Summary
In species with complex life cycles, population dynamics result from a combination of intrinsic cycles arising from delays in the operation of negative density-dependent processes (e.g., intraspecific competition) and extrinsic fluctuations arising from seasonal variation in the abiotic environment. Abiotic variation can affect species directly through their life history traits and indirectly by modulating the species’ interactions with resources or natural enemies.
We investigate how the interplay between density-dependent dynamics and abiotic variability affects population dynamics of the bordered plant bug (Largus californicus), a Hemipteran herbivore inhabiting the California coastal sage scrub community. Field data show a striking pattern in abundance: adults are extremely abundant or nearly absent during certain periods of the year, leading us to predict that seasonal forcing plays a role in driving observed dynamics.
We develop a stage-structured population model with variable developmental delays, in which fecundity is affected by both intra-specific competition and temporal variation in resource availability and all life history traits (reproduction, development, mortality) are temperature-dependent. We parameterize the model with experimental data on temperature-responses of life history and competitive traits and validate the model with independent field census data.
We find that intra-specific competition is strongest at temperatures optimal for reproduction, which theory predicts leads to more complex population dynamics. Our model predicts that while temperature or resource variability interact with development-induced delays in self-limitation to generate population fluctuations, it is the interplay between all three factors that drive the observed dynamics. Considering how multiple abiotic factors interact with density-dependent processes is important both for understanding how species persist in variable environments and predicting species’ responses to perturbations in their typical environment.
Keywords: competition, ectotherms, environmental variability, life history traits, mathematical modeling, population dynamics, resource variability, temperature variation
Introduction
Elucidating the mechanisms that drive species’ population dynamics is a central challenge in ecology. In organisms with complex life cycles, time delays due to juvenile development lead to delays in the operation of negative feedback processes (e.g., intraspecific competition), which can generate population cycles (Gurney et al., 1983; Nisbet & Gurney, 1983; Murdoch et al., 1987; Murdoch & Walde, 1989; Nisbet, 1997; Gurney & Nisbet, 1998; Murdoch et al., 2003).
It is well known that species’ responses to abiotic environmental variation can interact with density-dependent feedback processes to drive population dynamics (Kingsolver, 1989; Urbaneja et al., 1999; Huey & Berrigan, 2001; Crozier, 2004; Savage et al., 2004; Frazier et al., 2006; Zamani et al., 2006a; Amarasekare & Sifuentes, 2012). Abiotic variation can have both direct and indirect effects on population dynamics. Direct effects arise from the abiotic factor’s impact on species’ life history traits, such as reproduction, development, and mortality (Dreyer & Baumgartner, 1996; Liu & Tsai, 2000; Morgan et al., 2001; Urbaneja et al., 2001; Medeiros et al., 2003; Matadha et al., 2004; Bommiredyy et al., 2004; Castillo et al., 2006; Ulmer et al., 2006; Parajulee, 2006; Huang et al., 2008; Ragland & Kingsolver, 2008; De Conti et al., 2010; Hou & Weng, 2010; Jandricic et al., 2010; Nishikawa et al., 2010), and interaction traits, such as competition coefficients and attack rates (Zamani et al., 2006b; Dannon et al., 2010; Englund et al., 2011; Lang et al., 2011; Amarasekare & Coutinho, 2014). Temperature is perhaps the most important abiotic factor that exerts such direct effects. Indirect effects arise from the impacts of an abiotic factor on other species (resources, natural enemies, competitors, mutualists) with which a focal species interacts. For example, rainfall and/or temperature may drive the phenology of a plant species on which an herbivore feeds, and temporal variation in resource availability arising from the plant’s phenological response can, in turn, affect the herbivore’s population dynamics. Understanding how direct and indirect effects of abiotic variation influence density-dependent population dynamics is crucial in predicting how species may respond to atypical environmental variability such as climate change (Bale et al., 2002; Walther et al., 2002; Root et al., 2003; Parmesan, 2006; Deutsch et al., 2008; Tewksbury et al., 2008; Kingsolver, 2009; Kingsolver et al., 2011; McMahon et al., 2011; Sheldon et al., 2013).
Here, we use the bordered plant bug (Largus californicus; Fig. 1), a Hemipteran herbivore inhabiting the California coastal sage scrub, as a model system to investigate this issue. Motivated by a distinctive pattern observed in the bug’s population dynamics, we develop a mathematical model to generate predictions for two hypotheses about the underlying mechanisms. While the model is motivated by the biology of the bordered plant bug, the theory is more general and can be applied to any ectotherm whose dynamics are influenced by direct and indirect abiotic effects.
Figure 1.
Illustration of an adult bordered plant bug (Largus californicus).
Materials and Methods
Study System
We studied the bordered plant bug at the Main Campus Reserve of the University of California, Santa Barbara. This is essentially a closed population as the reserve is a small (150m by 250m) region of coastal bluffs bounded by the Pacific Ocean and a lagoon. The population was studied in 1986 by Booth (1990) and 25 years later by us (see Appendix S1 in Supporting Information).
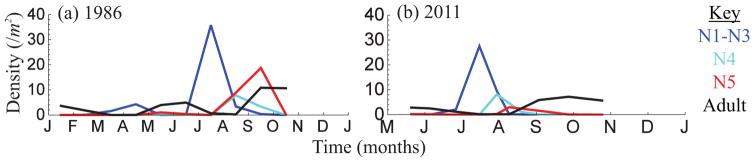
The bordered plant bug is a generalist herbivore (Booth, 1990) whose main food source at this site is bush lupine (Lupinus arboreus). Abundance patterns in the field exhibit a distinctive pattern that cannot be explained by density-dependent dynamics alone: adults are extremely abundant in summer and fall, but are almost completely absent in late-spring and late-summer (Fig. 2). This suggests that bug dynamics may be subject to seasonal environmental forcing.
Figure 2.
Time-series plots show the density of bordered plant bug life stages at the Main Campus Reserve at the University of California, Santa Barbara. Panel (a) is census data collected in 1986 by Booth (1990) and panel (b) is data that we collected in 2011. There is no census data of egg density in the field. As it is impossible to distinguish between pre-reproductive and reproductive adults in the field, the two life stages are combined into a single adult class.
Bordered plant bugs are attacked by three parasitoid species: an egg parasitoid (Gryon largi) and a tachinid fly (Trichopoda pennipes) (Booth, 1990) as well as an unidentified parasitoid wasp. Here, we do not include the effects of natural enemies on plant bug dynamics. We discuss how incorporating parasitoids in this framework offers promising future directions in the Discussion.
Conceptual Framework
Based on the population dynamics observed in the field, we can make two hypotheses about the mechanisms underlying the observed abundance patterns. First, if abundance patterns result from developmental time lags that cause delays in the operation of negative feedback, one would expect to see population cycles if adult longevity is short relative to the juvenile developmental period (Murdoch et al., 2003). Otherwise, one would expect stable (non-oscillatory) dynamics. Second, if abundance patterns result from the interplay between developmental delay-driven cycles and direct and/or indirect effects of abiotic variation, we expect more complex dynamics due to the effects of seasonal forcing on density-dependent population dynamics.
To test which hypothesis better explains the observed census data, we develop a mathematical framework that can accommodate both time delays in the operation of density-dependence and seasonal forcing of key parameters. To do this, we first quantify the temperature-responses of life history and competitive traits via laboratory experiments (see Appendix S2). We then incorporate these responses within a variable delay model. Finally, we explain how seasonal variation in temperature and resource availability is quantified in the field and incorporated within the model.
Effects of temperature on life history traits
The per capita birth rate of most ectotherms exhibits a symmetric and unimodal response to temperature that is well-described by a Gaussian function:
| (1) |
where b(T) is the per capita birth rate at temperature T (in degrees Kelvin), bTopt is the maximum birth rate, attained at an optimal temperature Topt, and σb is the variability about the optimum. Plant bug reproduction exhibits a unimodal temperature response with data providing a significant fit to Eq. 1 (Table S1). Reproduction is therefore greatest at intermediate temperatures and declines at higher and lower temperatures (Fig. 3a). The optimum temperature for reproduction is 23.9±0.3°C, which is near the maximum temperature in the field (1986: 24.5°C, 2011: 25.7°C).
Figure 3.
Temperature responses of life history traits. Reproduction (panel a) is described by a Gaussian function (Eq. 1), while development rate (panels b) and mortality (panels c) are described by the Boltzmann-Arrhenius function (Eq. 2). See Tables S1 for parameter estimates.
In ectotherms, development and mortality exhibit monotonic temperature-responses (Gillooly et al., 2001; Brown et al., 2004; Savage et al., 2004) given by the Boltzmann-Arrhenius function:
| (2) |
where ki(T) is the trait value (i.e., k = m for maturation rate and k = d for mortality) for stage i at temperature T, ki,T is the trait value k at a reference temperature Ti,k for stage i, and Ai,k is the Arrhenius constant of trait k for stage i, measuring its temperature sensitivity (how fast it changes with varying temperature). We find that development rate and mortality increase monotonically with temperature in a manner described by the Boltzmann-Arrhenius function (Eq. 2; Table S1).
Effects of temperature on competitive traits
To our knowledge, there are no empirical data on the temperature response of competitive traits; however, theory offers two hypotheses for how temperature affects the strength of competition. First, metabolic scaling theory predicts that the strength of competition increases monotonically with temperature within biologically-realistic temperature ranges (Savage et al., 2004). Second, ecological theory predicts that competitive traits exhibit a unimodal response to temperature such that competition is strongest near the optimal temperature for reproduction (Begon et al., 2005).
We find that the strength of competition is a unimodal function of temperature with data providing a significant fit to the following Gaussian function (Table S1):
| (3) |
where α(T) is the competitive effect at temperature T, αTmax is the maximum competitive effect, occurring at temperature Tmax, and σα is the variability about the maximum. This suggests that in plant bugs, competition is strongest at intermediate temperatures (23.1±0.3°C) near the optimum for reproduction (23.9±0.3°C) and declines at both higher and lower temperatures (Fig. 4). This is an important finding because theory predicts that when competition is strongest near the optimal temperature for reproduction, the effects of temperature and competition interact antagonistically, driving more complex dynamics (Amarasekare & Coutinho, 2014).
Figure 4.

Temperature response of competitive traits. Intra-specific competition (quantified by the decline in fecundity with adult density; see Fig. S1) exhibits a unimodal response to temperature, which is well-described by a Gaussian function (Eq. 3). See Table S1 for parameter estimates.
Now that the temperature-responses of life history and competitive traits have been quantified, the next step is to incorporate these responses into a mathematical framework to generate predictions about population-level outcomes.
Mathematical Framework
We develop a stage structured delay-differential equation (DDE) model to investigate bordered plant bug population dynamics. DDE models provide a natural way to describe the dynamics of species with stage structured life cycles (Gurney et al., 1983; Nisbet & Gurney, 1983; Murdoch et al., 1987; Murdoch & Walde, 1989; Nisbet, 1997; Gurney & Nisbet, 1998; Murdoch et al., 2003). The model is mechanistic because all parameters are explicitly temperature-dependent and temperature-driven variability in developmental delays are explicitly modeled. Although motivated by the biology of the bordered plant bug, the model can be easily modified to investigate the dynamics of other ectotherms that inhabit variable environments.
Population Dynamics
The bug’s life cycle consists of six life stages: eggs (E), early nymphal instars (N1 – N3; denoted NE in the model), 4th and 5th nymphal instars (N4 and N5), pre-reproductive adults (P), and reproductive adults (R). Population dynamics are given by the following system of DDEs:
| (4) |
where b(T(t)) is the temperature response of the birth rate and Q(t) depicts the time-varying effect of resource variability on the birth rate (i.e., Q(t) = 1 if resource availability remains constant over time and Q(t) ≠ 1 if resource availability varies seasonally). The function α(T(t)) is the temperature response of intra-specific competition; gi(t) depicts maturation through stage i (gR describes adult senescence); and di(T(t)) depicts the temperature response of mortality of stage i.
There are three key points to note about this model. First, resource variability (Q(t)) affects reproduction rather than development or mortality because laboratory experiments show that reproduction is the only trait exhibiting density-dependence (Fig. S1; Table S2). Because it is difficult to experimentally quantify how resource variability affects reproduction, we assume that laboratory estimates of reproduction reflect the maximum values possible.
The second point we want to emphasize concerns development (gi(t)). Because development is temperature-dependent, stage duration is not constant, and thus, developmental delays vary over time. We use the following maturation functions based on previous theory on dynamically varying time delays (Gurney et al., 1983; Nisbet & Gurney, 1983):
| (5) |
where
| (6) |
Note that t′ denotes the time at which eggs hatching at time t were laid (where τE is the time delay involved in egg development), si(t) describes through-stage survivorship of stage i, and τi(t) is the developmental time delay of stage i. Maturation of eggs to 1st nymphal instars (gE(t)) is a function of the rate at which eggs were laid a time τE(t) ago and survivorship through the egg stage (sE(t)). Similarly, maturation of successive stages (gi(t)) are functions of the rate at which individuals mature from the previous life stage and through-stage survivorship. The ratio mi(T(t))/mi(T(t – τi(t))) determines how temperature affects maturation. If temperature increases over the duration of stage i, this ratio is greater than one, stage duration is shorter, and more individuals survive. If temperature decreases over the stage duration, this ratio is less than one, stage duration is longer, and fewer individuals survive. Note that survivorship (si(t)) and developmental time delays (τi(t)) are time-varying differential equations (see Nisbet & Gurney (1983) for derivation). Appendix S3 provides more information about the DDE model developed here and Appendix S4 and Fig. S2 discuss the results of a simplified ODE version of the model.
The third point is about environmental variability. Note that the model incorporates both direct effects of seasonal temperature variation (T(t)) on life history traits and intra-specific competition, and indirect effects arising from resource variability (Q(T)) that affect the birth rate. Below we explain how environmental variability is quantified in the field and incorporated into the model.
Environmental Variability
We quantify seasonal temperature variation (T(t)) via the following sinusoidal function:
| (7) |
where mT is the mean temperature, aT is the amplitude of seasonal temperature variation, and δT gives the shift in the function. We fitted this function to data from the Western Regional Climate Center (wrcc.dri.edu) from 1986–1987 and 2011–2012, which coincide with census data (Fig. 5).
Figure 5.
Seasonal variation in temperature and resource availability are quantified by fitting functions to data on monthly temperatures in 1986 (panel a) and 2011 (panel b) and to field data on resource availability (panel c). See Table S3 for parameter estimates.
Because plant bugs mainly consume bush lupine (Booth, 1990), the availability of the preferred food resource varies seasonally based on bush lupine phenology. Food availability dramatically increases at the end of January following the winter rains (Harrison et al., 1986) and remains high until the end of the flowering season in July (Kittelson & Maron, 2000) when bush lupine wilts and drops its seed pods (Strong et al., 1995). We measured resource availability in the field as the average percent leaf-cover of 25 marked bush lupine shrubs. Thus, leaf-cover varies from 0 to 1.
We quantify temporal variation in food availability (Q(t)) by fitting the following sinusoidal function to the leaf-cover data obtained in the field:
| (8) |
where δR is the shift in the cosine function. Note that when Eq. 8 is negative, we set Q(t) = 0. We find that Q(t) captures the resource availability observed in the field (Fig. 5c; Table S3).
Results
Hypothesis 1: Abundance patterns result from density-dependent dynamics
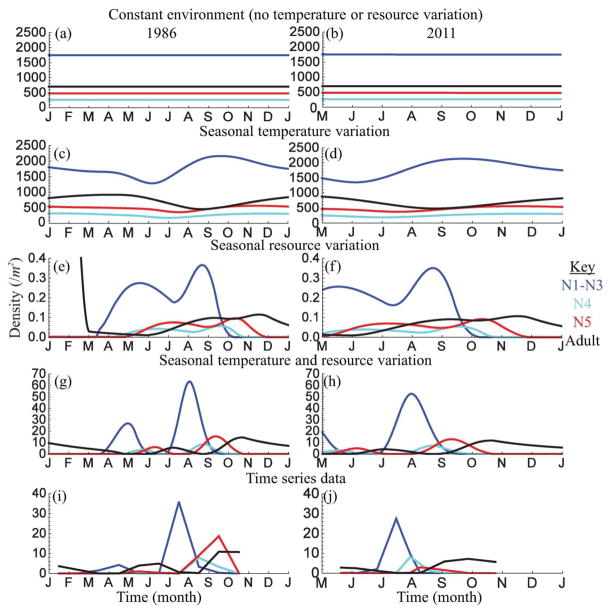
To predict plant bug dynamics in the absence of temperature and resource variability, we analyzed the stage structured model (Eq. 4) in a constant environment (T(t) = mT and Q(t) = 1). The model predicts a stable steady state (Fig. 6a,b), which is approached via damped oscillations. This is markedly different from the pattern of population dynamics observed in the field (Fig. 6i,j). Note that the predicted abundances are much higher than those observed in the field because adults reproduce year-round in the model, while adults reproduce only seasonally in nature.
Figure 6.
Plant bug population dynamics predicted by stage structured DDE models (Eq. 4). Left panels show model predictions for the 1986 census period and right panels show model predictions for the 2011 census period: panels (a,b): constant environment (no temperature or resource variation), panels (c,d): seasonal temperature variation, panels (e,f): seasonal resource variation, panels (g,h): seasonal temperature and resource variation, panels (h,i): census data.
Hypothesis 2: Abundance patterns result from the interplay between density-dependent dynamics and environmental variability
Seasonal temperature variation
A model with seasonal temperature forcing (Eq. 7) but no variation in resource availability (Q(t) = 1) causes fluctuations in abundance within a year. Adult density is greatest during spring and early-summer (Fig. 6c,d) when temperatures approach the optimal for reproduction. Because adult senescence is very sensitive to increasing temperature (high Arrhenius constant; Table S1), adult lifespan is relatively short and thus adult density declines in late-summer. The density of early nymphal stages is greatest in fall following peak reproduction in late-summer and is lowest in early-summer when adult density declines and nymphal mortality is relatively high due to high temperatures. As a result, successive nymphal stages peak in density during winter in the model.
Comparison of the predicted time-series with field census data reveals two mismatches. First, the model predicts that nymphs are present in winter, when in the field nymphs are completely absent in winter. Second, the predicted abundances of all life stages are much greater than are observed in the field. These mismatches are likely due to the model assumption that resources remain plentiful year-round, allowing adults to reproduce, and nymphs to survive, throughout the year. Thus, seasonal temperature variation alone does not explain the observed dynamics.
Seasonal resource variation
A model with seasonal variation in resource availability (Eq. 8) but no seasonal temperature variation (T(t) = mT) captures the gross patterns observed in the field, but greatly underestimates bug abundances (Fig. 6e,f). In the model, overwintering adults reproduce in March when resource availability increases. This initial juvenile cohort develops during the spring and adults reproduce during the summer. Reproduction ceases in August as resource availability declines. The second juvenile cohort develops during the summer/fall and matures into adults by November.
While this version of the model captures the overall trend in plant bug dynamics, it greatly underestimates abundances, as a result of which the population goes extinct within a few years. Extinction likely occurs because mean annual temperatures are sub-optimal for reproduction (recall that reproduction is optimal near the maximum, not mean, field temperatures). This perhaps signifies an important role for seasonal temperature variation. The crucial significance of this model version is the prediction that seasonal variation in resource availability determines the period of the year during which reproduction occurs and hence when nymphal stages are present. Resource variation alone, however, is insufficient to explain the observed time-series data.
Seasonal variation in temperature and resource availability
The full model with seasonal variation in temperature (Eq. 7) and resource availability (Eq. 8) captures both the qualitative pattern of population dynamics and the magnitude of bug abundances observed in the field (Fig. 6g,h). Adults cannot reproduce during the fall or winter due to insufficient resource availability; thus, nymphs are absent in the winter. Overwintering adults have a relatively long lifespan due to reduced mortality as a result of low temperatures. Thus, adults survive long enough to reproduce when resource availability increases in February before senescing by April. The initial juvenile cohort develops fairly slowly in the spring when development rates are low due to low temperatures and matures into adults by July. Reproduction ceases in August as resource availability declines and adults senesce by September as a result of increased mortality due to elevated summer temperatures. The second juvenile cohort develops relatively quickly in late-summer when development rates are faster due to higher temperatures and matures into adults by October. Bug abundances are greater in the second cohort because reproduction is greatest in the summer as temperatures approach the optimum for reproduction. Model dynamics lag slightly behind field census data, perhaps due to uncertainty around the fit of seasonal resource variability (Eq. 8) to the start of the growing season (Fig. 4c). This lag is slightly greater in 2011 than in 1986, likely due greater uncertainty around the fit of season temperature variation (Eq. 7) to temperature data in 2011 (Fig. 4b).
The full model yields two key findings. First, density is driven by both resource availability (via its effects on reproduction) and temperature (via its effects on reproduction, development and mortality). Second, density-dependent population dynamics are influenced by both resource availability (which determines when reproduction occurs) and temperature (which determines stage duration). These findings suggest that plant bug population dynamics result from the interplay between development-driven time delays in the operation of density-dependence and life history traits’ responses to seasonal variation in both temperature and resource availability.
Discussion
In species with complex life cycles, juvenile development leads to time delays in the operation of negative feedback processes (e.g., intra-specific competition), leading to population cycles (Murdoch et al., 2003). Environmental variability can interact with such delays, leading to patterns of population dynamics that deviate from those expected under density-dependent processes alone. Understanding how environmental variability interacts with density-dependent processes is important for predicting population dynamics not only under typical environmental regimes, but also under perturbations, natural or anthropogenic, to the typical environment. Here, we investigate this issue using the bordered plant bug (Largus californicus) as a model system.
We report two key results. The first result pertains to the joint effects of temperature and intraspecific competition on fecundity. We find that competition is strongest at temperatures optimal for reproduction and declines at higher and lower temperatures. This is an important finding because theory predicts that, in such a case, the effects of temperature and competition interact antagonistically, resulting in more complex dynamics than when the strength of competition increases monotonically with temperature (Amarasekare & Coutinho, 2014).
Our second result illustrates the complex interplay between environmental variability and delays in density-dependent feedback in driving population dynamics. While either temperature or resource availability can interact with density-dependent processes to induce population fluctuations, it is the interplay between density-dependence and seasonal variation in temperature and resource availability that generates the distinctive abundance patterns observed in the field. Specifically, density-dependent dynamics are modified by seasonal variation in resource availability (which determines the timing and magnitude of reproduction) and temperature (which affects life history traits both directly and indirectly via its effects on competition).
At a more conceptual level, we develop a theoretical framework for simultaneously considering the direct and indirect effects of abiotic variation on ectotherm population dynamics. There is growing emphasis on the importance of indirect effects of environmental variation on species’ phenology and population dynamics (Araujo & Luoto, 2007). Quantifying and modeling these indirect effects, however, can be challenging. This framework provides a natural way to incorporate both direct and indirect effects of abiotic variation. The direct effects of abiotic variation (here, in temperature) are quantified via the responses of life history and competitive traits. In the case of the bordered plant bug, indirect effects of abiotic factors such as temperature and rainfall are likely manifested via the effects of resource phenology on fecundity (which is the only trait that exhibits density dependence; Fig. S1). Thus, incorporating temperature-dependent parameters and the effects of resource availability on fecundity allows the simultaneous consideration of direct and indirect effects of abiotic factors on plant bug dynamics.
It is important to discuss our results in light of previous studies investigating the joint effects of temperature and resource variation on population dynamics. We discuss three studies. Ritchie (1996) studied the effects of temperature and resource limitation on the grasshopper Melanoplus sanguinipes. He used a non-delay model in which a fixed supply of resources are allocated to maintenance or growth at temperature-dependent rates. The model predicts greater mortality and lower density under elevated temperatures. Our model also predicts that mortality increases with increasing temperatures; however, temperature effects on population density are more complex as the underlying life history and competitive traits are also temperature-dependent.
Reigada and Godoy (2006) studied the effects of larval density on the dynamics of the fly Chrysomya megacephala at two temperatures in a laboratory environment and found that fecundity declines with increasing density and temperature, which may lead to a transition from a two-point limit cycle to a stable equilibrium. While plant bug fecundity also declines with increasing density, fecundity exhibits a unimodal temperature-response. While we consider temperature variation, not increasing temperature, it predicts more complex dynamics when temperature is considered.
Finally, Law and Belovsky (2010) studied the effects of density and temperature on the dynamics of the grasshopper Camnula pellucida in the field. They found that peak survival in low-density treatments occurs at higher temperatures than for high-density treatments, indicating that the strength of intra-specific competition varies with temperature; however, the temperature response of competition was not quantified. Our model explicitly incorporates the temperature response of intra-specific competition, which likely leads to more complex population dynamics.
In summary, previous studies often incorporate only a few (2–3) temperatures, do not quantify the temperature responses of both life history and competitive traits, and fail to explicitly consider temperature effects on the developmental delays that characterize ectotherm life cycles. Our framework differs from these previous studies in that it incorporates measurable temperature response functions for all parameters, explicitly considers variability in developmental delays due to temperature variation, and is well-linked with independent field census data.
The work presented here suggests several future directions. First, our study underscores the importance of considering the role of abiotic factors on bottom-up processes such as resource availability. It does not, however, consider the effects of abiotic factors on top-down processes such as natural enemies, which are likely important to gain a full understanding of how abiotic factors affect species’ population dynamics. Indeed, natural enemies can interact with intrinsic delays in density-dependence to drive more complex dynamics (Murdoch et al., 1987; Murdoch & Walde, 1989; Nisbet, 1997; Gurney & Nisbet, 1998; Murdoch et al., 2003). A key question for future research is how direct and indirect effects of abiotic variation influence consumer-resource dynamics. This framework provides a theoretical foundation for investigating these issues because it incorporates mechanistic descriptions of trait responses to abiotic variation.
The bordered plant bug offers an intriguing case study for investigating the effects of abiotic variation on bottom-up and top-down processes as we have documented variation in resource availability due to bush lupine phenology (bottom-up effects) and plant bugs are attacked by multiple ectothermic natural enemies (top-down effects). Our model suggests that, in this system at least, bottom-up processes are important in driving the observed abundance pattern. Intriguingly, while the full model captures the overall patterns observed in nature, it tends to overestimate bug density, perhaps signifying a key role for natural enemies in suppressing bug density. Future work should therefore incorporate natural enemies within the mathematical framework described here.
The second future direction involves predicting how ectotherms respond to perturbations in their typical thermal environment, such as climate warming (Bale et al., 2002; Walther et al., 2002; Root et al., 2003; Parmesan, 2006; Deutsch et al., 2008; Tewksbury et al., 2008; Kingsolver, 2009; McMahon, & Prentice, 2011; Kingsolver et al., 2011; Sheldon et al., 2013). The framework we have developed here is particularly amenable to investigating the effects of climate change on ectotherm population dynamics because the temperature responses of life history traits can be empirically quantified and climate change scenarios can be easily incorporated into the model.
In conclusion, this study serves as a first step towards a mechanistic understanding of how the interplay between density-dependent processes and abiotic variation affects ectotherm population dynamics. It also provides a case study of a variable delay model with mechanistic descriptions of trait responses to temperature. The model is readily amenable to incorporating empirically-derived temperature-response functions and yields predictions about abundance patterns that can be tested with census data. We have shown that this framework, parameterized with empirical data on life history traits, can capture very complex dynamics observed in the field. This is a key development as mechanisms underlying patterns of population dynamics cannot be elucidated from time-series analysis alone (Knape & de Valpine 2010). Thus, our framework offers the conceptual foundation and mathematical tools to investigate ectotherm population dynamics under climate warming.
Supplementary Material
Figure S1. Density-dependence of life history traits. Fecundity declines with adult density, likely due to intraspecific competition with data providing a significant fit (p < 0.001) to the equation f(A) = be−αA where f(A) gives the fecundity at adult density A, b is the per capita reproductive rate, and α is the strength of intra-specific competition. Density does not have a statistical effect on development (panels b) or mortality (panels c); see Table S2 for statistics.
Figure S2. Plant bug population dynamics predicted by stage-structured ODE models (see Appendix S4). Left panels show model predictions for the 1986 census period and right panels show model predictions for the 2011 census period: panels (a,b): stage-structured ODE model with seasonal temperature and resource variation, panels (c,d): census data.
Table S1. Parameter estimates (nonlinear regression; see main text) for temperature responses.
Table S2. Effects of density on development and mortality, which were quantified using a linear (k(D) = k+αD) and nonlinear (k(D) = keαD) function where k(D) gives the density-dependence of trait k (i.e., k = m, d); D is the density of the life stage at which competition arises; k is the trait value in the absence of competition; and α is the per capita competitive effect. We determine whether density has a statistical effect on development or mortality by whether the per capita competitive effect (α) provides a significant fit to the data.
Table S3. Parameter estimates for the temperature (Eq. 7) and resource availability (Eq. 8) functions. All parameters were estimated via nonlinear regression (see main text for details).
Appendix S1: Census protocols
Appendix S2: Experiment methods
Appendix S3: Effects of Density on Life History Traits
Appendix S4: DDE Model Description
Appendix S5: Non-Delay ODE Model
Acknowledgments
We thank S. Hubbell, J. Lloyd-Smith, V. Savage, S. Bewick, and an anonymous reviewer for helpful comments that substantially improved the manuscript. A special thanks to Julie Himes for the beautiful illustration of a bordered plant bug. The authors have no conflicts to declare. Support was provided by Science Foundation Arizona Grant BSP 0528-13 to C.A.J. and NSF grant DEB-0717350 and a Complex Systems Scholar Grant from the James S. McDonnell Foundation to P.A.
Footnotes
Data Accessibility
The python script for the DDE models (Variable DDE.py) and all data used to parameterize and validate models in this manuscript (Plant Bug Data.xlxs) are uploaded as supporting information.
Additional supporting information may be found in the online version of this article.
References
- Amarasekare P, Coutinho R. Effects of temperature on intraspecific competition in ectotherms. The American Naturalist. 2014;184:E50–E65. doi: 10.1086/677386. [DOI] [PubMed] [Google Scholar]
- Amarasekare P, Sifuentes R. Elucidating the temperature response of survivorship in insects. Functional Ecology. 2012;26:959–968. [Google Scholar]
- Araújo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Global Ecology and Biogeography. 2007:16. [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, JB, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology. 2002;8:1–16. [Google Scholar]
- Begon M, Harper J, Townsend C. Individuals, Populations and Communities. Blackwell Science; Oxford: 2005. [Google Scholar]
- Bommiredyy PL, Parajulee MN, Porter DO. Influence of constant temperatures on life history of immature Lygus elisus (Hemiptera: Miridae) Environmental Entomology. 2004;33:1549–1553. [Google Scholar]
- Booth CL. Biology of Largus californicus (Hemiptera: Largidae) Southwestern Association of Naturalists. 1990;35:15–22. [Google Scholar]
- Brown J, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- Castillo J, Jacas JA, Pena JE, JUB, JHD Effect of temperature on life history of Quadrastichus haitiensis (hymenoptera: Eulophidae), an endoparasitoid of Diaprepes abbreviatus (Coleoptera: Curculionidae) Biological Control. 2006;36:189–196. [Google Scholar]
- Constantino RF, Desharnais RA. Population dynamics and the Tribolium model: genetics and demography. Springer-Verlage; New York: 1991. [Google Scholar]
- Crozier L. Warmer winters drive butterfly range expansion by increasing survivorship. Ecology. 2004;85:231–241. [Google Scholar]
- Dannon EA, Tamo M, van Huis A, Dicke M. Functional response and life history parameters of Apanteles taragamae, a larval parasitoid of Maruca vitrata. BioControl. 2010;55:363–378. [Google Scholar]
- De Conti B, Bueno VHP, Sampaio MV, VSL Reproduction and fertility life table of three aphid species (Macrosiphini) at different temperatures. Revista Brasileira de Entomologia. 2010;54:654–660. [Google Scholar]
- Deutsch CJ, Tewksbury J, Huey RB, Sheldon K, Ghalambor C, Haak D, Martin PR. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer H, Baumgartner J. Temperature influence on cohort parameters and demographic characteristics of the two cowpea coreids Clavigralla tomentosicollis and C. shadabi. Entomologia Experimentalis et Applicata. 1996;78:201–213. [Google Scholar]
- Englund G, Ohlund G, Hein C, SD Temperature dependence of the functional response. Ecology Letters. 2011;14:914–921. doi: 10.1111/j.1461-0248.2011.01661.x. [DOI] [PubMed] [Google Scholar]
- Frazier M, Huey R, Berrigan D. Thermodynamic constraints on the evolution of insect growth rates: ’warmer is better’. American Naturalist. 2006;168:512–520. doi: 10.1086/506977. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. Effects of size and temperature on developmental time. Science. 2002;293:224–2251. doi: 10.1038/417070a. [DOI] [PubMed] [Google Scholar]
- Gurney W, Nisbet R. Ecological Dynamics. Oxford University Press; 1998. [Google Scholar]
- Gurney W, Nisbet R, Lawton J. The systematic formulation of tractable single-species population models incorporating age structure. Journal of Animal Ecology. 1983;52:479–495. [Google Scholar]
- Harrison S, Karban R, Url S. Folivorous moth on the success of a later-season species, mediated by a change in the quality of the shared host, Lupinus arboreus sims. Oecologia. 1986;69:354–359. doi: 10.1007/BF00377056. [DOI] [PubMed] [Google Scholar]
- Hou Y, Weng Z. Temperature-dependent development and life table parameters of Octodonta nipae (Coleoptera: Chrysomelidae) Environmental Entomology. 2010;39:1676–1684. doi: 10.1603/EN10015. [DOI] [PubMed] [Google Scholar]
- Howe RW. The effect of temperature and humidity on the rate of development and mortality of Tribolium castaneum (herbst) (Coleoptera: Tenebrionidae) Annuals of Applied Biology. 1956;44:356–368. [Google Scholar]
- Huang Z, Ren S, PM Effects of temperature on development, survival, longevity, and fecundity of the Bemisia tabaci gennadius (Homoptera: Aleyrodidae) predator, Axinoscymnus cardilobus (Coleoptera: Coccinellidae) Biological Control. 2008;46:209–215. [Google Scholar]
- Huey R, Berrigan D. Temperature, demography, and ectotherm fitness. American Naturalist. 2001;158:204–210. doi: 10.1086/321314. [DOI] [PubMed] [Google Scholar]
- Jandricic SE, Wraight SP, Bennett KC, Sanderson JP. Developmental times and life table statistics of Aulacorthum solani (Hemiptera: Aphididae) at six constant temperatures, with recommendations on the application of temperature-dependent development models. Environmental Entomology. 2010;39:1631–1642. doi: 10.1603/EN09351. [DOI] [PubMed] [Google Scholar]
- Kingsolver J. Weather and the population dynamics of insects: integrating physiological and population ecology. Physiological Zoology. 1989;62:314–334. [Google Scholar]
- Kingsolver J. The well-temperatured biologist. American Naturalist. 2009;174:755–768. doi: 10.1086/648310. [DOI] [PubMed] [Google Scholar]
- Kingsolver J, Woods A, Buckley LB, Potter L, MacLean H, Higgins J. Complex life cycles and the responses of insects to climate change. Integrative and Comparative Biology. 2011;51:719–732. doi: 10.1093/icb/icr015. [DOI] [PubMed] [Google Scholar]
- Kittelson PM, Maron JL. Outcrossing rate and inbreeding depression in the perennial yellow bush lupine, Lupinus arboreus (Fabaceae) American Journal of Botany. 2000;87:652–660. [PubMed] [Google Scholar]
- Knape J, de Valpine P. Effects of weather and climate on the dynamics of animal population time series. Proceedings of the Royal Society B. 2010;278:985–992. doi: 10.1098/rspb.2010.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B, Rall B, Brose Y, Rall B. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. Journal of Animal Ecology. 2011;81:516–523. doi: 10.1111/j.1365-2656.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- Liu YH, Tsai JH. Effects of temperature on biology and life table parameters of the Asian citrus psyllid, Diaphorina citri kuwayama (Homoptera: Psyllidae) Annals of Applied Biology. 2000;137:201–206. [Google Scholar]
- Matadha D, Hamilton GC, HLJ Effect of temperature on development, fecundity, and life table parameters of Encarsia citrina Craw (Hymenoptera: Aphelinidae), a parasitoid of euonymous scale, Unaspis euonymi (Comstock), and Quadarspidiotus perniciosus (Comstock) (Homoptera: Diaspididae) Environmental Entomology. 2004;33:1185–1191. [Google Scholar]
- McMahon SM, Harrison SP, Armbruster WS, Bartlein PJ, Beale CM, Edwards ME, Kattge J, Midgeley G, Morin X, Prentice IC. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends in Ecology & Evolution. 2011;26:249–259. doi: 10.1016/j.tree.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Medeiros RS, Ramalho FS, Zanuncio JC, Serrao JE. Effect of temperature on life table parameters of Podisus nigrispinus (Heteroptera, Pentatomidae) fed with Alabama argillacea (Lepidoptera, Noctuidae) larvae. Journal of Applied Entomology. 2003;127:209–213. [Google Scholar]
- Morgan D, Walters KFA, Aegerter JN. Effect of temperature and cultivar on the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae) life history. Bulletin of Entomological Research. 2001;91:47–52. [PubMed] [Google Scholar]
- Murdoch AWW, Walde SJ. Analysis of insect population dynamics. In: Grubb PJ, Whittaker JB, editors. Towards a more exact ecology. Blackwell Science; Oxford: 1989. [Google Scholar]
- Murdoch AWW, Nisbet RM, Blythe SP, Gurney WSC, Reeve JD. An invulnerable age class and stability in delay-differential parasitoid-host models. The American Naturalist. 1987;129:263–282. [Google Scholar]
- Murdoch W, Briggs CJ, Nisbet R. Consumer-resource dynamics. Princeton University Press; Princeton New Jersey: 2003. [Google Scholar]
- Nisbet R. Delay-differential equations for structured populations. Chapman and Hall; New York: 1997. [Google Scholar]
- Nisbet RM, Gurney W. The systematic formulation of population-models for insects with dynamically varying instar duration. Theoretical Population Biology. 1983;23:114–135. [Google Scholar]
- Nishikawa H, Shimada T, Nakahira K, RA Thermal effect on the development and reproduction of an indigenous mirid bug, Pilophorus typicus distant (Heteroptera: Miridae), a potential biological control agent in japan. Applied Entomology and Zoology. 2010;45:313–318. [Google Scholar]
- Parajulee M. Influence of constant temperatures on life history parameters of the cotton aphid, Aphis gossypii, infesting cotton. Environmental Entomology. 2006;36:666–672. doi: 10.1603/0046-225x(2007)36[666:ioctol]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Syst. 2006;37:637–669. [Google Scholar]
- Ragland GJ, Kingsolver JG. The effect of fluctuating temperatures on ectotherm life-history traits: comparisons among geographic populations of Wyeomyia smithii. Evolutionary Ecology Research. 2008;10:29–44. [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Savage VM, Gillooly JF, Brown JH, West GB, Charnov EL. Effects of body size and temperature on population growth. American Naturalist. 2004;163:429–441. doi: 10.1086/381872. [DOI] [PubMed] [Google Scholar]
- Sheldon KS, Yang S, Tewksbury JJ. Climate change and community disassembly: impacts of warming on tropical and temperate montane community structure. Integrative and Comparative Biology. 2013;14:1191–1200. doi: 10.1111/j.1461-0248.2011.01689.x. [DOI] [PubMed] [Google Scholar]
- Soliman MH, Lints FA. Longevity, growth rate and related traits among strains of Tribolium castaneum. Gerontologia. 1975;21:102–116. doi: 10.1159/000212037. [DOI] [PubMed] [Google Scholar]
- Strong ADR, Maron JL, Connors PG, Whipple A, Harrison S, Jefferies RL, Url S. High mortality, fluctuation in numbers, and heavy subterranean insect herbivory in bush lupine, Lupinus arboreus. Oecologia. 1995;104:85–92. doi: 10.1007/BF00365566. [DOI] [PubMed] [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch C. Climate warming puts the heat on tropical ectotherms. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- Ulmer BJ, Jacas JA, Pena JE, Duncan RE, Castillo J. Effect of temperature on life history of Aprostocetus vaquitarum (hymenoptera: Eulophidae), an egg parasitoid of Diaprepes abbreviatus (coleoptera: Curculionidae) Biological Control. 2006;39:19–25. [Google Scholar]
- Urbaneja A, Llacer E, Garrido A, Jacas JA. Effect of temperature on the life history of Cirrospilus sp. near lyncus (Hymenoptera: Eulophidae), a parasitoid of Phyllocnistis citrella (Lepidoptera: Gracillariidae) Applied Entomology and Zoology. 2001;45:313–318. doi: 10.1603/0022-0493-95.2.250. [DOI] [PubMed] [Google Scholar]
- Urbaneja A, Llacer E, Tomas O, Garrido A, Jacas JA. Effect of temperature on development and survivorship of Cirrospilus sp. near lyncus (hymenoptera: Eulophidae), a parasitoid of Phyllocnistis citrella (Lepidoptera: Gracillariidae) Environmental Entomology. 1999;28:339–344. [Google Scholar]
- Van der Have T. A proximate model for thermal tolerance in ectotherms. Oikos. 2002;98:141–155. [Google Scholar]
- Van der Have TM, de Jong G. Adult size in ectotherms: temperature effects on growth and differentiation. Journal of Theoretical Biology. 1996;183:329–340. [Google Scholar]
- Walther GR, Post E, Convery P, Menzel A, Parmesan C, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Zamani A, Talebi A, Fathipour Y, Baniameri V. Effect of temperature on biology and population growth parameters of Aphis gossypii Glover (Homoptera, Aphididae) on greenhouse cucumber. Journal of Applied Entomology. 2006a;130:453–460. [Google Scholar]
- Zamani A, Talebi A, Fathipour Y, Baniameri V. Temperature-dependent functional response of two aphid parasitoids, Aphidius colemani and Aphidius matricariae (Hymenoptera: Aphidiidae), on the cotton aphid. Journal of Pest Science. 2006b;79:183–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Density-dependence of life history traits. Fecundity declines with adult density, likely due to intraspecific competition with data providing a significant fit (p < 0.001) to the equation f(A) = be−αA where f(A) gives the fecundity at adult density A, b is the per capita reproductive rate, and α is the strength of intra-specific competition. Density does not have a statistical effect on development (panels b) or mortality (panels c); see Table S2 for statistics.
Figure S2. Plant bug population dynamics predicted by stage-structured ODE models (see Appendix S4). Left panels show model predictions for the 1986 census period and right panels show model predictions for the 2011 census period: panels (a,b): stage-structured ODE model with seasonal temperature and resource variation, panels (c,d): census data.
Table S1. Parameter estimates (nonlinear regression; see main text) for temperature responses.
Table S2. Effects of density on development and mortality, which were quantified using a linear (k(D) = k+αD) and nonlinear (k(D) = keαD) function where k(D) gives the density-dependence of trait k (i.e., k = m, d); D is the density of the life stage at which competition arises; k is the trait value in the absence of competition; and α is the per capita competitive effect. We determine whether density has a statistical effect on development or mortality by whether the per capita competitive effect (α) provides a significant fit to the data.
Table S3. Parameter estimates for the temperature (Eq. 7) and resource availability (Eq. 8) functions. All parameters were estimated via nonlinear regression (see main text for details).
Appendix S1: Census protocols
Appendix S2: Experiment methods
Appendix S3: Effects of Density on Life History Traits
Appendix S4: DDE Model Description
Appendix S5: Non-Delay ODE Model