Abstract
Findings of increased hemoglobin inside the HDL proteome among persons with diabetes who have haptoglobin 2-2 genotype suggests that iron-induced lipid peroxidation may be involved in diabetic kidney disease. We investigated the relationships of serum hemoglobin and iron with kidney function, and whether this varied by level of HDLc, in 5,296 adults with and 49,161 without diabetes. Estimated eGFR was our marker of kidney function. Hemoglobin was positively associated with eGFR among those with diabetes and inversely among those without diabetes (interaction p-value <0.0001). Iron was inversely associated with eGFR regardless of diabetes status. When stratified by median HDLc and median hemoglobin, among persons with diabetes mean eGFR was highest in those with high hemoglobin and low HDLc and lowest in those with both low hemoglobin and low HDLc. This divergent relationship was not observed in the non-diabetic population. In contrast to hemoglobin, high iron and low HDLc was associated with a lower mean eGFR regardless of diabetes status. Our data suggests that among persons with diabetes, both hemoglobin and iron are harmful to kidney function at high levels. Our data also suggests that HDLc may play a role in the relationship of high hemoglobin in kidney function in diabetes.
Diabetes is the primary cause of end-stage kidney disease in developed nations. Microvascular disease is common among persons with type 2 diabetes and typically develops with few symptoms until irreversible damage is done1. Chronic kidney disease (CKD) has been associated with elevated triglycerides and diminished high density lipoprotein cholesterol (HDLc). Reduced HDLc, which is characteristic of type 2 diabetic dyslipidemia, is a risk factor for macrovascular complications. However, the association of HDLc with renal disease is controversial.1
Increased hemoglobin levels have been linked to adverse CKD related outcomes, including; cardiovascular complications, poor quality of life, and prolonged rehabilitation.2 Many of these complications have been attributed to decreased oxygen delivery, or hypoxia.2 Studies have indicated that hypoxia mediates the progression of diabetic nephropathy. Although the kidneys make up less than 1% of the body, they consume 10% of the body’s total oxygen. Diabetes induces hyperfiltration of the kidneys leading to an excess oxygen delivery and consumption, and ultimately, a greater need for oxygen.3 Without proper oxygen delivery, kidney damage is likely to occur.
A potentially harmful effect of hemoglobin is excess iron. Iron and iron containing molecules, such as hemoglobin, have been reported to cause kidney injury to cells in vitro and in vivo.4 Individuals with CKD have been reported as having higher kidney and urinary iron content. Furthermore, CKD severity has been shown to diminish with treatment for excess iron.4
The haptoglobin 2-2 genotype among those with diabetes has been shown to increase risk of CKD by two to five-fold.5 Findings of increased hemoglobin inside the HDL proteome among persons with diabetes who have the haptoglobin 2-2 genotype suggests that iron-induced lipid peroxidation may be involved in diabetic kidney disease.5 In the following study, hemoglobin, iron, and HDLc and the interaction between them was evaluated in relation to estimated glomerular filtration rate in a large population of adults with diabetes.
Methods
Data from the C8 Health Project were used for this study. The C8 Health Project was a community-based study that investigated the effects of exposure to perfluorooctanioic acid (PFOA/C8) contaminated drinking water.6 Data were collected from August 2005 to August 2006 on 69,030 individuals who had a history of living, working, or going to school in areas of Ohio and West Virginia with PFOA contaminated water from DuPont’s Washington Works facility near Parkersburg, West Virginia.6,7 All participants completed a comprehensive health survey. Standard consent and release forms were used by the phlebotomy laboratory and an additional consent form was used to obtain medical records.6 Institutional review board (IRB) approval to use this data set was granted by the West Virginia University IRB.
Data collected included that on demographic and lifestyle factors, medical conditions, clinical blood profiles, and a wide range of serum concentrations. Clinical laboratory measurements were performed at an accredited clinical diagnostic laboratory (LabCorp Inc, Burlington, North Carolina).8 The sample for this study was restricted to C8 Health Project participants at least 20 years of age (n=54,457) and who self-reported a physician diagnosed diabetes (n=5296). EGFR was calculated using the modification of diet in renal disease (MDRD) formula.9 For the purposes of our study, individuals with an eGFR of less than 60 mL/min/1.73 m2 were defined as having CKD.
The student’s t-test was used to test for differences among continuous variables and a chi-squared test was used to test for differences among categorical variables. Linear regression analysis was conducted using general linear models to determine the multivariable association between concentrations of HDLc, hemoglobin, and iron with eGFR. Covariates controlled for included age, sex, and diabetes duration. Effect modification was examined with the addition of cross products for hemoglobin and iron, hemoglobin and HDLc, and iron and HDLc. The criterion for statistical significance was a p-value of <0.10 for multiplicative interaction and <0.05 otherwise. All statistical analyses were done using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the study population are presented in Table 1. The population was 49% male and 96% white. The mean age of the population was 58 years with an average diabetes duration of 10 years. The majority of the study population had a high school diploma or less and reported an average family income of $30,000 or less per year. Mean HDLc was 46 mg/dL, iron 79 ug/dL and hemoglobin 14 g/dL. CKD was present in 26% of the population.
Table 1.
Characteristics of Study Population by Diabetic Status Mean (Std), n (%)
| Total | With Diabetes | Without Diabetes | P-value | |
|---|---|---|---|---|
| 54457 | 5296 (90%) | 49161 (10%) | ||
| Sex (Male) | 25845 (47%) | 2620 (49%) | 23225 (47%) | 0.002 |
| Age, years | 46.31 ± 15.73 | 57.83 ± 13.54 | 45.07 ± 15.45 | <.0001 |
| Diabetes Duration | 9.97 ± 10.57 | 9.97 ± 10.57 | NA | NA |
| Ethnicity (White) | 52652 (97%) | 2620 (96%) | 47596 (97%) | <.0001 |
| Current Smoker | 14299 (26%) | 980 (19%) | 13319 (27%) | <.0001 |
| Current Alcohol Consumer* | 26679 (49%) | 1510 (29%) | 25169 (51%) | <.0001 |
| Cholesterol Medication† | 10730 (10%) | 2979 (56%) | 7751 (16%) | <.0001 |
| Education | ||||
| Less Than HS | 6186 (11%) | 962 (18%) | 5224 (11%) | <.0001 |
| HS Diploma or GED | 22799 (42%) | 2345 (44%) | 20454 (42%) | |
| Some College | 17973 (33%) | 1502 (28%) | 16471 (34%) | |
| Bachelor’s Degree or Higher | 7237 (13%) | 446 (8%) | 6791 (14%) | |
| Average Household Income‡ | ||||
| $10,000 or less | 5025 (9%) | 583 (11%) | 4442 (9%) | <.0001 |
| $10,001 – $30,000 | 15711 (29%) | 1822 (34%) | 13889 (28%) | |
| $30,001 – $60,000 | 17393 (32%) | 1636 (31%) | 15757 (32%) | |
| $60,001 and above | 10906 (20%) | 729 (14%) | 10177 (21%) | |
| BMI | 28.81 ± 7.64 | 33.17 ± 9.18 | 28.34 ± 7.30 | <.0001 |
| Creatinine mg/dL | 0.95 ± 0.28 | 1.02 ± 0.42 | 0.94 ± 0.26 | <.0001 |
| Cholesterol | ||||
| Total mg/dL | 198.54 ± 42.53 | 190.52 ± 48.90 | 199.41 ± 41.69 | <.0001 |
| HDL mg/dL | 49.70 ± 14.42 | 45.65 ± 12.19 | 50.13 ± 14.57 | <.0001 |
| LDL mg/dL | 112.69 ± 35.18 | 98.57 ± 36.51 | 114.10 ± 34.73 | <.0001 |
| Triglycerides mg/dL | 192.04 ± 156.11 | 250.91 ± 217.05 | 185.69 ± 146.64 | <.0001 |
| Estimated Glomerular Filtration (eGFR) | ||||
| Less than 60 mL/min/1.73 m2 | 6550 (12%) | 1372 (26%) | 5178 (11%) | <.0001 |
| 60 – 89 mL/min/1.73 m2 | 35218 (65%) | 2990 (57%) | 32228 (66%) | |
| 90 – 110 mL/min/1.73 m2 | 6541 (12) | 395 (8%) | 6146 (13%) | |
| 110 mL/min/1.73 m2 and above | 5685 (11%) | 500 (10%) | 5185 (11%) | |
| Hemoglobin g/dL | 14.52 ± 1.44 | 14.08 ± 1.54 | 14.57 ± 1.42 | <.0001 |
| Iron ug/dL | 86.62 ± 34.46 | 78.53 ± 29.88 | 87.50 ± 34.70 | <.0001 |
| C8 ng-mL | 86.75 ± 262.32 | 89.59 ± 369.64 | 86.45 ± 247.98 | <.0001 |
| White Blood Cell Count | 7.36 ± 2.32 | 7.58 ± 3.08 | 7.34 ± 2.22 | <.0001 |
| x10e3-uL Potassium mmol-L | 4.16 ± 0.35 | 4.25 ± 0.40 | 4.15 ±0.34 | <.0001 |
0.15% provided no response
0.36% provided no response
missing data on 13% for annual household income
The main effects multivariable adjusted associations of HDLc, serum hemoglobin, and serum iron with eGFR among those with diabetes are presented in Table 2. With increasing hemoglobin, a significant increase can be seen in age and sex adjusted eGFR. Model 2 shows that with increasing iron, age and sex adjusted eGFR increased as well. No significant relationship is seen between HDLc and eGFR (model 3). Additionally controlling for HDLc (model 4) or iron (model 5) did not affect the hemoglobin relationship with eGFR. However, model 5 shows that after controlling for hemoglobin, the relationship of iron with eGFR went from a positive association to an inverse association. A marginal effect modification between HDLc and iron was observed (p=0.08), but this disappeared after further controlling for diabetes duration.
Table 2.
Multivariate analysis of hemoglobin, iron, and HDLc with eGFR in persons with diabetes
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| β ± SE | β ± SE | β ± SE | β ± SE | β ± SE | β ± SE | |
| Age, years | −0.73 ± 0.02d | −0.80 ±0.02d | −0.80 ± 0.02d | −0.73 ± 0.02d | −0.73 ±0.02d | −0.71 ± 0.02d |
| Sex, male | 0.89 ± 0.55 | 3.52 ± 0.52d | 3.83 ± 0.53d | 1.12 ±0.57 | 0.95 ±0.55 | 0.94 ± 0.61d |
| Diabetes duration, years | −0.10 ± 0.03d | |||||
| Hemoglobin, g/dL | 2.20 ±0.18d | 2.22 ± 0.18d | 2.37 ±0.19d | 2.40 ± 0.21 | ||
| Iron, ug/dL | 0.02 ± 0.01a | −0.02 ±0.01a | −0.03 ± 0.01b | |||
| HDLc, mg/dL | 0.01 ± 0.02 | 0.03 ±0.02 | 0.03 ± 0.02 |
<0.05
<0.01
<0.001
<0.0001
To determine whether the observed relationship between hemoglobin and eGFR, and the modifying effect of HDLc, was specific to those with diabetes, we also tested these relationships in the non-diabetic population (Table 3). A significant effect modification by diabetes status was observed in the relationship between hemoglobin with eGFR (p-interaction term <0.0001). In contrast to the positive association of hemoglobin with eGFR in the diabetic population, among those without diabetes higher hemoglobin levels were associated with lower eGFR levels. This modifying effect of diabetes on the relationship of hemoglobin with eGFR is also depicted in Figure 1, where the modifying effect on the relationship between hemoglobin and HDLc can also be observed. Figure 1 shows that among both those with and without diabetes, mean eGFR was lower in those with both high HDLc levels and high hemoglobin levels (defined as values above the median) compared to those with high hemoglobin and low HDLc (values at or below the median). In contrast to those with diabetes, among those without diabetes where those with both low hemoglobin and low HDLc had the lowest mean eGFR, in the non-diabetic population those with low hemoglobin and low HDLc had the highest mean eGFR. In a full model combining both the diabetic and nondiabetic population and including interaction terms for diabetes-by–hemoglobin, diabetes-by-HDLc, and hemoglobin-by –HDLc, all interaction terms were significant.
Table 3.
Multivariate analysis of hemoglobin, iron, and HDLc with eGFR in persons without diabetes
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| β ± SE | β ± SE | β ± SE | β ± SE | β ± SE | β ± SE | |
| Age, years | −0.58 ± 0.005d | −0.57 ± 0.004d | −0.57 ± 0.004d | −0.58 ± 0.005d | −0.58 ± 0.005d | −0.58 ± 0.005d |
| Sex, male | 3.55 ± 0.18d | 1.86 ± 0.14d | 1.88 ± 0.15d | 3.70 ± 0.19d | 3.35 ± 0.18d | 3.71 ± 0.19d |
| Hemoglobin, g/dL | −1.05 ± 0.06d | −1.04 ± 0.06d | −1.01 ± 0.07d | −0.99 ± 0.07d | ||
| Iron, ug/dL | 0.01 ± 0.002d | −0.004 ± 0.002a | −0.006 ± 0.002b | |||
| HDLc, mg/dL | 0.02 ± 0.01c | 0.01 ± 0.01b | 0.02 ± 0.005c |
<0.05
<0.01
<0.001
<0.0001
Figure 1.
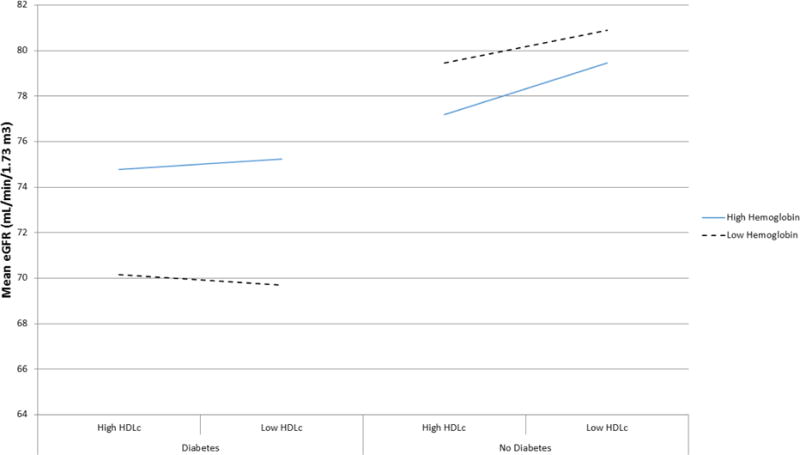
Mean eGFR by High and Low Serum Hemoglobin and HDLc Level in those with and without Diabetes.
Median HDLc = 44 mg/dL in diabetic population and 48 mg/dL in non-diabetic population. Low HDLc: HDLc ≤ population specific median. High HDLc: HDLc > population median. In full model adjusting for age, sex, iron, hemoglobin, HDLc, diabetes status, and including interaction terms for diabetes*hemoglobin, diabetes*HDLc, and hemoglobin*HDLc, p-values for interaction terms were: diabetes*hemoglobin p<0.0001; diabetes*HDLc p=0.03; hemoglobin*HDLc p=0.0006.
In contrast with the hemoglobin-HDLc relaltionship with eGFR, Figure 2 shows that the effect modification of HDLc on iron’s relationship with eGFR did not vary by diabetes status. In both those with and without diabetes, those with both low HDLc and lower iron levels had the lowest mean eGFR. However, those with high HDLc and high iron levels had similar mean eGFRs.
Figure 2.
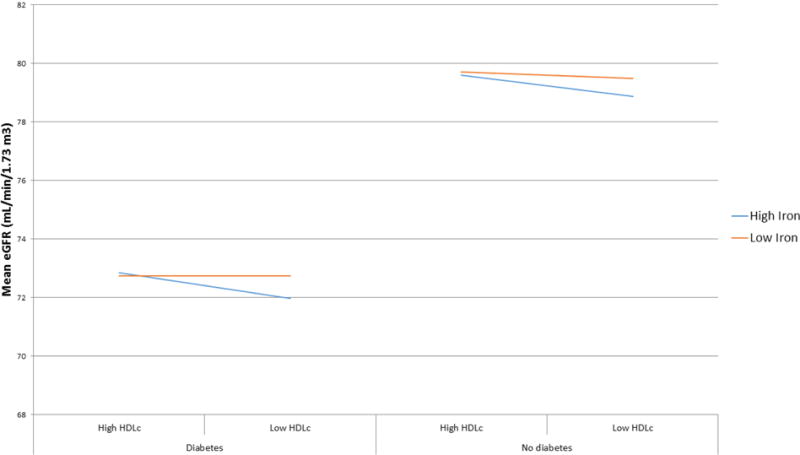
Mean eGFR by High and Low Serum Iron and HDLc Level in those with and without Diabetes
Median HDLc = 44 mg/dL in diabetic population and 48 mg/dL in non-diabetic population. Low HDLc: HDLc ≤ population specific median. High HDLc: HDLc > population median. In full model adjusting for age, sex, iron, hemoglobin, HDLc, diabetes status, and including interaction terms for diabetes*iron, diabetes*HDLc, and iron*HDLc, p-values for interaction terms were: diabetes*iron p<0.0001; diabetes*HDLc p=0.07; iron*HDLc p=0.02.
Conclusion
In this large population based study of over 5,000 adults with diabetes, we investigated the modifying effect of HDLc on the relationship of iron and hemoglobin with kidney function. The major finding of this study was that while hemoglobin was positively associated with eGFR and iron was inversely associated with eGFR, in the presence of high HDLc levels, high hemoglobin was also inversely associated with kidney function. Our data builds on the growing evidence of a role of HDLc in iron induced kidney damage.
The hypothesis that HDLc modifies the relationship of iron and hemoglobin with kidney damage stems from findings of increased hemoglobin inside the HDL proteome of diabetic individuals with the haptoglobin 2-2 genotype10 and that this genotype in persons with diabetes increases the risk of kidney disease.11–14 It is thought that iron induced peroxidation, resulting from the hemoglobin inside the HDL proteome, may be involved in diabetic kidney disease.5 Inside the HDL proteome, heme can become destabilized from its hemoglobin pocket. The heme derived iron can catalyze oxidation reactions leading to extensive oxidation of the HDL proteins and lipids, resulting in lipid peroxidation and advanced lipoxidation/glycoxidation products. Among our diabetic population, we found that for those with both high hemoglobin and high HDLc, mean eGFR was lower compared to those with high hemoglobin and low HDLc. This was also observed in our non-diabetic population, but only among those with diabetes was a suggestive protective relationship observed for hemoglobin which high HDLc appeared to impair (in the non-diabetic population higher hemoglobin levels were associated with a lower eGFR). Our finding lends support to this hypothesis of iron induced lipid peroxidative damage to the kidneys.
The primary purpose of hemoglobin is to transport oxygen to tissues. Poor renal tissue oxygenation is thought to mediate the progression of chronic kidney disease.15–18 However, erythropoietin is the hormone that stimulates the production of red blood cells and most of the erythropoietin produced in the body is produced in peritubular fibroblasts of the kidney, thus renal damage itself causes decreases in circulating hemoglobin levels.19 In results not stratified by HDLc level, we found that hemoglobin levels were positively associated with kidney function in our diabetic population. Our results are consistent with others who have also shown positive relationships between hemoglobin in kidney function among persons with diabetes. In a study of 174 individuals with childhood-onset Type 1 diabetes and overt nephropathy followed for 12 years, Conway et al. found hemoglobin to inversely predict end stage renal disease (ESRD) and mortality.20 In another study Thomas et al. followed 503 individuals with type 2 diabetes for five years to identify risk factors for decreases in hemoglobin levels in diabetic patients. Decreases in hemoglobin were most significant among patients with renal impairment. Patients with an eGFR above 90 mL/min/1.73 m2 had stable hemoglobin levels.21 In 130 Korean patients with diabetic nephropathy, anemia was associated with a rapid decline in renal dysfunction and faster initiation of dialysis.22 Mohanram et al investigated the impact of anemia on progression to ESRD and found that even mild anemia increased risk of ESRD and that hemoglobin was inversely related to progression to ESRD.23
While some of the body’s circulating iron is in the form bound to hemoglobin, the majority of the body’s circulating iron is bound to transferrin. Increased iron in renal tissues has been found in persons with acute kidney injury,24 chronic kidney disease25,26 and proteinuria25–27 and is associated with renal disease progression.27 This metal has been shown to be directly injurious to renal tubular cells.28–30 We found higher levels of iron to be associated with lower kidney function, building on the growing evidence of the role of iron in the incidence and progression of acute kidney injury and chronic kidney disease.
Our study showed no real main effects relationship between HDLc and kidney function. In other studies, HDLc has been shown to have a positive, an inverse, and no relationship with kidney function. In persons with overt diabetic nephropathy, hypoproteinemia increased LDLc. Renal failure specifically increased remnant lipoproteins and decreased LDLc and HDLc.31 A protective association against the progression of diabetic nephropathy with higher mean HDLc was observed in 864 patients with Type 2 diabetes participating in a comprehensive diabetic care program.32 In adult patients with Type 1 diabetes, a significantly lower multivariable adjusted likelihood of having albuminuria was associated with higher HDLc levels.33 A study by Bentley et al. investigating the association between HDLc and eGFR found significant differences by race/ethnicity. Although HDLc was positively associated with kidney function in non-diabetic Han Chinese, it was negatively associated with kidney function among African Americans.34 Krikken et al. also observed a negative association between HDLc and eGFR during animal experiments testing relationships of HDLc and apo-I with kidney function in subjects with severe chronic kidney disease.35 While our data is largely consistent with the literature in showing generally increased eGFR with increasing HDLc levels, this relationship was not significant in our diabetic population but appeared to vary by level of hemoglobin. The weakness and heterogeneity of the association of HDLc with eGFR in our population is also consistent with HDL cholesterol raising trials in persons with Type 2 diabetes. These trials have suggested that raising HDL cholesterol in the subgroup with kidney disease may not be beneficial for kidney function36–38 or mortality.39
There were several limitations to this study that should be noted. First, diabetes was self-reported. Another limitation of our study is the lack of information on iron supplementation. Were unable to assess the potential influence of iron supplementation on the relation of hemoglobin concentration and eGFR. An additional limitation was a lack of racial diversity in the study population. With 96% of the study population being white, possible racial differences couldn’t be accounted for. It is possible that stronger relationships than observed in our population may be evident in African Americans due to the generally lower hemoglobin levels and higher CKD risk in that population. Finally, ours was a cross-sectional study. Although the association between hemoglobin and eGFR is likely due to decreased synthesis of erythropoietin in persons with advanced CKD, it might also, at least in part, be due to a protective effect of hemoglobin against hypoxia. Due to the cross sectional nature of the data, we were unable to determine if hemoglobin was responsible for changing eGFR or vice versa. This is difficult to determine in prospective studies as well.
In conclusion, our data suggests that in persons with diabetes high hemoglobin in the context of high HDL cholesterol is harmful to the kidney. Our data also suggests that after controlling for hemoglobin, iron is also harmful to kidney function at high levels. The jury is still out on how HDLc itself affects kidney health, but our data supports the growing evidence of HDLc in a role of iron induced damage to kidney tissues in diabetes. Further studies are needed in this area.
Acknowledgments
A. N. Williams wrote the manuscript and analyzed the data. B.N.C. conceived the study, directed the analyses, analyzed the data, and wrote the manuscript. B.N.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported in part by the National Institutes of Health grant U54GM1049 to the West Virginia University CTSI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
This work was presented in part at the 75th Annual American Diabetes Associations Scientific Sessions, June 2015.
References
- 1.Morton J, Zoungas S, Li Q, et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes care. 2012;35:2201–6. doi: 10.2337/dc12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan JA, Levin A. Sex, haemoglobin and kidney disease: new perspectives. European journal of clinical investigation. 2005;35(Suppl 3):52–7. doi: 10.1111/j.1365-2362.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 3.Takiyama Y, Haneda M. Hypoxia in diabetic kidneys. BioMed research international. 2014;2014:837421. doi: 10.1155/2014/837421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martines AM, Masereeuw R, Tjalsma H, Hoenderop JG, Wetzels JF, Swinkels DW. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nature reviews Nephrology. 2013;9:385–98. doi: 10.1038/nrneph.2013.98. [DOI] [PubMed] [Google Scholar]
- 5.Asleh R, Blum S, Kalet-Litman S, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes. 2008;57:2794–800. doi: 10.2337/db08-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisbee SJ, Brooks AP, Jr, Maher A, et al. The C8 health project: design, methods, and participants. Environmental health perspectives. 2009;117:1873–82. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan H, Ducatman A, Zhang J. Perfluorocarbons and Gilbert syndrome (phenotype) in the C8 Health Study Population. Environmental research. 2014;135:70–5. doi: 10.1016/j.envres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Frisbee SJ, Shankar A, Knox SS, et al. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Archives of pediatrics & adolescent medicine. 2010;164:860–9. doi: 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estimating Glomerular Filtration Rate (GFR) (Accessed Apeil 18, 2016 at http://www.niddk.nih.gov/health-information/health-communication-programs/nkdep/lab-evaluation/gfr/estimating/pages/estimating.aspx.)
- 10.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circulation research. 2005;96:435–41. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 11.Costacou T, Ferrell RE, Ellis D, Orchard TJ. Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes. 2009;58:2904–9. doi: 10.2337/db09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orchard TJ, Sun W, Cleary PA, et al. Haptoglobin genotype and the rate of renal function decline in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes. 2013;62:3218–23. doi: 10.2337/db13-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Lee CC, Huang CY, et al. Haptoglobin polymorphism as a risk factor for chronic kidney disease: a case-control study. American journal of nephrology. 2011;33:510–4. doi: 10.1159/000327822. [DOI] [PubMed] [Google Scholar]
- 14.Conway BR, Savage DA, Brady HR, Maxwell AP. Association between haptoglobin gene variants and diabetic nephropathy: haptoglobin polymorphism in nephropathy susceptibility. Nephron Experimental nephrology. 2007;105:e75–9. doi: 10.1159/000098563. [DOI] [PubMed] [Google Scholar]
- 15.Palm F, Nordquist L. Renal Tubulointerstitial Hypoxia: Cause and Consequence of Kidney Dysfunction. Clin Exp Pharmacol Physiol. 2011;38:424–30. doi: 10.1111/j.1440-1681.2011.05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine LG, Orphanides C, Norman JT. Progressive renal disease: The chronic hypoxia hypothesis. Kidney International. 1998;53:S74–S8. [PubMed] [Google Scholar]
- 17.Eckardt K-U, Bernhardt WM, Weidemann A, et al. Role of hypoxia in the pathogenesis of renal disease. Kidney International. 2005;68:S46–S51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- 18.Norman JT, Fine LG. Intrarenal Oxygenation in Chronic Renal Failure. Clin Exp Pharmacol Physiol. 2006;33:989–96. doi: 10.1111/j.1440-1681.2006.04476.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas S, Rampersad M. Anaemia in diabetes. Acta diabetologica. 2004;41(Suppl 1):S13–7. doi: 10.1007/s00592-004-0132-4. [DOI] [PubMed] [Google Scholar]
- 20.Conway B, Fried L, Orchard T. Hemoglobin and overt nephropathy complications in type 1 diabetes. Annals of epidemiology. 2008;18:147–55. doi: 10.1016/j.annepidem.2007.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas MC, Tsalamandris C, MacIsaac RJ, Jerums G. The epidemiology of hemoglobin levels in patients with type 2 diabetes. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;48:537–45. doi: 10.1053/j.ajkd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Lee KA, Jin HY, Baek HS, Park TS. The Relationship between Anemia and the Initiation of Dialysis in Patients with Type 2 Diabetic Nephropathy. Diabetes & metabolism journal. 2015;39:240–6. doi: 10.4093/dmj.2015.39.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2004;66:1131–8. doi: 10.1111/j.1523-1755.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 24.Moreno JA, Martin-Cleary C, Gutierrez E, et al. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clinical journal of the American Society of Nephrology: CJASN. 2012;7:175–84. doi: 10.2215/CJN.01970211. [DOI] [PubMed] [Google Scholar]
- 25.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. Journal of the American Society of Nephrology: JASN. 2007;18:16–28. doi: 10.1681/ASN.2006050500. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Nishiya K, Ito H, Hosokawa T, Hashimoto K, Moriki T. Iron deposition in renal biopsy specimens from patients with kidney diseases. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2001;38:1038–44. doi: 10.1053/ajkd.2001.28593. [DOI] [PubMed] [Google Scholar]
- 27.Harris DC, Tay C, Nankivell BJ. Lysosomal iron accumulation and tubular damage in rat puromycin nephrosis and ageing. Clinical and experimental pharmacology & physiology. 1994;21:73–81. doi: 10.1111/j.1440-1681.1994.tb02472.x. [DOI] [PubMed] [Google Scholar]
- 28.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood. 2010;116:6054–62. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–43. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 30.Sponsel HT, Alfrey AC, Hammond WS, Durr JA, Ray C, Anderson RJ. Effect of iron on renal tubular epithelial cells. Kidney Int. 1996;50:436–44. doi: 10.1038/ki.1996.334. [DOI] [PubMed] [Google Scholar]
- 31.Hirano T. Abnormal lipoprotein metabolism in diabetic nephropathy. Clinical and experimental nephrology. 2014;18:206–9. doi: 10.1007/s10157-013-0880-y. [DOI] [PubMed] [Google Scholar]
- 32.Chang YH, Chang DM, Lin KC, Hsieh CH, Lee YJ. High-density lipoprotein cholesterol and the risk of nephropathy in type 2 diabetic patients. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2013;23:751–7. doi: 10.1016/j.numecd.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Molitch ME, Rupp D, Carnethon M. Higher levels of HDL cholesterol are associated with a decreased likelihood of albuminuria in patients with long-standing type 1 diabetes. Diabetes Care. 2006;29:78–82. doi: 10.2337/diacare.29.01.06.dc05-1583. [DOI] [PubMed] [Google Scholar]
- 34.Bentley AR, Doumatey AP, Chen G, et al. Variation in APOL1 Contributes to Ancestry-Level Differences in HDLc-Kidney Function Association. International journal of nephrology. 2012;2012:748984. doi: 10.1155/2012/748984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krikken JA, Gansevoort RT, Dullaart RP. Lower HDL-C and apolipoprotein A-I are related to higher glomerular filtration rate in subjects without kidney disease. J Lipid Res. 2010;51:1982–90. doi: 10.1194/jlr.M005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen S, Schmitz O, Moller N, et al. Renal function and insulin sensitivity during simvastatin treatment in type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1993;36:1079–86. doi: 10.1007/BF02374502. [DOI] [PubMed] [Google Scholar]
- 37.Atthobari J, Brantsma AH, Gansevoort RT, et al. The effect of statins on urinary albumin excretion and glomerular filtration rate: results from both a randomized clinical trial and an observational cohort study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:3106–14. doi: 10.1093/ndt/gfl244. [DOI] [PubMed] [Google Scholar]
- 38.Ruggenenti P, Perna A, Tonelli M, et al. Effects of add-on fluvastatin therapy in patients with chronic proteinuric nephropathy on dual renin-angiotensin system blockade: the ESPLANADE trial. Clinical journal of the American Society of Nephrology: CJASN. 2010;5:1928–38. doi: 10.2215/CJN.03380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. The New England journal of medicine. 2005;353:238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 40.Morris A, Ferdinand KC. Hyperlipidemia in Racial/Ethnic Minorities: Differences in Lipid Profiles and the Impact of Statin Therapy. Clin Lipidology. 2009;4:741–54. [Google Scholar]


