Abstract
Hannen et al. report that cutaneous glucocorticoidogenesis and expression of glucocorticoid receptors are inhibited in psoriatic skin. These findings substantiate the previous concept that deficient feedback of local proopiomelanocortin and glucocorticoids on cutaneous immunity contributes to inflammatory and autoimmune dermatoses. Restoration of efficient endogenous glucocorticoid signaling represents a realistic goal in treating psoriasis.
Psoriasis and cutaneous corticosteroidogenesis
The investigative team led by Dr Hannen (Hannen et al., 2017) reports that synthesis of glucocorticoids (GC) and expression of nuclear GC receptors (GR) are inhibited in lesional and perilesional skin of patients with psoriasis. In a series of elegant experiments, they also provide proof that GC produced locally by epidermal keratinocytes protect the skin against inflammatory insults. Specifically, using GR epidermal knockout mice with adrenalectomy, they were able to demonstrate local glucocorticosteroidogenesis and enhanced levels of epidermal GC, which, in turn, protected skin against induced inflammation. These findings are not only consistent with the therapeutic role of GC in psoriasis and other inflammatory disorders, but also support the concept that defective cutaneous GC signaling may underlie the pathogenesis of inflammatory dermatoses in general (Figure 1).
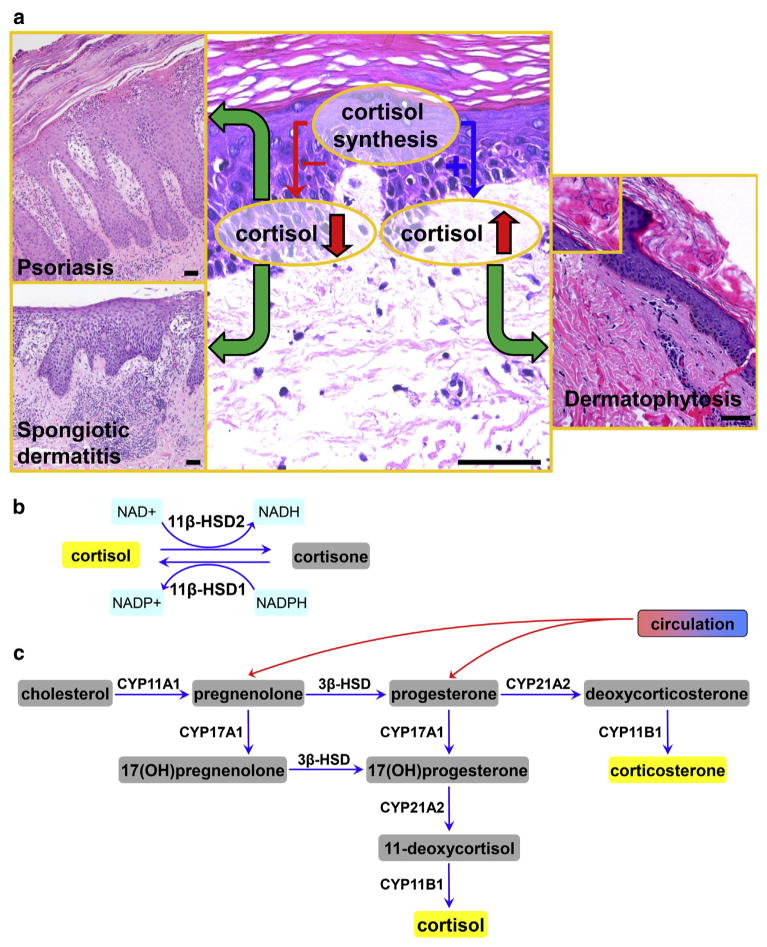
Figure 1. Defective local glucosteroidogenesis and skin pathology.
(a) Decreased local synthesis of cortisol or corticosterone can promote the development of psoriasis, spongiotic dermatitis (allergic contact dermatitis, nummular dermatitis, id reaction, atopic dermatitis, etc.), and other inflammatory skin diseases or autoimmune processes. Increased epidermal cortisol or corticosterone accumulation would inhibit antimicrobial or antiviral defenses. Scale bars = 50 μm. (b) Interconversion between hormonally active cortisol and inactive cortisone in skin cells. Isoenzyme 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) activates cortisol from hormonally inactive cortisone and 11β-HSD2 inactivates hormonally active cortisol to cortisone. (c) Glucocorticosteroidogenic pathway in the skin. NAD, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate.
Skin as a steroidogenic organ
Overview
Almost 20 years have passed since molecular evidence was presented for cutaneous corticosteroidogenesis (Slominski et al., 1996), a metabolic pathway that is regulated by local hormonal signaling induced by various stressors (Slominski et al., 2013). Local levels of GC are regulated by delivery from the adrenal cortex, activation of the 11-keto-precursor or oxidative inactivation by 11β-hydroxysteroid dehydrogenase type 1 and 2, and local synthesis either from cholesterol initiated by CYP11A1, or from GC precursors of systemic origin that are further hydroxylated by locally expressed CYP17A1, CYP21A2, and CYP11B1. All are expressed in skin (reviewed in Slominski et al., 2015b) (Figure 1).
Cutaneous CYP11A1
We have revised the traditional role of CYP11A1 (Slominski et al., 2015a), the mitochondrial enzyme catalyzing the rate-limiting step in steroidogenesis during which the side chain of cholesterol is cleaved to produce pregnenolone (Miller and Auchus, 2011), the precursor of all steroid hormones. It is now apparent that in addition to adrenal glands, gonads, and placenta (Miller and Auchus, 2011; Tuckey, 2005), CYP11A1 is also expressed in all of the major cellular elements that reside in skin, including keratinocytes, melanocytes, fibroblasts, and adipocytes. The locally produced pregnenolone undergoes further transformation to GC in all skin compartments, including the epidermis, dermis, hypodermis, and adnexal structures (Slominski et al., 2004, 2015a, 2015b). Interestingly, CYP11A1 protein expression was reduced in psoriatic skin and increased in the skin of adrenalectomized GR epidermal knockout mice (Hannen et al., 2017). These considerations indicate that CYP11A1 activity may play an important role in preventing or attenuating the presentation of psoriasis or other inflammatory skin disorders. This hypothesis is supported indirectly by the upregulation of CYP11A1 expression in the skin by UVB radiation, which is accompanied by increased production of cortisol (Skobowiat et al., 2011). UVB phototherapy is a therapeutic option for psoriasis.
It should be noted that in the skin CYP11A1 metabolizes 7-dehydrocholesterol (Slominski et al., 2004), a precursor to cholesterol and vitamin D3 (Slominski et al., 2015a). The latter observation led to the discovery of an alternative pathway of D3 activation, with intermediates showing anti-inflammatory properties and being detectable in the human blood (Slominski et al., 2014, 2015a). The transformation of 7-dehydrocholesterol to 7-dehydropregnenolone opens an alternative pathway of production of Δ7steroids that, if subjected to UVB, will be transformed to vitamin D-like molecules with a short side chain that are biologically active in the skin (Slominski et al., 2004, 2015b). Thus, the role of CYP11A1 in inflammatory disorders can be extended to include production of alternative secosteroids with the vitamin D3 derivatives showing anti-inflammatory properties, while being noncalcemic (Slominski et al., 2014).
Cutaneous equivalent of the hypothalamo-pituitary-adrenal axis (cHPA)
General considerations
The cHPA enables a highly organized response by the body to sustained stress involving the hypothalamus, pituitary, and adrenals, with cortisol (corticosterone in rodents) acting as a potent immunosuppressor and terminator of corticotropin releasing hormone (CRH), proopiomelanocortin (POMC), and proinflammatory cytokine activities (Chrousos, 2009). The latter, including IL-1, IL6, and tumor necrosis factor-α, are stimulators of CRH and POMC expression. The skin also expresses all elements of the HPA, which can be organized along the classical HPA algorithm or context-dependent elements of it (Slominski et al., 2013).
Dysfunctional cHPA as the origin of inflammatory disorders
CRH, while being a regulatory arm of the HPA, has direct proinflammatory effects in the periphery, effects that are enhanced further by positive feedback and self-amplifying interactions with locally produced proinflammatory cytokines (Slominski, 2009; Slominski et al., 2013). These properties are also shared by CRH-related urocortins. The conclusion of the paper by Hannen et al. (2017) that deficient GC signaling underlies the pathogenesis of psoriasis represents validation of the original theory concerning involvement of the HPA. That is, that defective attenuation of the CRH signaling system acting in concert with proinflammatory cytokines, and defective downstream immunosuppressive signaling composed of POMC peptides and GC, underlies the development and progression of inflammatory dermatoses, including psoriasis and autoimmune diseases, as had been proposed (Slominski, 2009; Slominski et al., 2013).
The opposite is that overstimulation of local glucocorticoid signaling can lead to skin infection (Figure 1), a weakened epidermal barrier and skin atrophy, as indicated by studies on psychological stress downregulating epidermal barrier function and antimicrobial defenses through the action of GC (Aberg et al., 2007). Such increased GC signaling may be secondary to increased cutaneous glucosteroidogenesis or an increased rate of cortisone reduction to cortisol (Figure 1), or enzymatic defects in GC inactivation through sulfatation or metabolic degradation. These events can be regulated in the skin. For example, UVB radiation, but not UVA, can stimulate production of cortisol, with attendant enhancement of CYP11A1 and 11β-hydroxysteroid dehydrogenase type 1 protein levels in human skin (Skobowiat et al., 2011, 2013). These effects may in part explain the well-recognized immuno-suppressive properties of UVB radiation. Interestingly, UVB downregulates GR expression in epidermal keratinocytes (Skobowiat et al., 2013). This would shield keratinocytes from the negative effects of enhanced cortisol levels, but protect UV-damaged epidermis from autoimmune attack by migrating immune cells that have an intact GR. A similar explanation was offered for enhanced protection of GC in GR epidermal knockout mouse skin against proinflammatory reactivity (Hannen et al., 2017). A possible role for hyperactivity of endogenous epidermal glucocorticosteroidogenesis in infection could be tested in impetiginized versus nonimpetiginized skin. In this context, weakening of antimicrobial activity and of barrier function could be counteracted by cytokines, CRH, urocortins, and POMC-derived peptides that would act as builders of the barrier and protective function of the skin (Slominski, 2007; Slominski et al., 2013). Therefore, optimal functioning of the sHPA is crucial for effective skin barrier function and prevention of inflammatory disorders or infection.
Cutaneous GC signaling: clinical and pharmacological implications
Based on the above, the optimal activity of the local glucocorticoidal system, which is composed of GC synthesizing and inactivating enzymes, and the corresponding nuclear and membrane bound receptors, is required for healthy skin. Glucocorticoid activity would be enhanced or counterbalanced by CRH, cytokines, urocortins, and POMC peptides. Taken together, the work by Hannen et al. (2017) and the studies reviewed above offer guidance for alternative strategies in treating inflammatory dermatoses. For example, specific enzyme inhibitors could be used to regulate the enzymatic steps outlined in Figure 1b and c, or to inhibit enzymes that either esterify or degrade cortisol or corticosterone or to increase the intracellular NADPH/NADP+ ratio favoring formation or reactivation of cortisol. More challenging would be gene therapy or molecular approaches targeting specific enzymes in the epidermis, with a special focus on CYP11A1 because of its pleiotropic activity (Slominski et al., 2015a). This strategy could also be applied to the regulation of CRH or POMC activity as proposed previously (Slominski, 2009; Slominski et al., 2013). Thus, pharmacological regulation of cutaneous GC activity using existing or newly designed chemical molecules that could selectively modify enzymatic activities represents a promising area of research.
Clinical Implications.
Cutaneous glucocorticoidogenesis and cortisol levels are low in psoriatic skin.
Glucocorticoid receptor expression is also decreased in psoriatic skin.
Restoration of local glucocorticoid signaling may represent a novel therapy for psoriasis.
Acknowledgments
Per journal’s regulations, we are restricted to a limited number of references; however, many original papers are cited in the listed references and reviews. We acknowledge a partial support by grants R21AR066505 and 1R01AR056666 from National Institutes of Health to ATS.
References
- Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–49. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Hannen R, Udeh-Momoh C, Upton J, Wright M, Michael A, Gulati A, et al. Dysfunctional skin-derived glucocorticoid synthesis is a pathogenic mechanism of psoriasis. J Invest Dermatol. 2017;137:1630–7. doi: 10.1016/j.jid.2017.02.984. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–93. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117:3166–9. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol. 2009;160:229–32. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A novel pathway for sequential transformation of 7-dehy-drocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144PA:28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015a;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015b;103:72–88. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–84. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–81. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]