Abstract
We have generated a humanized anti-cocaine monoclonal antibody (mAb), which is at an advanced stage of pre-clinical development. We report here in vitro binding affinity studies, and in vivo pharmacokinetic and efficacy studies of the recombinant mAb. The overall aim was to characterize the recombinant antibody from each of the three highest producing transfected clones and to select one to establish a master cell bank. In mAb pharmacokinetic studies, after injection with h2E2 (120 mg/kg iv) blood was collected from the tail tip of mice over 28 days. Antibody concentrations were quantified using ELISA. The h2E2 concentration as a function of time was fit using a two-compartment pharmacokinetic model. To test in vivo efficacy, mice were injected with h2E2 (120 mg/kg iv), then one hour later injected with an equimolar dose of cocaine. Blood and brain were collected 5 minutes after cocaine administration. Cocaine concentrations were quantified using LC/MS. The affinity of the antibody for cocaine was determined using a [3H] cocaine binding assay. All three antibodies had long elimination half-lives, 2–5 nM Kds for cocaine, and prevented cocaine’s entry into the brain by sequestering it in the plasma. Pharmacokinetic and radio-ligand binding assays supported designation of the highest producing clone (85) as the master cell bank candidate. Overall, the recombinant h2E2 showed favorable binding properties, pharmacokinetics, and in vivo efficacy.
Keywords: Cocaine, addiction, monoclonal antibody, immunotherapy, master cell bank
Introduction
Immunotherapy has shown potential as a treatment for cocaine abuse. Anti-cocaine antibodies have been shown to bind to cocaine, preventing its entry into the brain [1,2]. In clinical trials, active immunization with an anti-cocaine vaccine induced the production of anti-cocaine antibodies [3,4]. However, effectiveness in inhibiting cocaine use was variable, likely due to the inability to control antibody titers. In the first trial some level of effectiveness was observed that depended on the level of antibody titers raised. The minimum effective plasma concentration of anti-cocaine antibodies was achieved in only a sub-population of subjects. However, in a subsequent larger trial, though some patients generated levels of anti-cocaine antibodies proposed as adequate, no significant effect on cocaine usage was observed. An alternative immunotherapy using passive immunization with an anti-cocaine monoclonal antibody should provide a more consistent clinical response, as the dose can be directly controlled and the affinity of the antibody is constant and known.
The recombinant humanized anti-cocaine antibody, h2E2, has previously been reported to have high affinity and selectivity for cocaine over its inactive metabolites [5,6]. It is able to prevent cocaine entry into the brain in rats [5], and cocaine’s active metabolite, cocaethylene, entry into the brain in mice [7]. It has a long half-life in both rats and mice [5,7]. This mAb also increased the amount of cocaine needed to reinstate self-administration behavior in a rat model of relapse by 3-fold [8], which should translate into a decrease in the probability of cocaine induced relapse. On the basis of these findings h2E2 is a lead candidate as an immunotherapeutic for cocaine abuse.
The recombinant h2E2 mAb is currently being produced in g/L quantities from Chinese Hamster Ovary (CHO) clones [5]. In order to advance h2E2 toward clinical trials, it is necessary to establish a master cell bank to permit the production of protein suitable for use in humans [9]. The selection of a single clone is a critical step in establishing this cell bank [10]. It was shown, using the same material as in the current study, that post-translational modifications (glycosylation) can vary between h2E2 produced by different clones (cell lines) with different production yields [11]. The effects of these differences in glycosylation on the pharmacokinetics of monoclonal antibodies are unclear, since contradictory results have been obtained in previous studies [11, 12]. A recent review by Higel et al [12] discusses how increasing levels of high mannose glycans can decrease the pharmacokinetic half-life of antibodies, whereas other types of N-glycosylation have been found to have opposite effects. This review concluded that high mannose glycan levels influence the pharmacokinetics of IgGs by increasing the clearance rate via the mannose receptor, which is highly expressed on immune cells. For other N-glycans and glycoforms the results were less clear [12]. With respect to our h2E2 mAb, Kirley et al did not show any difference in the mannose levels in the clones, however, there were differences observed some neutral biantennary N-glycan levels, especially in G0F in antibody from clone 85 when compared to that from clones 188 and 323 [10]. Although there is no reason to believe that this heterogeneity of glycosylation of h2E2 should affect the affinity for cocaine, it is important to characterize the h2E2 protein produced from potential clones for in vitro and in vivo efficacy before selecting a clone to establish the master cell bank.
It is generally assumed that an ideal anti-drug antibody used for the prevention of relapse should have a high affinity for its target, a long biological half-life, and be able to rapidly alter the pharmacokinetics of the target drug [12]. This can be tested by in vitro binding studies to determine the Kd (a measure of binding affinity) of the antibody for its target, an in vivo pharmacokinetic study to determine the half-life, and an in vivo study of the tissue distribution of the target drug, particularly to the brain, in the presence of the antibody. Combined, this battery of tests can provide confidence that the antibody has appropriate pharmacokinetic (PK) and pharmacodynamics (PD) properties. Therefore, material from the top three producing h2E2 clones were screened using the described battery of tests in order to determine which of these clones would be appropriate candidates to establish a master cell bank.
Materials and Methods
Antibody production and preparation
A series of h2E2-producing clones were generated by Catalent Pharma Solutions (Madison, WI) using their GPEx technology [13] to permanently transfect Chinese Hamster Ovary (CHO) cells with multiple copies of the cDNAs for the heavy and light chains of h2E2. The top three producing clones (85, 188 and 323) were selected for further optimization of h2E2 production levels. The clones were passaged every 3–4 days during the exponential phase, maintaining a viability of 90% or better in either CD OptiCHO (Life Technologies), PowerCHO-2 (Lonza) or G12.1 (Lonza) growth media. Cells were inoculated at a cell density of 300,000 cells/mL in each media and incubated in a Multitron shaking incubator. To find the optimal growth conditions three different culture conditions using the feed supplements Efficient C, F12.2 or F12.7 were performed in each growth media for each clone. Additional feed supplements including Cell Boost 4 PS307, l-glutamine, and glucose were included in all growth flasks. Duplicate 500 mL capacity flasks (190 mL total volume) were inoculated for each condition. The duplicate shaker flasks for each set of conditions were sampled on alternate days and were harvested when viabilities were approximately 50%, up to a maximum of 20 days. The secreted recombinant h2E2 mAb was purified using a series of filtrations and chromatography purifications using protein A. For animal studies, the purified h2E2 was tested for sterility to ensure low virus and endotoxin levels. To decrease the injection volume, the h2E2 was concentrated using size-exclusion filtration concentration with a 3,000 molecular weight cutoff. Final protein concentrations were determined by 280 nm absorbance.
[3H]Cocaine-binding
Cocaine binding studies were carried out by immunoprecipitation of h2E2 bound to [3H]cocaine as previously described by Norman et al 2014 [5], except that the incubations were done at 4°C instead of room temperature, and all reagents were added at once instead of sequential additions and incubations. In summary, a fixed concentration (0.4 nM) of h2E2 mAb was incubated with a serially diluted range of [3H]cocaine concentrations (0.5–100 nM) with the specific activity diluted with unlabeled cocaine to 1μCi/200 pmole. A goat anti-human antibody (4 nM) and a rabbit anti-goat antibody (40 nM) were added and the samples were incubated overnight (18–20 hours) at 4° C. Bound ligand was separated by filtration through Whatman GF/F filters using a cell harvester and a single wash with 2 mL of cold PBS. Filters were placed in scintillation fluid and radioactivity was measured using a Beckman Coulter LS6500 Multi-Purpose Scintillation Counter. Nonspecific binding was determined by the counts measured in the absence of h2E2. Specific binding was calculated by subtracting the non-specific counts from the samples containing the h2E2. CPM specifically bound as a function of total cocaine concentration was fit to a saturation binding curve to determine Kd and Bmax. Curves displayed on the figures were generated by taking the mean of all the replicate curve fits. Both fitted parameters (Kd and Bmax) from at least three sets of experiments were statistically compared using a one-way ANOVA.
Animals
All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals [14] and under a protocol approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. Jugular vein catheterized Swiss-Webster mice (19–22 g) were purchased from Taconic Farms. Mice were housed individually on a 14/10-hour light/dark schedule with unrestricted access to food and water.
Pharmacokinetics of h2E2 mAb in mice
Three groups of mice were injected with h2E2 produced from one of the three clones (n=6 mice per clone) at a dose of 120 mg/kg i.v. At times from 7 min to 21 days, 10 μL of blood was collected from a small incision at the tip of the tail. Blood was immediately diluted into 90 μL of citrate buffer (0.1 M, pH 4) and stored at 4°C. Within a week of collection, blood was further diluted for storage at 4°C at a final dilution of 1:100 in phosphate-buffered saline (PBS, pH = 7.2) containing BSA (0.5%).
Blood h2E2 concentrations were quantified using ELISA as described previously [15]. Briefly, h2E2 was captured by binding to a benzoylecgonine 1,4-butanediamine-BSA (BE-BSA) conjugate adsorbed onto 96 well plates, which were then blocked using BSA (0.5%). The bound h2E2 was detected by incubation with biotinylated goat anti-human polyclonal antibodies and the colorimetric signal was generated using a streptavidin-alkaline phosphatase conjugate hydrolysis of 4-nitrophenyl phosphate di (tris) salt. The optical density was measured at 405 nm after addition of 1N NaOH. All samples were quantified at two different dilutions by comparing to a standard curve of known h2E2 concentrations ranging from 0 to 0.4 ug/mL. All standards and samples were assayed in triplicate.
Pharmacokinetic data was analyzed using Phoenix WinNonlin software and PK curves for individual mice were fit using a two-compartment model and a 1/y2 data weighting scheme. The mean and standard error of the mean for the distribution and elimination half-lives and volume of distribution were estimated for each antibody. Graphs represent the mean ± SEM of concentrations and curves, and the table represents mean ± SEM parameter values. Final parameter estimates were compared using a one-way ANOVA.
The effect of h2E2 mAb on the distribution of cocaine and metabolites to the plasma and brain in mice
Three groups of mice (n=5 per group) were injected with h2E2 (120 mg/kg i.v) from one of the clones and one group with vehicle (PBS). One hour later, mice were injected with an equimolar dose of cocaine HCl (0.56 mg/kg i.v) containing 100 units/mL of heparin and decapitated 5 minutes later. About four minutes prior to decapitation, mice were anesthetized with sodium pentobarbital (60 mg/kg i.p.). Trunk blood was collected and plasma was separated by centrifugation in heparinized tubes for 5 min at 5000 xg. Brains were also harvested. Tissues were placed immediately on dry ice and then stored at −20°C. Cocaine and metabolite concentrations were quantified using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) as previously described for cocaine and benzoylecgonine (BE) [16]. Conditions for the measurement of the metabolites ecgonine methyl ester (EME) and norcocaine were as described previously [2], with the only difference being that electrospray ionization was used instead of atmospheric pressure chemical ionization. The concentrations of compounds were compared to the vehicle control values using a one-way ANOVA on ranks with a Dunnett’s post-hoc test.
Materials
Cocaine HCl was provided by the Research Triangle Institute (Chapel Hill, NC) under the National Institute on Drug Abuse Drug Supply Program. Sodium pentobarbital was purchased from Patterson Veterinary supply company. Cocaine, levo-[benzoyl-3,4-3H(N)] (32 Ci/mmol) was purchased from Perkin Elmer. Recombinant h2E2 was manufactured by Catalent PharmaSolutions (Madison, WI) using their GPEx technology. Cell growth media were CD OptiCHO (Life Technologies; Catalog # 12681-011), PowerCHO-2 (Lonza; Catalog # 12-771Q) or G12.1 (Irvine; Catalog # 98945). The feed supplements used were: Cell Boost 4 PS307 (HyClone; Catalog # SH30857.03), Efficient Feed C (Irvine), F12.2 and F12.7 (Irvine; catalog #98943 and 98944), l-glutamine and glucose (Mallinckrodt Baker; catalog #JT2078-6 and JT-1920-5). Goat anti-human purified antibody to human IgG Fc was purchased from MP Biomedicals, and the rabbit anti-goat IgG was from EMD Millipore. Glass microfiber filters were from GE Healthcare Life Sciences.
Results
Production of h2E2 mAb
Clone 85 had the highest maximum production with h2E2 protein levels of 4.1 g/L in growth media G12.1 using the feed supplement F12.7 and L-glutamine. This is compared with maximum productions of 3.8 g/L for clone 188, and 3.3 g/L for clone 323. Under all culture conditions tested, clone 85 had higher production levels than the other two clones.
[3H]Cocaine-binding
The mean ± SEM binding affinity (Kd) of h2E2 from the three clones was 2.2 ± 0.3, 5.2 ± 0.7, 4.2 ± 1.2 nM for 85, 188, and 323, respectively. The Bmax’s were 4007.1 ± 479.3, 4707 ± 223, and 3026 ± 178 counts per minute (CPM) (n=3 for 85 and 323, and 4 for 188) (Fig 1). The mean Kd and Bmax values were not significantly different between the clones (ANOVA, p>0.05).
Fig. 1.
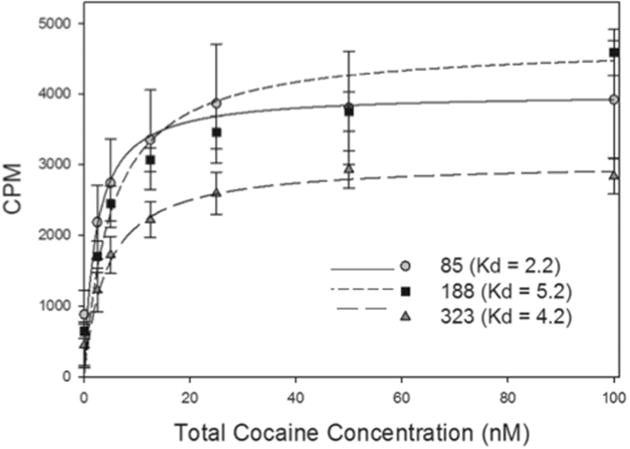
[3H]Cocaine binding curves for h2E2 mAb from the three clones. The y-axis represents specific counts per minute. The x-axis represents total cocaine concentration (nM). Each point represents the mean ± SEM from n=3 for 85 and 323, and n = 4 for 188. The curves represent the mean fit from all replicates for each clone.
Pharmacokinetics of h2E2 mAb in mice
The h2E2 produced from the three clones each displayed bimodal pharmacokinetics that were adequately described by a two-compartment model with a mean distribution half-life (t1/2α) of 4.27 – 5.35 hours and a terminal elimination half-life (t1/2β) of 4.91 – 7.14 days (Table 1, Fig. 2). The volume of distribution (Vd) was low (as expected) for all of the recombinant antibodies, with mean values ranging from 0.13 – 0.27 L/kg (Table 1, Fig. 2). Data from one mouse in the clone 85 group was excluded due to high variability in the ELISA assay between multiple repeat runs. None of the pharmacokinetic parameter estimates were significantly different for any of the clones as determined by a one-way ANOVA.
Table 1.
The pharmacokinetic parameter estimates of h2E2 mAb from the three clones. Pharmacokinetic parameter estimates were generated from a two-compartment pharmacokinetic model in WinNonlin. The mean values and mean fits are plotted in Fig. 2. Each value represents the mean ± SEM from n=6 for 188 and 323, and n=5 for 85 mice samples were collected from 7 min to 28 days after h2E2 injection. The fitted parameter estimates did not significantly differ for any of the three clone mAbs on any of the parameters.
| Parameter | ||||
|---|---|---|---|---|
| t1/2α (hours) | t1/2β (days) | VD (L/kg) | ||
| Clone | 85 | 5.35 ± 1.55 | 7.14 ± 1.61 | 0.13 ± 0.02 |
| 188 | 4.27 ± 2.62 | 5.17 ±1.66 | 0.19 ± 0.01 | |
| 323 | 4.34 ± 2.32 | 4.91 ± 1.86 | 0.27 ± 0.07 | |
Fig. 2.
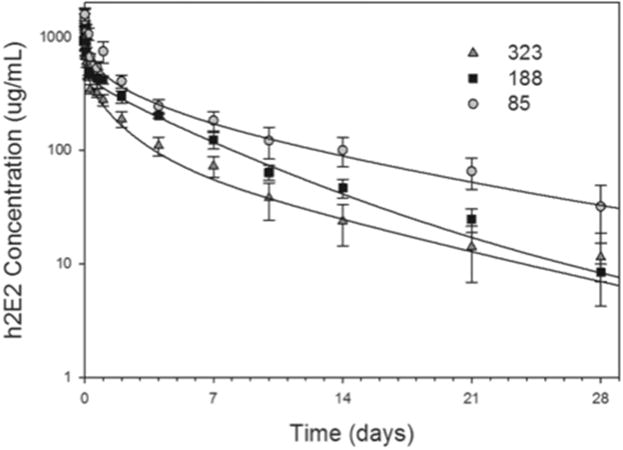
The pharmacokinetics of h2E2 mAb produced from three clones. The blood h2E2 concentrations over time following a single injection of h2E2 (120 mg/kg i.v) from each of the clones are plotted. Each point represents the mean ± SEM from 6 mice for 188 and 323, and 5 mice for 85. The curves are the mean fit from all animals using a two-compartment pharmacokinetic model.
The effect of h2E2 mAb on the distribution of cocaine in the plasma and to the brain in mice
Plasma cocaine concentrations in the presence of h2E2 from all three clones were significantly higher than in the vehicle control group, increasing between 21.4 and 34.5-fold. Furthermore, the mAb from clone 85 produced a statistically significant increase (4.1-fold) in plasma BE concentrations compared to control values (Fig. 3A).
Fig. 3.
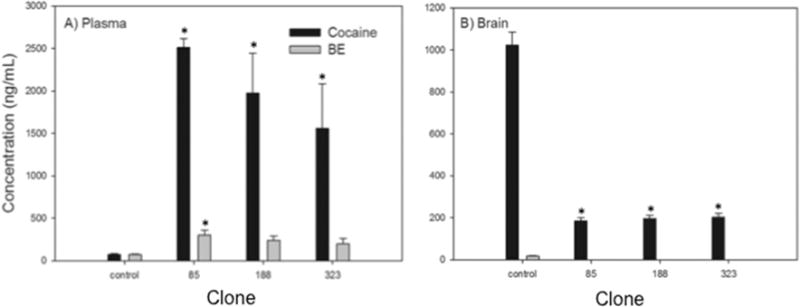
The effect of h2E2 mAb from three clones on the distribution of cocaine and metabolites in mice. Mice (n = 5 per group) were injected with h2E2 (120 mg/kg i.v.) from each of the three clones and one hour later were injected with cocaine HCl (0.56 mg/kg i.v.). After 5 minutes plasma and brain were collected. The bars represent the mean ± SEM concentration of cocaine (black bars) or benzoylecgonine (BE) (grey bars) in plasma (A) or brain (B). BE was not detectable in any of the brain samples from h2E2-treated mice, and control concentrations were only 18 ng/mL, so these data are not displayed for clarity. Asterisks(*) indicate values significantly different from the vehicle control (p<0.05, one-way ANOVA on ranks with Dunnett’s post-hoc test).
Brain cocaine concentrations were significantly lower than controls in all three groups (Fig. 3B) with decreases ranging from 80.1–80.7 %. BE was not detectable in any of the treated brains, and was only 18 ng/mL in the control group, so this data is not shown. Cocaine’s other inactive metabolite, ecgonine methyl ester, was also detected, but was only at low levels (20 ± 5 ng/mL in plasma, 34.9 ± 3 ng/mL in brain) in control samples, and overall not detectable in the h2E2-treated groups. The active metabolite norcocaine was not detectable in any control samples, but was present at low levels in the treatment groups.
Discussion
The experiments described in this study provide us with a useful characterization of the ability of the recombinant h2E2 mAb to bind cocaine in vitro, to prevent cocaine from entering the brain in vivo and to assess the pharmacokinetic half-life and low volume of distribution of the mAb in vivo. These measurements facilitated the selection of a clone for establishing a master cell bank.
All three antibodies bound cocaine with high affinity (Fig 1) consistent with previous estimates of the binding affinity for this mAb based on quenching of tyrosine and tryptophan fluorescence by bound cocaine [6] and previous radioligand binding studies [5]. It has previously been shown with anti-methamphetamine mAbs that a higher affinity mAb was more effective at antagonizing methamphetamine effects in both pretreatment and overdose models [17]. Therefore, it would be predicted that all three of these high affinity anti-cocaine mAbs should be equally effective.
Measuring the in vivo pharmacokinetics is a particularly important step in the characterization of antibody produced by different clones or under different growth conditions. This is because different clones (referred to in the referenced paper as cell lines) have been shown to produce this recombinant antibody with different post-translational glycosylation patterns [11], and it is therefore possible that the pharmacokinetics could differ [12]. However, that was not observed in this mouse PK study (Fig 2, Table 1). The mAbs had uniformly long elimination half-lives consistent with previous reports in mice of h2E2 produced from a mixture of clones [7]. This predicts a long duration of action in humans, which is again favorable for a long-term immunotherapy of cocaine abuse. The uniformly low volume of distribution is also desirable, since it makes it likely that the antibody will be able to better prevent the distribution of cocaine to the brain by sequestering it in the plasma.
Lastly, the antibodies were tested for in vivo efficacy. This test is arguably the most important, as the ultimate goal of this therapy is to prevent cocaine distribution to the brain. All three mAbs significantly decreased brain concentrations, with corresponding increases in plasma concentrations. It has been previously shown that h2E2 binds to BE with a moderate affinity (3.4 to 7.6-fold lower affinity than cocaine) [5,18]. It is likely that this BE-h2E2 binding accounts for the higher BE concentrations in the plasma, where the BE formed is sequestered in the plasma, rather than an increased rate of metabolism of cocaine to BE in the presence of the antibody. Interestingly, the metabolite EME was not detected at substantial levels in any samples either in the absence or presence of h2E2. This is in contrast to previous studies, which reported that EME, not BE, was the major metabolite of cocaine in mice [19]. Further PK studies on the metabolism of cocaine in mice over time are needed to resolve this discrepancy.
Overall, the mAbs derived from the three clones were indistinguishable in all assays, and thus all of these three clones produce recombinant h2E2 with properties predictive of an effective cocaine immunotherapeutic agent. On the basis of clone 85 exhibiting the highest production of h2E2 mAb, this clone was selected for establishing a master cell bank and advancement towards clinical trials. However, in the unlikely event that problems should arise with the stability of production from this clone, the other two clones could also be used.
Highlights.
Three different clones in batch cultures produced the h2E2, in g/L quantities
All mAbs had high affinity for cocaine and antagonized cocaine entry into the brain
All mAbs had long in vivo half-lives, predicting a long duration of action
Clone 85 was selected to establish a master cell bank based on high production levels
Acknowledgments
LC/MS analyses were conducted through NIDA contract N01DA-14-7788 by Dr. David Andrenyak and Dr. David Moody at the University of Utah. We would like to thank Purabi Dey for technical assistance. This work was supported by the National Institutes of Health, National Institute on Drug Abuse grant number U01DA039550.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Norman is named as a co-inventor on a patent application for the matter and use of the h2E2 humanized anti-cocaine monoclonal antibody.
Citations
- 1.Norman AB, Ball WJ., Jr Predicting the clinical efficacy and potential adverse effects of a humanized anticocaine monoclonal antibody. Immunotherapy. 2012;4:335–343. doi: 10.2217/imt.12.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin SN, Walsh SL, Moody DE, Foltz RL. Detection and time course of cocaine N-oxide and other cocaine metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Anal Chem. 2003;75:4335–4340. doi: 10.1021/ac030037c. [DOI] [PubMed] [Google Scholar]
- 3.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, Sekerka R, Levin FR, Mariani JJ, Stitzer M, Tompkins DA, Rotrosen J, Thakkar V, Smoak B, Kampman K. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42–47. doi: 10.1016/j.drugalcdep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman AB, Gooden FCT, Tabet MR, Ball WJ. A Recombinant Humanized Anti-Cocaine Monoclonal Antibody Inhibits the Distribution of Cocaine to the Brain in Rats. Drug Metabolism and Disposition. 2014;42:1125–1131. doi: 10.1124/dmd.114.057034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirley TL, Norman AB. Characterization of a recombinant humanized anti-cocaine monoclonal antibody and its Fab fragment. Hum Vaccin Immunother. 2015;11:458–467. doi: 10.4161/21645515.2014.990856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wetzel HN, Tabet MR, Ball WJ, Norman AB. The effects of a humanized recombinant anti-cocaine monoclonal antibody on the disposition of cocaethylene in mice. Int Immunopharmacol. 2014;23:387–390. doi: 10.1016/j.intimp.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetzel HN, Tsibulsky VL, Norman AB. The effects of a repeated dose of a recombinant humanized anti-cocaine monoclonal antibody on cocaine self-administration in rats. Drug Alcohol Depend. 2016;168:287–292. doi: 10.1016/j.drugalcdep.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Biologic Evalulation and Research, editor. FOR THE SUBMISSION OF CHEMISTRY, MANUFACTURING, AND CONTROLS INFORMATION FOR A THERAPEUTIC RECOMBINANT DNA-DERIVED PRODUCT OR A MONOCLONAL ANTIBODY PRODUCT FOR IN VIVO USE. 1996. [Google Scholar]
- 10.Elgundi Z, Reslan M, Cruz E, Sifniotis V, Kayser V. The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Kirley TL, Greis KD, Norman AB. Structural characterization of expressed monoclonal antibodies by single sample mass spectral analysis after IdeS proteolysis. Biochem Biophys Res Commun. 2016;477:363–368. doi: 10.1016/j.bbrc.2016.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higel F, Seidl A, Sorgel F, Friess W. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur J Pharm Biopharm. 2016;100:94–100. doi: 10.1016/j.ejpb.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Bleck GT. Consistent production of genetically stable mammalian cel llines. Biopharm International. 2012;25:56–59. [Google Scholar]
- 14.Institute for Laboratory Animal Research, Guide for the care and use of laboratory animals. National Research Council of the National Academies. 2011 [Google Scholar]
- 15.Paula S, Tabet MR, Farr CD, Norman AB, Ball WJ., Jr Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 16.Lin SN, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J Anal Toxicol. 2001;25:497–503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- 17.Byrnes-Blake KA, Laurenzana EM, Landes RD, Gentry WB, Owens SM. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol. 2005;521:86–94. doi: 10.1016/j.ejphar.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Kirley TL, Norman AB. Characterization of a recombinant humanized anti-cocaine monoclonal antibody and its Fab fragment. Hum Vacc Immunother In press; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warner A, Norman AB. Mechanisms of cocaine hydrolysis and metabolism in vitro and in vivo: a clarification. Ther Drug Monit. 2000;22:266–270. doi: 10.1097/00007691-200006000-00006. [DOI] [PubMed] [Google Scholar]


