrophages potentiate the renin angiotensin aldosterone system (RAAS) (25). Ang II receptors have been documented in glomerular epithelial cells (26). Podocytes respond to Ang II with an increase in free cytosolic Calcium (Ca) concentration via release of Ca from intracellular stores and influx of Ca from the extracellular space (27). An increase of the cytosolic Ca activates Cl− channels in podocytes in vivo and in vitro, resulting in a depolarization of podocytes (28). Expecially in the rat there is a considerable evidence that Ang II modulates directely the podocyte function via on the calcium and TRPC6 (Transient Receptor Potential Canonical channel- 6). (29) In podocytes TRCP6 is located in the slit diaphragm (30). This ion channel expression is increased in acquired human kidney disease and the overexpression of TRCP6 in podocytes is sufficient to induce actin reorganization and proteinuria (31). Foot process contains an actin cytoskeleton-based contractile apparatus comparable to that of smooth muscle cells or pericytes and its regulation is vital to the kidney glomerular function (32). The examination of electrophysiologic properties of podocytes in the intact glomerulus, demonstrates that Ang II depolarizes podocytes in the isolated rat glomerulus, and Ang II increases the inward current of podocytes (33). Interestingly, in cultured differentiated podocytes, flufenamate, an inhibitor of nonselective channels such as transient receptor potential canonical (TRCP) channels, inhibithed AngII-mediated increase of intracellular Ca (34).
Figure 1.
Principal mechanisms of podocyte injury in obesity.
3.2. Aldosterone
Aldosterone (Ald) blockade reduces renal injury. This benefit is independent of the antihypertensive effect and may be related to the blocking of Ald effects on plasminogen-activator inhibitor-1 and on transforming growth factor beta, on reactive oxygen intermediates, on inflammatory mediators and on podocyte function (35,36,37) Adipose tissue is capable of stimulating AngII-independent Ald production; at least one oxidized derivative of linoleic acid is able to stimulate Ald production (38). Furthermore complement-C1q TNF-related protein 1 (CTRP1), which in part mediates Ang II stimulation of Ald, is greatly increased in adipose tissue of db/db mice and Zucher diabetic fatty (fa/fa) rats (39). This novel adipokine stimulates Ald production in Male Sprague-Dawley rats in the zona glomerulosa of the adrenal cortex through induction of CYP11B2 gene expression (40). Mineralcorticoid receptor (MR) was detected in the podocytes in vivo and in vitro and Ald induces its effector Kinase SgK1, activates NADPH oxidase and generates reactive oxygen species (35,41). Therefore, CTRP1 may represent a molecular link between obesity-related hypertension, ORG and Ald blockers. CTRP1 may be renoprotective in patients with activated MR signaling in target tissue and chronic kidney disease (42). In the transgenic Ren2 rat, podocyte foot process effacement is normalized by treatment with spironolactone and is accompanied by a reduction in albuminuria as well as attenuated NADPH oxidase activity (43). In uninephrectomized rats continuously infused with Ald proteinuria, podocyte damage and SgK1 upregulation are significantly alleviated by tempol, a membrane –permeable superoxide dismutase. That suggests a pathogenetic role of oxidative stress (41). Furthermore, Ald antagonism in dogs attenuates obesity-induced arterial hypertension and glomerular hyperfiltration (44).
3.3. Plasminogen activator inhibitor-1 (PAI-1)
PAI-1 as the primary physiological inhibitor of plasminogen activators, inhibits fibrinolysis and proteolysis, has a key role in obesity as well as insulin resistance and has been associated with complications of these conditions such as atherosclerosis, myocardial infarction, cardiovascular disease (45, 46,47). PAI-1 modulates adipocyte differentiation (48). Recently, Kishore P et al have demonstrated that the action of free fatty acids on adipose tissue macrophages dinamically regulates the expression of PAI-1 (49). PAI-1 is a multifunctional glycoprotein with impressive fibrosis-promoting effects in the kidney.(50,51,52,53). How PAI-1 promotes renal fibrosis is not clear. Recent studies suggest that PAI-1 inhibits serine protease activity within vascular and extracellular compartments; directly modulates inflammatory cells leading to a vicious cycle of inflammatory cell recruitment, fibroblast activation and scar tissue accumulation.(54). Obesity increases PAI-1 in adipose tissue and in glomerular cells (49). Human idiopatic FSGS shows intrarenal PAI-1 expression (51). In experimental kidney disease the genetic or therapeutic manipulation of PAI-1 may reduce or increase fibrosis (54). The presence of PAI-1 is an independent risk factor for the renal damage due to decreased protease-dependent matrix degradation and cellular migration (55). Peroxisome proliferator-activated receptor (PPAR) is a large nuclear receptor family whose main role is to activate genes involved in fatty acid oxidation (56). In a podocyte injury-associated glomerulosclerosis model, renoprotection conferred by PPAR-gamma agonist is achieved, in part, through decreased PAI-1(57). Among the many renoprotective properties of the AngII inhibitors there is their ability to suppress PAI-1 (58). These observations suggest that PAI-1 may modulate podocyte injury. The development of selective anti-PAI-1 therapeutic agents is under study, however the ideal antifibrotic agent has not yet been discovered (59). Much still remains to be disclosed about the role of PAI-1 in kidney disease and much remains to be learned about the cellular receptor-dependent biologic effects of PAI-1 that may modulate fibrosis severity and be relevant to renal fibrogenesis and regression (54).
3.4. Lipid metabolism
Hyperlipidemia has been identified as a causative factor of obesity-related FSGS and may directly contribute to renal damage. The term lipotoxicity refers to an intracellular shunting of free fatty acids excess, towards synthesis of lipid products. In advanced stages of intracellular lipid overload lipotoxicity is capable of inducing cell damage such as diacylglicerol, tryglicerides and ceramides. These products induce apoptosis of many cell types (60). Obese hyperlipidemic mice (young C57BL/6 mice) induced by high-fat diet show elevated glicemia, insulinemia, tryglicerides, cholesterol and low circulating adiponectin levels. These obese mice become preoteinuric and develop kidney morphological abnormalities including glomerulomegaly, expanded mesangial matrix, glomerular basement membrane thickening and podocyte effacement (61). Young obese Zucker rats fed a high-fat diet show an increase in mesangial area which is normalized by treatment with rosuvastatin (62). Lipid moieties can directely injury renal parenchimal cells. Human mesangial cells exposed to LDL, or oxidized LDL, increase the synthesis of mesangial matrix components as fibronectin and laminin. The lipid moieties also promove mesangial production of macrophage migration inhibitory factor and increased expression/release of the inflammatory activators CD40 and IL-6 (63). Hyperlipidemic mice treated with anti-IL-6 monoclonal antibody ameliorates lipid-induced renal toxicity (64). Lipids also damage podocytes. Oxidized LDL causes redistribution and loss of nephrin as well as podocyte apoptosis by decreasing phosphorylation of Akt, a prominent pathway for cell survival (65). Rosuvastatin protects against podocyte apoptosis in vitro through a p21-dependent pathway (66). Cultured human podocytes exposed to the satured fatty acid palmitate show decreased insulin-stimulated glucose uptake (insulin resistance). Podocyte palmitate exposure is associated with increased ceramide production. The ceramide inhibitors myoricin and fumonosin B1 partially recovered the insulin sensitivity-reduced expression of genes associated with insulin sensitivity, reduced phosphorylation of the insulin receptors IRS1 and PKB and impaired translocation of GLUT4 (67). Sterol regulatory element binding protein-1 (SREBP-1) appears to play a critical role in the renal lipid accumulation and the consequent injury. Renal effects of a high-fat diet are not observed in SREBP-1c −/− mutant mice, whereas SREBP-1a transgenic mice has increased glomerular lipid accumulation, glomerulosclerosis and albuminuria (68,69). In a rodent model of high-fat diet induced proteinuria and glomerular disease, farnesoid X receptor activation ameliorates trygliceride accumulation, podocyte loss, mesangial expansion, proteinuria as well as inflammatory and oxidative stress (70). Finally, SREBP-1 glomerular expression is upregulated two-fold in glomeruli from patients with obesity-related glomerulopathy (71). Further studies are necessary to explain how the genetic manipulation of SREBP using SREBP-1c(−/−) mice prevents renal deposition of lipids.
3.5. Adiponectin
3.5.1. General aspects
Adiponectin (Adipo) levels are depressed in obesity (72) because fetuin-A, a glycoprotein produced exclusively by the liver (73) and hypersecreted during caloric excess, suppresses Adipo transcription in adipocytes directly and indirectly through expansion of adipose tissue (74). Adipo is a bioactive substance secreted by adipose tissue toghether with others such as leptin, resistin, visfatin –so called adipokines-that are very late markers of adypocyte cell differentiation during adipogenesis in adipose tissue (75). These adipokines, secreted products of preadipocytes and mature adypocytes, regulate energy homeostasis, appetite/satiety, reproduction, bone turnover, insulin sensitivity and influence neuroendocrine, endothelial, immunological, hematological, angiogenic, vascular functions in an endocrine, paracrine, autocrine manner (76). The adipose tissue, once considered as a passive type of connective tissue storing excess of energy, has now been established as a real endocrine organ coupling (neuro)-endocrine and metabolic signaling (77). Adipo is a 30 k-Da protein secreted mainly by adipocytes but also by skeletal muscle cells, cardiac myocytes, endothelial cells. It circulates in multimeric forms as low/middle/high molecular Adipo and globular Adipo. Globular Adipo is insulin-sensitizing, anti-inflammatory, anti-atherogenic, immunodepressant and vasculo-protective (78,79). Two receptors for Adipo have been identified: Adipo R1 and Adipo R2 (80). Adipo R1/R2 do not seem to be coupled with G protein transmembrane system. These receptors activate PPAR alpha, 5′ adenosin monophosphate protein kinase(AMPK), p38 mitogen-activated protein kinase (MAPK) signaling pathways with consequent glucose uptake, gluconeogenesis and fatty acid oxidation (81). Interaction of adaptor protein containing a pleckstrin homology domain, phosphtyrosine-binding domain (PTB) and leucine zipper motif (APPL1) with AdipoR1 appears to play an important role in Adipo signalling and Adipo mediated downstream events such as lipid oxidation and glucose uptake. Adipo enhances the binding of APPL1 to both Adipo R1/R2 and this interaction is essential for the subsequent phosphorilation of AMPK (82). Adipo R1 is primarly responsible for the AMPK signalling pathway activation whereas AdipoR2 for PPAR-alpha activation (83).
3.5.2. Adiponectin and podocytes
Glomerular cells express a functional adiponectin receptor AdipoR1 which trough activation of AMPK plays an important role in the control of oxidative stress and cell survival within the glomerulus (84). Adipo null mutant mice show podocyte foot process fusion, oxidant stress and albuminuria (85). The mice also show an exaggerated response to renal injury (subtotal renal ablation model) including glomerulomegaly, glomerular collagen deposition, podocyte foot process effacement, increased TGF beta and albuminuria (86). The treatment with Adipo normalizes podocyte effacement and albuminuria (85) indicating that Adipo supports normal function of the podocyte. At least in part, Adipo benefit may occur through reduction of oxidant stress (85,84). Conversely, Adipo deficiency leads to augmentation of NADPH oxidase and increase of urinary reactive oxygen species. The increased production of ROS from renal NADPH oxidase could cause ROS to enter the circulation, contributing to the systemic inflammation that accompanies obesity 85). The positive effect of Adipo administration to null mice is mediated through the Adipo stimulation of AMPK pathway, a key regulator of intracellular energy status with potent antiproliferative effects. AMPK suppression of an isoform of NADPH oxidase (Nox 4) may account for the improvement in podocyte cytostructure in Adipo-treated animals (85). In obesity low Adipo (72) triggers oxidative stress via NADPH oxidase 4 (Nox 4) enhancement and induces podocyte damage (extensive foot process effacement). On the other hand the reduced activation of 5′ AMP protein kinase (AMPK) determines podocyte zona occludens-1 (ZO-1) internalization, a tight junction protein highly espressed adjacent to the insertion of the slit diaphragm of the foot process, a condition that together with foot process effacement contributes to proteinuria (85,88,89). Using conditionally differentiated podocytes, inhibition of AMPK dramatically impairs podocyte morphology. Activation of AMPK with its analogue aminoimidazole carboxamide ribonucleotide restores podocyte morphology in vitro and normalizes albuminuria in vivo in the Adipo null mice (74,85). Collectively, these data suggest that Adipo protects against albuminuria trough AdipoR1 receptor pathway by stimulating AMPK and inhibiting reactive oxygen species. Whether additional renal effects are mediated through the AdipoR2 is currently unknown.
3.6. Macrophages and proinflammatory cytokines
Adipose tissue not only secretes bioactive substances but also promotes a low-grade chronic inflammatory state (90). Obesity-related macrophages infiltration of adipose tissue is believed to be the key of inflammation and insulin resistance (91,92). Depending on the local micro-environment and stage of tissue injury, macrophages display heterogeneity in functions (93,94). Thus, M1 or ‘classically activated’ macrophages are induced by classical immune pathways and function to enhance proinflammatory cytokine production (IL1-beta, TNF-alpha, Il-6). By contrast, M2 or ‘alternatively activated’ macrophages synthesize antiinflammatory cytokines IL-10 and IL-1 during resolution of inflammation and tissue repair. They also possess high endocytic clearance capacities (95,96). Obesity induces the macrophage phenotypic switch in adipose tissue (97) shifting from M2 phenotype predominating in lean rodents to a robust increase in the proinflammatory M1 macrophage population in obese animals (96,98). Experimental approaches to inhibit proinflammatory macrophages have been successful in reducing kidney injury (99,100). Macrophages have also a reciprocal relationship with adipocytes. For example, fatty acids released from adypocytes stimulate TNF-alpha release by macrophages which, in turn, can stimulate production of IL-6 by fat cells, further amplifying the inflammatory response in adipose tissue as well as in the kidney (101). As the adipose tissue expands during periods of nutritional excess, at least two inflammatory pathways are activated: the stress kinase JNK and the transcription factor NF-kB (102,103). Potential initiators of the inflammatory activation include Endoplasmic Reticulum (ER) and oxidative stress, ceramides and other lipids possibly activating toll-like receptors (104,105,106). Once activated, the downstream consequences include the production of proinflammatory cytokines, chemokines and cellular adhesion molecules that recruit and localize immune cells including monocytes and macrophages (91,92). Proinflammatory cytokines are key mediators of progressive renal fibrosis. In cultured mouse podocytes and rat glomeruli, activated macrophages downregulate podocyte nephrin and podocin expression via stress-activated protein kinases (107). In conditionally immortalized podocytes, the bystander macrophages as well as macrophage-derived cytokines IL-1beta and TNF-alpha markedly suppress activity of the nephrin gene promoter of the podocyte with involvement of the PI3K/Akt pathway (108). The suppression of nephrin expression by TNF-alpha involves the cAMP-retinoic acid receptor pathway (109). In cultured rat of glomerular epithelial cells TNF-alpha induces actin cytoskeleton reorganization (110). Toll like receptor 4 (TLR4) too seems to link podocytes with the innate immune system to mediate glomerular injury. In two mouse models, the TLR4 expression in podocyte, its activation by ligands such as LPS or Lipid A and induction of chemokine production have been observed (111). The physiological function of TLR4 in podocytes is unknown. We can speculate that it may enable podocytes, by virtue of their unique location in the urinary space, to perform surveillance functions and respond to the presence of pathogens or proteins normally foreign to this space by recruitment of leukocytes. Further studies are necessary.
3.7. Inflammation and oxidative stress
Obesity has been described as a cluster of metabolic derangements associated with disturbance in adipose tissue and abnormal visceral fat accumulation from physical inactivity and excess of calories in genetically susceptible individuals (112). Inflammatory abnormalities and oxidative stress are characteristic findings of both obesity and metabolic syndrome. The obese Zucker rats provide conclusive evidence for the role of oxidative and nitrosoactive stress (113). In this experimental model the treatment with a peroxinitrate scavenger, ebselen, ameliorates not only markers of oxidative stress but also histological and functional abnormalities linked to obesity. The implication of oxidative stress in the renal damage associated with obesity has also been suggested in another experimental model of obesity, the spontaneously hypertensive/NIH-corpulent rat: SHR/Ndmer-cp(cp/cp). A low caloric diet in these animals improved proteinuria, glomerulosclerosis and the renal content of pentosidine and advanced glycation end products (114). The study of gene expression profiles in renal biopsies of six patients with ORG compared to normal controls, shows that the expression of gene related to lipid metabolism, inflammation and insulin resistance is significantly increased (115). The list of these genes includes peroxisome proliferator-activated receptor, leptin receptor, glucose transporter 1, interferon gamma, vascular endothelial growth factor, interleukin-6 signal transducer, TNF-alpha, sterol regulatory element binding protein 1, fatty acid binding protein 3, low-density lipoprotein receptor. Heme oxygenase (HO) plays a key role in the renal function regulation by attenuating the production of reactive oxygen species because degradation products of HO possess potent antioxidant and antiapoptic activity (116). HO exists in two isoforms that are products of different genes: HO-1 inducible by oxidant stress and HO-2 (constitutive) that has in corticosteroids the major stimulus (117). The HO-1/HO-2 system is a regulator of both cardiovascular-renal system integrity and oxidative stress (118). Targeting of HO system by an up-regulation may provide therapeutic benefit for the cardio-renal disease ( 119,120).
4. PHARMACOLOGIC TARGETING OF PODOCYTE INJURY IN OBESITY-RELATED GLOMERULOPATHY
It is widely accepted that proteinuria reduction is an imperative therapeutic goal in chronic proteinuric kidney disease (121). Proteinuria is an hallmark of ORG and the podocyte is the common treath of proteinuric disease (122). Although lifestyle modification (salt restriction, hypocaloric diet and regular aerobic exercise) and angiotensin-converting enzyme inhibitors have shown benefical effects on obesity-associated proteinuria, only weight loss in early stages of the disease remains the most effective measure to definitely control proteinuria (124). Therephore, more selective drugs for the control of obesity-associated proteinuria are needed. Antiproteinuric strategies include: Angiotensin Converting Enzyme Inhibitor (ACEI) therapy, Angiotensin II type 1-receptor blocker (ARB) therapy, combination ACEI ARB therapy, beta-blocker therapy, control of protein and fluid intake, restriction of NaCl intake, nondihydropyrridine calcium-channel blocker therapy, control of blood lipids, aldosterone antagonist therapy, smoking cessation, decrease of elevated homocysteine (123). The improving knowledge of the pathophysiologic mechanisms of podocyte injury in obesity associated glomerulopathy suggests development of new antiproteinuric therapy. The following can be considered (Table 1).
Table 1.
Focal segmental glomerulosclerosis in obesity: interventions based on the mechanism of podocyte injury
| General Mechanism | Specific Intervention | |
|---|---|---|
| Currently available | Suggested | |
| RAAS | .ACE inhibitors .Ang II receptor block .Inhibition of aldosterone receptors |
.Selective TRCP6 inhibitors .AngI receptors block .Inhibition of non-genomic effect of aldosterone |
| PAI-1 | .Angiotensin II inhibitors | .Selective PAI-1 inhibitors |
| Lipids | .Body weight reduction .Statin |
.Ligands of farnesoid X receptor |
| Adiponectin | .Body weight reduction .RAAS blockade .Enhancement of Adiponectin intracellular signal pathway |
.Adiponectin administration .Agonists Adiponectin R1/2 receptor |
| Inflammation | .Steroids | .Selective inhibitors of TLR4 .Selective block of inflammatory kinases |
| Oxidative stress | .Antioxidants | .HO-1 overexpression .Inhibition of specific NADPH oxidase (nox-4 isoform) |
Abbreviations: RAAS:renin angiotensin aldosterone system, ACE:angiotensin converting enzyme, PAI-1:plasminogen activator inhibitor-1, TRCP6: transient receptor cacnonical potential-6, HO-1: heme oxygenase-1, TLR4: toll like receptor 4, NADPH: nicotinamide adenine dinucleotide phosphate oxidase
4.1. AT2 receptor blockers
Ang II modulates directly the podocyte function via calcium intracellular influx in cultured differentiated podocytes. This influx is absent after inhibition of non selective ion channels such as transient receptor potential canonical channel-6 (TRCP6) that is located in the slit diaphragm. The development of drugs inhibiting this non selective channel can be considered in proteinuric experimental animal model such as puromycin aminonucleoside-induced nephrosis (PAN) model (125). The development of ARBs selective for AT2 receptors can be considered, because cellular signalling through AT2 receptor regulates complex biological program such as embryonic development, cell differentiation tissue repair and programmed cell death while the AT1 receptor mediates the classical physiological effects of Ang II. AT2 receptors may mediate functions opposite to those mediated by the AT1 receptor (126).
4.2. Adipokine complement C19 TNF-related protein-1 blocker
Mineralcorticoid receptor are detected in podocyte and Ald causes podocyte injury via SgK1 and oxidative stress. Circulating Ald measurement can be performed in obese subjects for evaluating administration of anti-Ald drugs. The measurement is useful in patients treated with ACEIs or ARBs also, because of elevated Ald concentration for the phenomen called “aldosterone escape”(127). We believe that Ald blockers with antiproteinuric and podocyte-protective properties are promising drugs for prevention and treatment of ORG. The adipokine complement-C1qTNF-related protein-1(CTRP1) should be evaluated in obese subjects with arterial hypertension because in experimental model angiotensin II-induced aldosterone production is, at least in part, mediated by the stimulation of CTRP1 that is highly expressed in obese subjects (39,40).
4.3. Selective PAI-1 inhibitor
The renoprotective properties of the Ang II inhibitors include partial suppression of PAI-1. In the 5/6 nephrectomy glomerulosclerosis model the inhibitors of angiotensin or aldosterone given alone or in combination significantly decrease and sometimes reverse glomerulosclerosis (renal fibrosis regression). This beneficial effect is strongly linked to decreased glomerular PAI-1 expression (128). Much still remains to be disclosed about the role of PAI-1 because much remains to be learned about the knowledge of the cellular receptor-dependent biologic effects of PAI-1 such as its ability to promote detachment of cells from their anchors (i.e. dystroglycans/integrins in glomerular basement membrane for podocyte) or cells migration (monocytes/macrophages). So the development of more selective anti-PAI-1 therapeutic drugs may be useful for the control of profibrotic effects of PAI-1.
4.4. Farnesoid x receptor activation
Hyperlipidemia may mediate renal injury by increasing the expression of sterol-regulatory element-binding proteins (SREBPs) which is responsible for increasing synthesis of tryglicerides/cholesterol in the kidney and stimulates podocyte injury (65). Rosuvastatin protects against podocyte apoptosis in vitro (66). In human glomerular podocytes the treatment with statins reduces in a dose-dependent manner the oxLDL-induced apoptosis and loss of nephrin by activating the PI3K/AKT-dependent pathway (129). The farnesoid X receptor (FXR) is a bile acid-activated nuclear receptor which plays an important role in regulating bile acid metabolism. FXR has been shown to control lipid metabolism by a mechanism involving repression of SREBPs. FXR and SREBPs coexist in the glomeruli and proximal tubular cells and are expressed in cultured mouse mesangial cells and podocytes. FXR activation by highly selective ligands could represent an effective therapy for treatment of abnormal renal lipid metabolism with associated inflammation, oxidative stress and kidney pathology in patients affected by obesity.
4.5. Increase of circulating adiponectin
The link between adiponectin, podocyte dysfunction and proteinuria has been established in obesity (72,74). Adipo circulates in multimeric forms that are decreased in obesity (78,79). Because plasma Adipo concentration is inversely associated with body weight, triglicerides and LDL cholesterol levels and predicts incident type 2 diabetes, the monitoring of HMW Adipo levels – the most bioactive circulating form- and identification of subjects at low Adipo levels can be a good biomarker for evaluating the severity of obesity and predicting obesity-associated complications such as type 2 diabetes, cardiovascular disease, hepatic dysfunction and kidney disease (130). Increased albuminuria identifies also obese subjects at high risk profile with regard to chronic kidney disease and cardiovascular disease. Maneuvres to raise Adipo level may be weight reduction and RAS blockade (131,132). The administration of Adipo recombinant protein in humans is actually unlike to be cost effective even if it has been used extensively in preclinical models (133,134) Approaches that increase synthesis or administration of AdipoR1/R2 agonists – so called adiponectin mimetics- remain attractive but compounds that stimulate Adipo synthesis in a selective manner or receptor agonists are not available at present. Enhancement of Adipo intracellular signalling pathway (AMPK/PPAR) are available: metformin raises AMPK pathway activity, thiazolidine-dione (TZD) raises the PPARgamma, fibrates raise the PPARalpha (135,136,137).
4.6. Selective antiinflammatory drugs
The inflammatory obesity state is linked to podocyte disfunction by Toll like receptor (TLR) system. Proinflammatory cytokines trough the TLR activate the intracellular podocyte IkB complex that leads to NFkB activation and consequent nuclear translocation of NFkB with activation of target genes (138). Today the NFkB activity measurement in proteinuric obese subjects is needed. Steroids exert the antiinflammatory action by preventing the binding of NFkB to DNA and the subsequent gene transcription (139,140). However this is an unselective block of NFkB activation and further studies are necessary to provide a more selective blockade agonists of this relevant transcription factor. Phramacological manipulation may also be achieved by selective block of TLR system or several serine/threonine kinases such as JNK, IKK inhibitors of inflammatory kinases (141).
4.7. More potent antioxidants (Heme oxigenase, NOX4 inhibitors)
Oxidative stress is caused by an imbalance between the production of reactive oxygen and the detoxification by antioxidants: severe oxidative stress can trigger necrosis and apoptosis. In obese proteinuric subject, it is necessary to quantify by breath or plasma biomarkers oxidative stress and its severity. Then oxidative stress may be reversed by the overexpression of antioxidant enzymes and the administration of antioxidants. Among the antioxidants the heme degradation products CO (carbon monoxide), biliverdin/bilirubin, iron/ferritin possess potent antioxidant and antiapoptotic effects (118,142). Heme oxygenase (HO-1/2) degrades heme (116). Obesity is associated with a decrease in HO-1 and pharmacological or genetic approaches to induce HO-1 overexpression may provide therapeutic benefit in obese oxidative stress. This is observed in animal models of obesity where the up-regulation of HO-1 is associated with a concomitant decrease in the levels of O2 and iNOS which mark oxidative stress (116,120,143,144). A more selective therapeutic approach for podocyte dysfunction in obese subjects may be the inhibition of specific NADPH oxidase isoform such as Nox4.
5. CONCLUSION
Obesity predisposes towards renal disease independently of diabetes and arterial hypertension. In obesity-related glomerulopathy podocyte lesions are present with focal segmental glomerulosclerosis -classified as secondary FSGS-and glomerulomegaly. In ORG, the podocyte injury is linked to many pathophysiological mechanisms as above discussed. Improving knowledge of these mechanisms suggests development of more potent antiproteinuric drugs. However, because the incidence of obesity related glomerulopaty remains under 5% the need for a long term therapeutical approach in obese proteinuric patients should be carefully evaluated and limited to the cases with progressive loss of renal failure.
References
- 1.Visscher TSL, Seidel JC. The public health impact of obesity. Ann Rev Public Health. 2001;22:355–375. doi: 10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell MP, Kasiske BL, Cleary MP, Keane WF. Effects of genetic obesity on renal structure and function in the Zucker rat. II. Micropuncture studies. J Lab Clin Med. 1985;106:605–610. [PubMed] [Google Scholar]
- 3.Kasiske BL, Napler J. Glomerular sclerosis in patients with massive obesity. Am J Nephrol. 1985;5:45–50. doi: 10.1159/000166902. [DOI] [PubMed] [Google Scholar]
- 4.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight and body surface in normal man. Anat Rev. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 5.Wessen DE, Kurtzman NA, Pedro Frommer J. Massive obesity and nephrotic proteinuria with a normal biopsy. 1985;40:235–237. doi: 10.1159/000183467. [DOI] [PubMed] [Google Scholar]
- 6.Jennette JC, Charles L, Grubb W. Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep apnea syndrome. Am J Kid Dis. 1987;10:470–472. doi: 10.1016/s0272-6386(87)80196-8. [DOI] [PubMed] [Google Scholar]
- 7.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy:an emerging epidemi. Kid Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 8.Chagnac A, Weinstein T, Korzets A. Glomerular hemodynamics in severe obesity. Am J Physiol renal Physiol. 2000;278: F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 9.Praga M, Hernandez E, Morales E. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Trannspl. 2001;16: 1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 10.Praga M, Hernandez E, Andres A. Effects of body weight loss and captopril treatment on proteinuria associated with obesity. Nephron. 1995;70: 35–41. doi: 10.1159/000188541. [DOI] [PubMed] [Google Scholar]
- 11.Adelman RD. Obesity and renal disease. Curr Opin Nephrol Hypert. 2002;11: 331–335. doi: 10.1097/00041552-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta analysi. Kid Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 13.Ritz E, Kolegonova N. Obesity and chronic kidney disease. Semin Nephrol. 2010;29: 504–511. doi: 10.1016/j.semnephrol.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Kasiske BL, Umen AJ. The influence of age, sex, race and body habitus on kidney weight in humans. Arch Pathol Lab Med. 1986;110:55–60. [PubMed] [Google Scholar]
- 15.Kasiske BL, O’Donnell MP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia and renal injury. Hypertension. 1992;(Supp I):I110–I115. doi: 10.1161/01.hyp.19.1_suppl.i110. [DOI] [PubMed] [Google Scholar]
- 16.Dobrian AD, Davies MJ, Prewitt RL, Lanterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35:1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 17.Jiang T, Wang Z, Proctor G, Morkowitz S. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280: 32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AH. Massive obesity and the kidney. Am J Pathol. 1975;81:117–130. [PMC free article] [PubMed] [Google Scholar]
- 19.Morales E, Valero MA, Leon M, Hernandez E, Praga M. Beneficial effects of weight loss on overweight patients with chronic proteinuric nephropathies. Am J Kid Dis. 2003;41:319–327. doi: 10.1053/ajkd.2003.50039. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY, McCulloch C, Iribarren CE. Body mass index and risk for end-stage renal disease. Ann Int Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 21.Thorner PS, Ho M, Eremina V, Sado Y, Quaggin S. Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol. 2008;19:495–502. doi: 10.1681/ASN.2006101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bariety J, Bruneval P, Meyrier A, Mandet C, Jacquot C. Podocyte involvement in human immune crescentic glomerulonephritis. Kid Int. 2005;68:1109–1119. doi: 10.1111/j.1523-1755.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen HM, Liu ZH, Zeng CI, LISH, Wang QW, Li SH. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kid Dis. 2006;48: 772–779. doi: 10.1053/j.ajkd.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Schorr U, Blaschke K, Turan S. Relationship between angiotensinogen, leptin and blood pressure levels in young normotensive men. J Hypert. 1998;16:1475–1480. doi: 10.1097/00004872-199816100-00011. [DOI] [PubMed] [Google Scholar]
- 25.Frederich RC, Kahn BB, Pesch MJ, Flier JS. Tissue specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension. 1992;19:339–344. doi: 10.1161/01.hyp.19.4.339. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, Sharma R, Greene AS, McCarthy ET, Savin VS. Documentation of angiotensinogen II receptors in glomerular epithelial cells. Am J Physiol Renal Physiol. 1998;274: F623–F627. doi: 10.1152/ajprenal.1998.274.3.f623. [DOI] [PubMed] [Google Scholar]
- 27.Huber TB, Gloy J, Henger A. Catecholamines modulate podocyte function. J Am Soc Nephrol. 1998;9: 335–345. doi: 10.1681/ASN.V93335. [DOI] [PubMed] [Google Scholar]
- 28.Pavenstadt H, Kritz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83: 253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 29.Patel A, Sharif-Naeini R, Folgering JRH, Bichet D, Duprat F, Honorè E. Canonical TRP channels and mechanotransduction:from physiology to diseases states. Pfluf Arch Eur J Physiol. 2010;460:571–81. doi: 10.1007/s00424-010-0847-8. [DOI] [PubMed] [Google Scholar]
- 30.Reiser J, Polu KR, Moller CC. TRCP6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moller CC, Wei C, Altintas MM. Induction of TRCP6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 32.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends in Cell Biology. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Gloy J, Henger A, Fischer GK. Angiotensin II depoolarizes podocytes in the intact glomerular of the rat. J Clin Invest. 1997;99:2772–2781. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henger A, Huber T, Fischer KG. Angiotensin II increases the cytosolic calcium activity in rat podocytes in culture. Kid Int. 1997;52: 687–693. doi: 10.1038/ki.1997.383. [DOI] [PubMed] [Google Scholar]
- 35.Nagase M, Fujita T. Aldosterone and glomerular podocyte injury. Clin Exp Nephrol. 2008;12:233–242. doi: 10.1007/s10157-008-0034-9. [DOI] [PubMed] [Google Scholar]
- 36.De Paula RB, Da Silva AA, Hall JE. Aldosterone anatogonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–47. doi: 10.1161/01.HYP.0000105624.68174.00. [DOI] [PubMed] [Google Scholar]
- 37.Bomback AS, Klemmer PJ. Renal injury in extreme obesity: the important role of aldosterone. Kid Int. 2008;74:1216–1217. doi: 10.1038/ki.2008.429. [DOI] [PubMed] [Google Scholar]
- 38.Goodfriend TL, Ball DL, Egan BM. Epoxy-kedto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. doi: 10.1161/01.HYP.0000113294.06704.64. [DOI] [PubMed] [Google Scholar]
- 39.Kim KY, Kim JH, Lee CH, Kim DH, Han SH, Yang Y. Tumor necrosis factor-alpha and interleukin-1beta increases CTRP1 expression in adipose tissue. FEBS Lett. 2006;580:3953–3960. doi: 10.1016/j.febslet.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 40.Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Kim JW, Han SH, Lim JS, Yang Y. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J. 2008;22: 1502–1511. doi: 10.1096/fj.07-9412com. [DOI] [PubMed] [Google Scholar]
- 41.Shibata S, Nagase M, Yoshida S, Kawachi I, Fujita T. Podocyte as the target for aldosterone. Roles of oxidative stress and SgK1. Hypertension. 2007;49: 355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 42.Guney I. Antifibrotic effects of aldosterone receptor blocker (spironolactone) in patients with chronic kidney disaease. Renal Failure. 2009;31: 779–784. doi: 10.3109/08860220903150312. [DOI] [PubMed] [Google Scholar]
- 43.Whaley-Connell A, Habibi J, Wei Y. Mineralcortocoid receptor antagonism attenuates glomerular filtration barrier remodeling in the transgenic Ren2 rat. Am J Physiol Renal Physiol. 2009;296: F1013–F1022. doi: 10.1152/ajprenal.90646.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Paula RB, da Silva A, Hall JE. Aldosterone antagonism attenuates obeisty-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–47. doi: 10.1161/01.HYP.0000105624.68174.00. [DOI] [PubMed] [Google Scholar]
- 45.Ma LJ, Fogo AB. PAI-1 and kidney fibrosis. Front Biosc. 2009;14:2028–2041. doi: 10.2741/3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loskutoff DJ, Quigley JP. PAI-1, fibrosis and the elusive provisional fibrin matrix. J Clin Invest. 2000;106:1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma LJ, Mao SL, Taylor KL. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 48.Liang X, Kanjanabuch T, Mao SL. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am J Physiol Endocrinol Metab. 2006;290: E103–E113. doi: 10.1152/ajpendo.00605.2004. [DOI] [PubMed] [Google Scholar]
- 49.Kishore P, Li W, Tonelli J, Lee D, Koppaka S, Hawkins M. Adipocyte-derived factors potentiate nutrient-induced production of plasminogen activator inhibitor-1 by macrophages. Sci Transl Med. 2010;24: 20ra15. doi: 10.1126/scitranslmed.3000292. [DOI] [PubMed] [Google Scholar]
- 50.Lee HS, Park SY, Moon KC, Hong SY. mRNA expression of urokinase and plasminogen activator inhibitor-1 in human crescentic glomerulonephritis. Histop. 2001;39:203–209. doi: 10.1046/j.1365-2559.2001.01195.x. [DOI] [PubMed] [Google Scholar]
- 51.Hamano K, Iwano M, Akai Y, Yoshida Y, Kohono S, Dohi K. Expresion of glomerular plasminogen activator inhibitor type 1 in glomerulonephritis. Am J Kid Dis. 2002;39:695–705. doi: 10.1053/ajkd.2002.31986. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura T, Tanaka N, Higuma N, Yokota S. The localization of plasminogen activator inhibitor-1 in glomerular subepithelial deposits in membranous nephropathy. J Am Soc Nephrol. 1996;7:2434–2444. doi: 10.1681/ASN.V7112434. [DOI] [PubMed] [Google Scholar]
- 53.Paueksakon P, Revelo MP, Fogo AB. Microangiopathic injury and augmented PAI-1 in human diabetic nephropathy. Kid Int. 2002;61: 2142–2148. doi: 10.1046/j.1523-1755.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 54.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanism of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- 55.Yuan J, Jia R, Bao Y. Aldosterone up-regulates production of plasminogen activator inhibitor-1 by renal mesangial cells. J Biochem Mol Biol. 2007;40: 80–188. doi: 10.5483/bmbrep.2007.40.2.180. [DOI] [PubMed] [Google Scholar]
- 56.Francis GA, Knopp RH, Oram JF. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 57.Yang HC, Ma LJ, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kid Int. 2006;69:1756–1764. doi: 10.1038/sj.ki.5000336. [DOI] [PubMed] [Google Scholar]
- 58.Naito T, Ma LJ, Yang H, Zuo Y, Fogo AB. Angiotensin II receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298: F683–F691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gils A, Declerck PJ. Plasminogen activator inhibitor-1. Curr Med Chem. 2004;11: 323–2334. doi: 10.2174/0929867043364595. [DOI] [PubMed] [Google Scholar]
- 60.Bagby SP. Obesity-initiated metabolic syndrome and the kidney:a recipe for chronic kidney disease? J Am Soc Nephrol. 2004;15:2775–2791. doi: 10.1097/01.ASN.0000141965.28037.EE. [DOI] [PubMed] [Google Scholar]
- 61.Deji N, Kume S, Araki S. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol. 2009;296: F118–F126. doi: 10.1152/ajprenal.00110.2008. [DOI] [PubMed] [Google Scholar]
- 62.Reisin E, Ebenezer PJ, Liao J. Effect of the HMG-CoA reductase inhibitor rosuvastatin on early chronic kidney injury in obese Zucker rats fed with an atherogenic diet. Am J Med Sci. 2009;338:301–309. doi: 10.1097/MAJ.0b013e3181b27195. [DOI] [PubMed] [Google Scholar]
- 63.Santini E, Lupi R, Baldi S. Effects of different LDL particles on inflammatory molecules in human mesangial cells. Diabetologia. 2008;51: 2117–2125. doi: 10.1007/s00125-008-1127-4. [DOI] [PubMed] [Google Scholar]
- 64.Tomiyama-Hanayama M, Rakugi H, Fonsato V. Effect of interleukin-6 receptor blockage on renal injury in apolipoprotein E-deficient mice. AmJPhysiolRenalPhysiol. 2009;297: F679–F684. doi: 10.1152/ajprenal.90680.2008. [DOI] [PubMed] [Google Scholar]
- 65.Bussolati B, Deregibus MC, Fonsato V. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidyilinositol 3-kinase/Akt-signaling pathway. JamSocNephrol. 2005;16: 936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- 66.Cormack-Aboud FC, Brinkkoetter PT, Pippin JW. Rosuvastatin protects against podocyte apoptosis in vitro. Nephrol Dial Transpl. 2009;24:404–412. doi: 10.1093/ndt/gfn528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lennon R, Pons D, Sabin MA, Wei C, Shield JP, Coward RJ. Saturated fatty acids induce insulin resistance in human podocytes:implications for diabetic nephropathy. Nephrol Dial Transpl. 2009;24:288–3296. doi: 10.1093/ndt/gfp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun L, Halaihelk N, Zhang W. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919–18927. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 69.Jiang T, Wang Z, Proctor G. Diet-induced obesity in C57BL/6J mice causes increased renal accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280: 32317–32395. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 70.Wang XX, Jiang T, Shen Y. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297: F1587–F1596. doi: 10.1152/ajprenal.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Y, Liu Z, Xiang Z. Obesity-related glomerulopathy:insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147: 44–50. doi: 10.1210/en.2005-0641. [DOI] [PubMed] [Google Scholar]
- 72.Yatagai T. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 1 diabetes mellitus. Metabolism. 2003;52:1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 73.Swallow CC, Dennis J. Alpha2HS-glycoprotein, an antagonist of transforming growth factor beta in vivo, inhibits intestinal tumor progression. Cancer Res. 2004;64:6402–6409. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- 74.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease and fatty liver disease: the roles of fetuin-A, adiponectin and AMPK. J Am Soc Nephrol. 2010;21:406–412. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaffler A, Muller-ladner U, Sclomerich J, Buchler C. Role of adipose tissue as an inflammatory organ in human disease. Endocrine Rev. 2006;27: 449–467. doi: 10.1210/er.2005-0022. [DOI] [PubMed] [Google Scholar]
- 76.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocr Metab. 2004;89: 2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 77.Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Int Med. 2010 doi: 10.1111/j,1365-2796. [DOI] [PubMed] [Google Scholar]
- 78.Tilg H, Moschen AR. Adipocytokines:meddiators linking adipose tissue, inflammation and immunity. Nature Immun. 2006;6: 773–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 79.Simpson F, Whitehead JP. Adiponectin-It’s all about the modifications. Int J Biochem Cell Biol. 2010;42: 785–788. doi: 10.1016/j.biocel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 80.Yamauchi T, Kamon J, Ito Y, Tsuchida M. Cloning of adiponectin receptors that mediate antidiabetic affects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 81.Kadowaki T, Yamauchi T, Kubota N, Hara K. Diponectin and adiponectin receptors in insulin resistance, diabetes and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mao X, Kikani CK, Rijas RA, Langlais P, Wang L, Ramos FJ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nature Cell Biology. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 83.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 84.Cammisotto PG, Bendayan M. Adiponectin stimulates phosphorylation of AMP-activated protein kinase alpha in renal glomeruli. J Mol Histol. 2008;39: 579–584. doi: 10.1007/s10735-008-9198-6. [DOI] [PubMed] [Google Scholar]
- 85.Sharma K, Ramachadraro S, Qiu G. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118: 1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohashi K, Iwatani H, Kihara S. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knochout mice. Arterioscl Thromb Vasc Biol. 2007;27:1910–1917. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- 87.Sharma K. The link between obesity and albuminuria:adiponectin and podocyte dysfunction. Kid Int. 2009;76t:145–148. doi: 10.1038/ki.2009.137. [DOI] [PubMed] [Google Scholar]
- 88.Ahima RS. Linking adiponectin to proteinuria. J Clin Invest. 2008;118: 1619–1622. doi: 10.1172/JCI35655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zoccali C, Mallamaci F. Obesity, diabetes, adiponectin: a podocyte affair? Nephrol Dial Transpl. 2008;23: 3767–3770. doi: 10.1093/ndt/gfn517. [DOI] [PubMed] [Google Scholar]
- 90.Wellen KE, Hotarnisligil GS. Inflammation, stress and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weisberg SP, McCann D, Desai M. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu H, Barnes GT, Yang O. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 94.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118: 3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kluth DC. Pro-resolution properties of macrophages in renal injury. Kid Int. 2007;72: 234–236. doi: 10.1038/sj.ki.5002332. [DOI] [PubMed] [Google Scholar]
- 96.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3: 23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 97.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117: 175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lumeng CN, Del Proposto JB, Westcott DJ, Saltiel AR. Phenotype switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophages subtypes. Diabetes. 2008;57: 3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duffield JS, Tipping PG, Kipari T. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Wang YP, Zheng G. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kid Int. 2007;72: 290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 101.Lee DL, Leite R, Fleming C. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension. 2004;44: 259–263. doi: 10.1161/01.HYP.0000139913.56461.fb. [DOI] [PubMed] [Google Scholar]
- 102.Hirosumi J. A central role for JNK in obesity and insulin resistance. Nature. 2002;420: 333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 103.Yuan M. Reversal of obesity and diet-induced insulin resistance with salycilates or targeted disruption of IKKbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 104.Ozcan U. Endoplasmic reticulum stress links obesity, insulin action and type 2 diabetes. Science. 2004;306: 457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 105.Furukawa S. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi H. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ikezumi Y, Suzuki T, Karasawa T. Activated macrophages down-regulate podocyte nephrin and podocin expression via stress-activated protein kinases. Biochem Biophys Res Comm. 2008;376:706–711. doi: 10.1016/j.bbrc.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 108.Takano Y, Yamauchi H, Kayakawa K, Hramatsu N. Transcriptional suppression of nephrin in podocytes by macrophages:roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett. 2007;581: 421–426. doi: 10.1016/j.febslet.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 109.Saito Y, Okamura M, Nakajima S, Hayakawa K. Suppression of nephrin exprtession by TNF-alpha via interfering with the cAMP- retinoic acid receptor pathway. Am J Physiol Renal Physiol. 2010;298: F1436–F1444. doi: 10.1152/ajprenal.00512.2009. [DOI] [PubMed] [Google Scholar]
- 110.Koukouritaki SB, Vardaki EA, Papakonstanty EA, Lianos E. TNF-alpha induces actin cytoskeleton reorganization in glomerular epithelial cells involving tyrosine phosphorylation of paxillin and focal adhesion kinase. Mol Med. 1999;5: 382–392. [PMC free article] [PubMed] [Google Scholar]
- 111.Banas MC, Banas B, Hudkins L, Wietecha TA. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19: 704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Potenza MV, Mechanick JL. The metabolic syndrome:definition, global impact and pathophysiology. Nutr Clin Pract. 2009;24:560–577. doi: 10.1177/0884533609342436. [DOI] [PubMed] [Google Scholar]
- 113.Chander PN, Gealekman O, Brodsky SV. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosattive stress :prevention by chroni therapy with a peroxynitrate scavenger ebselen. J Am Soc Nephrol. 2004;15: 2391–2403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]
- 114.Nangaku M, Izuhara Y, Usuda N. Type 2 diabetic nephropathy rat model, the improvement of obesity by a low caloric diet reduces oxidative/carbonyl stress and prevents diabetic nephropathy. Nephrol Dial Transpl. 2005;20: 2661–2669. doi: 10.1093/ndt/gfi096. [DOI] [PubMed] [Google Scholar]
- 115.Wu Y, Liu Z, Xiang Z. Obesity-related glomerulopathy:insights from gene expression profiles of the glomeruli derived from renal biposy samples. Endocrinology. 2006;147: 44–50. doi: 10.1210/en.2005-0641. [DOI] [PubMed] [Google Scholar]
- 116.Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G. Heme oxygenase: the key to renal function regulation. Am J Physio Renal Physiol. 2009;297:F1137–F1152. doi: 10.1152/ajprenal.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cruse I, Mainess MD. Evidence suggesting that two forms of heme oxygenase are products of different genes. J Biol Chem. 1988;263: 3348–3353. [PubMed] [Google Scholar]
- 118.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Rad Biol Med. 2005;39: 1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 119.Peterson SJ, Frishman WH, Abraham NG. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev. 2009;17: 99–111. doi: 10.1097/CRD.0b013e31819d813a. [DOI] [PubMed] [Google Scholar]
- 120.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57: 1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 121.Reiser J, Mundel P. Dual effects of RAS blockade on blood pressure and podocyte function. Current Hypert Rep. 2007;9:403–408. doi: 10.1007/s11906-007-0074-7. [DOI] [PubMed] [Google Scholar]
- 122.Camici M, Carpi A, Cini G, Galetta F, Abraham N. Podocyte dysfunction in aging - related glomerulosclerosis. doi: 10.2741/204. In press on FBS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilmer JA, Rovin BH, Hebert CJ. Management of glomerular proteinuria; a commentary. J Am Soc Nephrol. 2003;14: 3217–3232. doi: 10.1097/01.asn.0000100145.27188.33. [DOI] [PubMed] [Google Scholar]
- 124.Rutkowski P, Klassen A, Sebekova K. Renal disease in obesity the need for greater attention. J Ren Nutr. 2006;16:216–223. doi: 10.1053/j.jrn.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 125.Lee DW, Kwak IS, Lee SB, Song SH, Seong EY, Chung HC, Yang BY, Lee MY, Sol MY. Effects of colecoxib and nordihydroguaiaretic acid on puromycin aminonucleoside-induced nephrosis in the rat. J Korean Med Sci. 2009;(24 Suppl):S183–8. doi: 10.3346/jkms.2009.24.S1.S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–63. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 127.Struthers AD. Aldosterone blockade in cardiovascular disease. Heart. 2004;90: 1229–1234. doi: 10.1136/hrt.2003.025312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma LJ, Nakamura S, Aldigier JC, Fogo AB. Regression of glomerulosclerosis with high-dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol. 2005;16: 966–976. doi: 10.1681/ASN.2004060492. [DOI] [PubMed] [Google Scholar]
- 129.Bussolati B, Deregibus MC, Fonsato V, Doublier S, Camussi G. Statins prevents oxidized LDL-induced injury of glomerular podocytes by activating the posphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16: 1936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- 130.Jalovaara K, Santaniemi M, Timonen M, Jokelainen J, Kesaniemi YA, Ukkola O, Keinanen-Kiukaanniemi S, Rajala U. Low serum adiponenctin levels as a predictor of impaired glucose regulation and type 2 diabetes mellitus in a middle-aged Finnish population. Metabolism. 2008;57: 1130–1134. doi: 10.1016/j.metabol.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 131.Linscheld P, Christ-Crain M, Stoeckli R. Increase in high molecular wieght adiponectin by bariatric surgery-induced weight. Loss Diabetes Obes Metab. 2008;10: 1266–1270. doi: 10.1111/j.1463-1326.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 132.Serra A, Granada ML, Romero R, Bayes B, Cantona A, Bonet J. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity:1-year follow up. Clin Nutr. 2006;23: 400–408. doi: 10.1016/j.clnu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 133.Kim CH, Pennisi P, Zhao H, LeRoth D. MKR mice are resistant to the metabolic actions of both insulin and adiponectin: discordance between insulin resistance and adiponectin responsiveness. Am J Physiol Endocrinol Metab. 2006;291: E298–E305. doi: 10.1152/ajpendo.00319.2005. [DOI] [PubMed] [Google Scholar]
- 134.Restituto P, Colina I, Varo JJ, Varo N. Adiponectin diminishes platelet aggregation and sCD40L release. Potential role in metabolic syndrome. J Physiol Endocrin Met. 2010;298: E1072–E1077. doi: 10.1152/ajpendo.00728.2009. [DOI] [PubMed] [Google Scholar]
- 135.Zhou G, Myers R, Li Y. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108: 1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kanjanbuch T, Ma LJ, Chen J. PPAR-gamma agonist protects podocytes from injury. Kid Int. 2007;71:1232–1239. doi: 10.1038/sj.ki.5002248. [DOI] [PubMed] [Google Scholar]
- 137.Lee CH, Olson MR. Minireview:lipidi metabolism. metabolic diseases and peroxisome proliferator-activated receptors. Endocrinology. 2003;144: 2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 138.Israel A. The IKK complex:an integrator of all signals that activate NF-kB? Trends in Cell Biology. 2000;10: 129–133. doi: 10.1016/s0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- 139.Wardle EN. Nuclear factor kB for the nephrologist. Nephrol Dial Transpl. 2001;16:1764–1768. doi: 10.1093/ndt/16.9.1764. [DOI] [PubMed] [Google Scholar]
- 140.Guijarro C, Egido J. Trnscription factor kB(NF-kB) and renal disease. Kid Int. 2001;59: 415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 141.Yamaguchi N, Argueta JGM, Mashiro Y, Yamashita M. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Letters. 2005;579: 6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 142.Juncos P, Grande JP, Murali N, Nath KA. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235: 1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 143.Kim DH, Burgess AP, Li M, Abraham NG. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduces adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325: 833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 144.Nicolai A, Li M, Kim DH, Peterson SJ, NG Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]