Abstract
Lighting technologies are rapidly evolving, creating many opportunities for good lighting within the NICU. With the widespread adoption of advanced solid-state lighting technologies, lighting no longer needs to be static. Rather, lighting systems can be more easily adjusted to the different and changing visual and non-visual needs of the professional staff, infants and family members throughout the 24-hour day. This paper provides a conceptual framework for defining good lighting in the NICU, recognizing the needs of various constituent groups, each with very different needs from the lighting. Several other papers on the topic of lighting for various constituent groups at different times of the day in the NICU are summarized. Attention is given specifically to the Recommended Standards for Newborn ICU Design, a consensus standard developed by a wide range of experts, to help the reader translate this conceptual framework to practice.
Keywords: NICU lighting, NICU design, recommended standards, lighting technologies, circadian rhythms, solid state lighting
Introduction
Creating good lighting for the neonatal intensive care unit (NICU) in a hospital can be complicated. The NICU will be simultaneously occupied by different constituent groups, from infants to professional staff to family members to business administrators to maintenance personnel, each with very different needs from the lighting. And since the NICU is operated 24 hours per day, the very same constituent group will have different lighting needs depending upon the time of day. The diversity of needs by the different groups at different times of the day and night requires deliberate and careful consideration of the lighting design.
Lighting most obviously affects visibility. Lighting needs will be very different for inserting a catheter into an infant's vein than that for viewing electronic records displayed on a computer screen. Clearly, lighting systems must be designed to support these very different visual requirements. However, lighting does not just affect visibility. Non-visual systems emanating from the retina, including the modulation of alertness and the regulation of circadian rhythms are also affected by the lighting. Responses to light by these non-visual systems vary rhythmically across the 24-hour day, so lighting needs during the day are not the same as that during the night. Here again, lighting systems must be designed to support the time-of-day differences in these non-visual system responses to light.
Finally, lighting also sends a psychological message to occupants of the NICU, conveying the values and concerns of the hospital to the different constituent groups. Exposed light sources that create glare send a very different message to family members than ones that are designed to be glare-free and enhance the furnishings and the colors of art on the wall.
Fortunately lighting technologies are rapidly evolving, creating many more opportunities for good lighting within the NICU. With the widespread adoption of solid-state lighting (SSL), lighting no longer needs to be static. Rather, lighting systems can be more easily adjusted to the different and changing visual and non-visual needs of the professional staff, infants and family members throughout the 24-hour day. Dynamic lighting, if done well, can send compelling messages to occupants, conveying warmth and comfort during the evening and bright daylight conditions during the day. Color rendering has also improved with the development of SSL. Decorative colors of furnishings or of art on the wall can be enhanced by new SSL light sources. More importantly, better color rendering can help professional staff make better and more subtle observations of infant skin tones. Certain unexpected problems can also arise from some SSL systems. In particular, attention must be given to potential flicker produced by SSL because flickering lights may be responsible for headaches and fatigue among the professional staff.
This paper provides a conceptual framework for defining good lighting in the NICU, recognizing the needs of the different constituent groups throughout the 24-hour day. Several other papers have been written on the topic of lighting for different constituent groups at different times of the day in the NICU and may be found in the references.1-10 Attention is given specifically to the Recommended Standards for Newborn ICU Design (http://www3.nd.edu/∼nicudes), a consensus standard developed by a wide range of experts, to help the reader translate this conceptual framework to practice.11
Visual System Requirements
Visual Performance
Visual performance is defined as the speed and accuracy of processing visual information.12 The human retinal architecture is such that some 2% of the retina, the macula, is specialized for high spatial resolution and color vision. Cone photoreceptors, of which there are three types: long-wavelength (L), middle-wavelength (M) and short-wavelength (S) sensitive cones, dominate this macular region of the retina. At the very center of the macula is the fovea, a small reduction in the thickness of the retina, comprised almost entirely of densely packed L- and M-cones that are used for fine spatial resolution and for reading. A disproportionate amount of the visual cortex, nearly 80%, is devoted to processing visual information obtained by the macula. The remaining 98% of the retina contains all three cone types as well as rods which are responsible for processing visual information at very low light levels like those experienced outdoors at night. This peripheral retina is largely responsible for detecting “anomalies” in the environment. The peripheral retina detects sudden movements or, for example when driving or walking, changes in the “flow” of relative object motion in the environment. Through dedicated neural channels reaching sub-cortical regions of the brain, eye movements are quickly initiated so that the fovea can inspect these potential hazards in the environment.
The macular and peripheral areas of the retina work seamlessly together and in parallel to help us perform a wide variety of tasks like maintaining balance while walking or climbing stairs, playing sports, visual inspection for defects, and even reading a book line by line. A quantitative model of visual performance for processing information by the fovea has been developed13 and validated in a number of contexts, from reading text to interpreting potential hazards on the roadway.14-16 The relative visual performance (RVP) model quantifies the functional relationship between speed and accuracy and three stimulus parameters, the size and the contrast of a target, such as a printed letter on a page, and the luminance of the target background, such as an illuminated page of paper.13
The model exhibits a “plateau and escarpment” characteristic such that speed and accuracy remain relatively constant (i.e., on the plateau) for most sizes, contrasts, and luminance levels that would be found in the NICU (Figure 1).13 When either the size or the contrast of the target is reduced, however, there is a rapid, precipitous drop in speed and accuracy (i.e., on the escarpment) to a point where the target can no longer be seen at all. Reductions in light level have a less pronounced impact on RVP, so it is important that every effort be given to enhance the contrast or increase the size of a target in the NICU environment. Nevertheless, it is sometimes difficult or impossible to increase object contrast or size, so providing high levels of illumination is the only practical means of increasing the speed and accuracy of processing the visual information. It might require increasing light levels by an order of magnitude to get a 2 or 3 percent increase in RVP, but this small change might be beneficial to the professional staff for a critical task such as inserting a catheter in an infant's vein.
Figure 1.
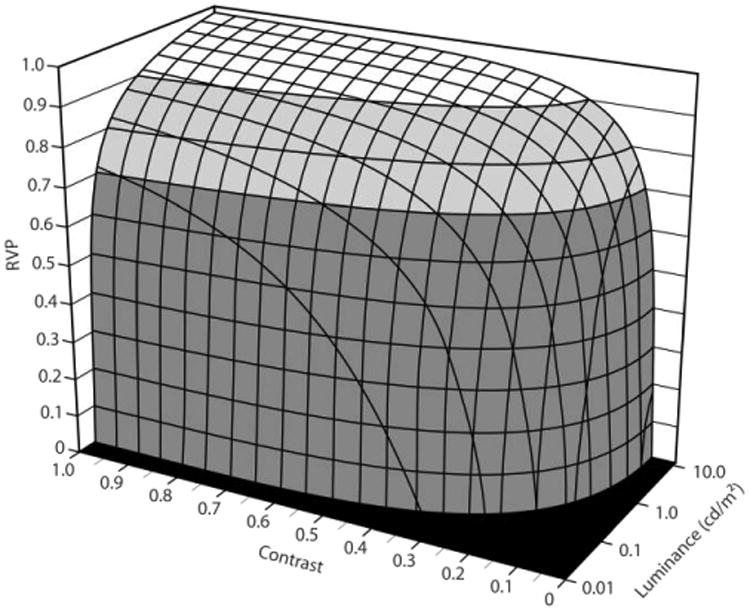
Relative visual performance (RVP) as a function of background luminance (light level) and target contrast for one target size.13 Large differences in light levels are needed to make even modest changes in the speed and accuracy of processing the visual task when on the “plateau” but small changes in contrast or target size near the “escarpment” can make the difference between seeing and not seeing an object.
Appearance
The macula is also critical for assessing the appearance of objects in the environment. As with visual performance, the macular cones are responsible for appearance in the NICU environment. However, information obtained from the very same cones responsible for visual performance transmit neural signals over different neural channels in the retina to other parts of the brain, including the visual cortex. Unlike the “plateau and escarpment” character of visual performance, the channel responsible for appearance exhibits, for example, a nearly linear response to contrast (Figure 2).14 Importantly, appearance and visual performance occur in parallel; a target that would appear to have moderate contrast would not necessarily be processed significantly slower.
Figure 2.
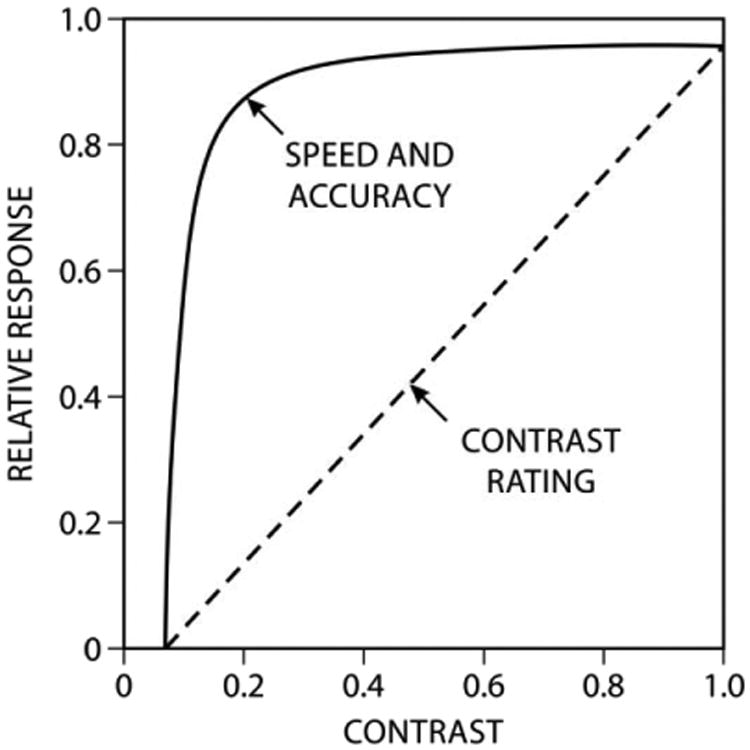
Relative visual performance (RVP) for one background luminance and target size (letters printed on white paper under one level of illumination) as a function of target contrast together with subjective ratings of contrast of the same stimulus (i.e., same background luminance and target size).14
The spectral sensitivities of the channels responsible for visual performance and for appearance also differ. Whereas visual performance is dominated by responses from the L- and M-cones, the appearance channel includes significant input from the S-cone as well. Indeed, appearance is very much dominated by S-cone response which actually grows stronger as light level increases. Two rooms illuminated by different light sources set to provide equal levels of RVP, a “cool” light source that appears blue-white and a “warm” light source that appears yellow-white, will not appear equally bright.17-21 The room illuminated by the “cool” source can appear as much as 35% brighter in the NICU environment because the S-cone contributes significantly to appearance, but not to visual performance.
Color Vision
Color appearance is important in the NICU. The visual system actually reduces the complex spectral composition of the light incident on the retina into neural signals carried by just three neural channels, two spectrally opponent channels, blue versus yellow (b-y) and red versus green (r-g), and a unidirectional, achromatic channel. These neural channels responsible for color appearance are, again, formed from the three cone types. The r-g channel is formed at the second stage of processing in the retina such that the L- and M-cones converge on a single so-called bipolar cell that can signal to the next level of neural processing in the retina either “red,” when input from the L-cone dominates, or “green,” when the M-cone input dominates. If the L- and M-cone inputs are equal, the r-g channel sends no signal at all. Similarly, the b-y channel places the S-cone response in opposition to the sum of the L- and M-cones to signal, respectively, either “blue” or “yellow.” Again, when the S-cone input and the L+M-cone input are equal, the b-y channel sends no signal to the next level of retinal processing. Like the “yellow” response, the achromatic channel is formed from the addition of input from the L- and M-cones, but does not signal any hue to the brain. Luminous objects appear “white,” “grey,” or “black” when the two color channels are in balance (i.e., neither blue nor yellow and neither red nor green).
The spectral composition of the light source interacting with the spectral reflectance of objects affects how these three channels respond and, thus, how we see, for example, the skin tone of an infant. “White” light sources used to illuminate the NICU do not render object colors equally. Many commercially available light sources can inherently limit the strength of a hue signal (e.g., “blue”) because the spectral power distribution of the light emitted by the source is biased toward one end of the visible spectrum or has limited spectral emission in some parts of the spectrum. Generally speaking, a light source for NICU applications should emit light across the entire visible spectrum. Dual metrics, used in combination, have been developed to characterize the color rendering capabilities of a light source. These metrics, Color Rendering Index (CRI) and Gamut Area Index (GAI), help ensure that objects illuminated by these sources will appear both natural and vivid (but not too vivid).22-24
CRI was developed in the 1960s with the aim of describing how similar a given light source makes illuminated objects appear relative to a reference light source.25,26 The implicit assumption is that the reference light source is “ideal” and any other light source can be rated as being equal to or worse than the reference source. So, for example, if a fluorescent lamp renders colors just like an incandescent light source, then it would have a CRI of 100, the maximum possible value. If it did not render the colors similarly, it would have a lower CRI value. Interestingly, the fluorescent light source with the lower value may actually render colors better than the incandescent lamp. For this reason a second metric that characterizes the ability of the lamp to provide saturated rather than muted hues was developed. GAI and similar metrics aimed at describing the saturating capabilities of a light source is based upon the idea that a light source that enables people to make finer distinctions in color will also produce colors of greater hue saturation. Research has shown that people's acceptance or preference for a light source is better predicted when CRI and GAI are used together to describe the color rendering properties of a light source, than either metric alone.22-24
Another important consideration is the color of the illumination itself. In the past, light sources have been described and sold as “cool” (blue-white), “warm” (yellow-white) or “neutral.” Correlated color temperature (CCT) has been used as the metric for the color of illumination; “warm” being associated with CCTs below 3000 K and “cool” above 5000 K. New metrics have been developed to more accurately characterize the color of the illumination which, together with the color rendering properties of the light source, better characterize how well people accept a given light source for general illumination in an architectural application (Figure 3). In general, people prefer light sources with minimum tint in NICU applications. Perhaps surprisingly, sources of illumination with minimum tint (i.e., “white”) are associated with virtually any CCT.27
Figure 3.
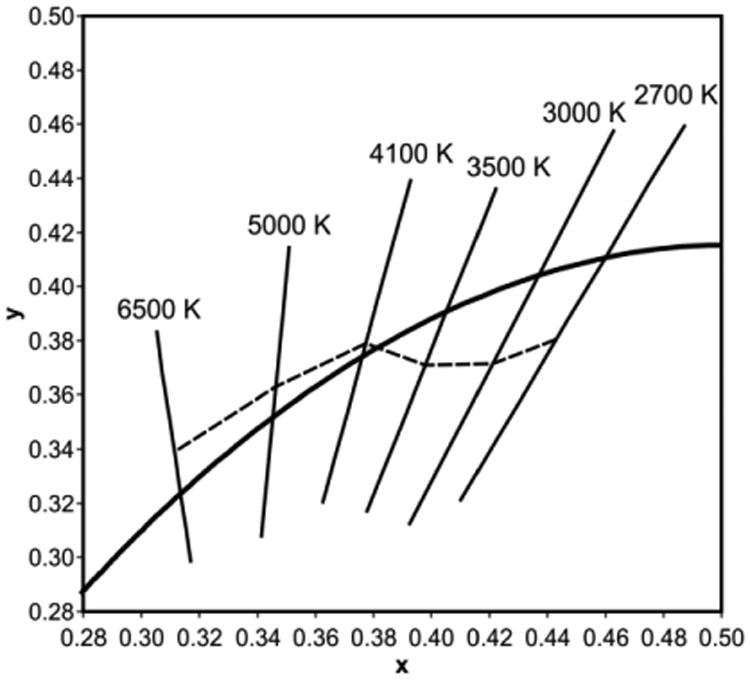
The CIE chromaticity diagram (1931) including the blackbody locus and lines of constant correlated color temperature (CCT). The dashed line indicates chromaticities of minimum tint, the “white body locus,” for each CCT.
Glare
There are two types of glare: disability glare reduces the apparent contrast of a visual task, while discomfort glare gives a sensation of discomfort to the viewer. Disability glare can be further refined into two types, reflected or veiling glare which is caused by light coming to the eyes off the task surface and entoptic glare which is caused by light scatter within the eye itself. Reflected glare can often be avoided by changing the specularity or glossiness of objects in the NICU. Unlike computer monitor technology of the last century, modern computer screens reduce reflected glare by scattering the incident light. Nevertheless, there are times when bright lights in the environment, particularly windows, can cause contrast-reducing reflections from computer screens and other display technologies. In those situations either the light from the window must be occluded by blinds or drapes or the display must be adjusted to minimize the reflections.
Entoptic scatter becomes more and more problematic with age. As one ages, the crystalline lens becomes less transparent and floaters, or myodesopsia, become more prevalent, scattering the light entering the eye and reducing retinal contrast. Windows can be particularly problematic for those individuals bothered by entoptic scatter. As with reflected glare, window blinds can reduce entoptic scatter. Unshielded, overhead lighting can also increase entoptic scatter if it is within the line of sight. This is particularly noticeable if the furnishings in the NICU are dark. For this reason it is better to use more indirect lighting and light finishes on the walls and the furnishings.
Discomfort glare is more complicated to predict and control than disability glare, requiring a more thoughtful lighting design for the NICU. Discomfort glare is related to three major factors, the brightness (luminance) of the light source itself, the illuminance at the eye from the light source alone, and the illuminance at the eye from the area surrounding the light source.28 A lighting design that minimizes discomfort glare will, first, hide direct views of the light source itself. Indirect lighting that primarily illuminates the vertical surfaces in the NICU will significantly reduce the likelihood of discomfort glare. Further, the contrast between the light source and its immediate background should be minimized. For this reason, and again, dark surfaces and furnishings should be avoided, particularly those close to the light source.
Shadows
As with glare, good lighting design should minimize shadows in the NICU. Diffuse lighting, rather than strong, direct lighting should be used, particularly in areas where critical visual tasks are performed. Strong directional light can be used successfully to highlight art on the wall or three-dimensional pieces of art. Often, however, directional light is selected to increase light levels on critical tasks. While this approach can in principle improve visibility by increasing the background luminance of the task, it will also create shadows if something like a hand or an instrument is placed between the light source, and the infant, thereby reducing visibility of the critical visual task.
The visual system has a momentary operating range of between 3-to-1 and 30-to-1, depending upon light level,29 thus large differences in the scene brightness can render objects in darker areas invisible. A strongly directional light source can create both a bright background and a dark shadow that exceeds the operating range of the visual system and, moreover, creates disability glare, further reducing the visibility of objects in the shadow. Where high light levels are achieved with strongly directional procedure lights in the NICU, provisions should be given to the professional staff to add diffuse auxiliary lighting adjacent to the critical task. This diffuse light will decrease the shadow contrast and thus bring the entire visual task within the operating range of the visual system.
Flicker
All electric light sources supplied by alternating current will flicker. The modulation frequency, modulation depth, duty cycle, light level, and region of the retina stimulated by the light source will determine whether the flicker will be perceived or not.30,31 In North America, electrical current alternates at 60 Hz. Flicker cannot be seen from most electric light sources used in the NICU, either because the modulation depth (temporal contrast) is too low and/or because the 60 Hz alternating current has been modified by the electronics to modulate the current frequency faster than can be processed by the visual system. Some data suggest, however, that a few people may be more sensitive to invisible flicker than others.32 For these people, even though the flicker cannot be seen, they may still experience headache and fatigue.
Flicker that might not be perceived directly may nevertheless be revealed to observers when the source illuminates a rotating or oscillating object.30,31 Under these conditions, the motion of the object will be seen in more discrete steps rather than continuous motion. This phenomenon is known as the stroboscopic effect but, unlike some factory or industrial applications with rotating machinery (e.g. a lathe), is unlikely to be a serious issue in the NICU.
Non-visual System Requirements
Circadian Entrainment
Circadian rhythms are sustained, endogenous oscillations of approximately 24 hours in biological systems, including physiology and behavior. Circadian rhythms are generated and regulated by a biological clock located in the suprachiasmatic nuclei (SCN) in the hypothalamus in the brain. In the absence of external time cues, the SCN will continue to run with a period slightly greater than 24 hours (on average 24.2 hours) in humans. The daily 24-hour light-dark pattern incident on the retinae resets the timing of the biological clock so that it runs with a period of 24 hours. After traveling multiple time zones, the new light-dark pattern will eventually synchronize, or entrain, a person's circadian rhythms to the new, local position on Earth. Thus, physicians and nurses in Australia are entrained to their local sunrise and sunset as are those in Boston who are entrained to their own times of sunrise and sunset.
The phototransduction mechanism regulating circadian rhythms, which is how the retina converts light signals into neural signals for the circadian system, have become clearer than they were 20 years ago. Intrinsically photosensitive retinal ganglion cells (ipRGCs) were identified in 2002 as a central neuron for converting light into neural signals for the circadian system.33 Rods and cones, particularly S-cones, have also been shown to play an important role in this process.34,35 The ipRGCs receive input from more distal neurons, and these vertical and lateral connecting neurons enable both rod and cone to participate in the spectral and absolute sensitivities of the circadian system.
A computational model for both the spectral and the absolute sensitivities of the human circadian system to light has been developed.35,36 This model has been used to successfully predict suppression of nocturnal melatonin, a marker for circadian system stimulation.37 Melatonin is known as the “hormone of darkness” because it is produced at night and under conditions of darkness. Light of sufficient amount and of the right spectrum will attenuate melatonin synthesis at night in a dose-dependent manner. Taking the neuroanatomy and neurophysiology of the retina into account and using published data on light's effect on acute melatonin suppression, the model was used to develop a metric for circadian-effective light called circadian stimulus (CS). Photometric equipment for measuring CS has also been developed.38 Recent studies have shown that exposure to a CS value of 0.3, measured at the plane of the cornea, for 2 to 4 hours in the morning will support circadian entrainment.39-41
Circadian Entrainment for Dayshift and Nightshift Workers
Light in the NICU should be designed to promote circadian entrainment of dayshift workers and minimize circadian disruption in nightshift workers. Generally, for those professionals working the dayshift, light exposures in the NICU during the day followed by dim or dark light exposures at home and while sleeping are sufficient to entrain circadian rhythms to local time. This is not always the case, however, if for example, nurses and staff work in very dim conditions during the day or if they are exposed to bright light during the night. Professionals who, for example, must view computer screens in windowless offices for most the day may not get enough daytime light for entrainment.42 Or the professional who reads a bright tablet before going to bed, may be delaying circadian rhythms, effectively moving the body clock one or two time zones to the west.43 Therefore, while the lighting in the NICU can be designed to promote entrainment in dayshift workers, it is important to keep in mind that entrainment will only occur if light exposure in the evening and at night is also managed. Educational materials with suggestions of how to manage light exposures throughout the day and night should be developed and distributed to dayshift workers (Appendix).
Promoting entrainment in nightshift workers is more challenging. Several studies have shown that most nightshift workers are, in terms of their circadian rhythms, dayshift workers trying to stay awake at night. This is because most nightshift workers also have dayshift families and daytime social obligations. These important commitments limit their ability to sleep during the day and work at night or, rather, entrain their circadian rhythms to working the nightshift. A lack of entrainment may be linked to the increased risk for maladies and diseases observed in rotating nightshift workers, including diabetes, obesity, and reproductive difficulties.44-46 In addition, epidemiological studies have shown that individuals working rotating nightshift for more than 20 years are at higher risk for breast and colorectal cancers.47-49 In 2007, the International Agency for Research on Cancer (IARC) classified shift work that involves circadian disruption as probably carcinogenic to humans.50 This classification was based on data from animal studies that showed that light exposure during the dark period is associated with the development and growth of tumors. However, the IARC felt that the epidemiological evidence for the carcinogenity of light during the dark period was still limited.
There are two parallel theories concerning the relationship between nightshift work and cancer risks. One focuses on light at night (LAN) as a suppressor of the normal nocturnal melatonin levels and the second theory focuses on the negative health-effects of light-induced circadian disruption.51 No field studies to date have been published where a large sample of personal light exposures have been directly related to health risks arising from suppression of nocturnal melatonin levels or to levels of circadian disruption. However, some studies suggest that nightshift workers are not exposed to enough light to acutely suppress their nocturnal melatonin, but the amplitude of their melatonin rhythm is reduced, suggesting some level of circadian disruption resulting from working the nightshift.52,53 In fact, one study using data from the Nurses' Health Study using calibrated personal light exposure sensors showed that circadian disruption for rotating-shift nurses working 3 or 4 nights per week was significantly greater than that measured for dayshift nurses or for nurses working one night per week.54
While there are no perfect solutions for minimizing circadian disruption in rotating or nightshift workers, compromise solutions have been proposed and tested under laboratory conditions. Smith et al. developed and tested a compromise work schedule that attempts to delay the workers' circadian rhythms rather than entrain their rhythms such that day is night and vice versa.55 In other words, the nightshift worker becomes more of a “night owl” than a completely nocturnal person. This solution will work best for permanent shift workers who still want to have daytime social and family interactions. The timing of the circadian clock can be delayed by exposure to high photopic light levels of a white light (>500 lux at the cornea) or lower levels of a narrowband 470-nm (blue) LED light (≈ 40 lux at the cornea) for the first half of the shift and by avoiding light exposures in the morning commute home by wearing dark or orange-filtered glasses.56 Thus, controlling both evening and morning light exposures will delay the endogenous drive for sleep onset from the early part of the night to the later part of the night. These “delayed” workers should not attempt to drive home when the shift has ended because they will be very tired (albeit less tired than they would have been without the delayed lighting protocol). Taking public transportation home is highly recommended for these workers, with or without a delaying light pattern. Again, educational materials aimed specifically to nightshift workers should be developed (Appendix).
Alertness
Keeping people in dim light can increase sleepiness and reduce alertness. This is particularly true at night. Several studies have shown that light can work like a cup of coffee both during the day and during the night to enhance alertness.57-64
Until recently, the alerting effect of light was associated with its ability to suppress nocturnal melatonin. More recently, it has been demonstrated that the photic pathway from the retina to the brain for acute melatonin suppression is not the same pathway for enhancing sympathetic system activation. For example, one hour exposure to saturated red light (at least 40 lux at the cornea or a 640-nm light) is quite effective at night for increasing heart rate, subjective alertness, and altering brain activity as measured with EEG but has no measurable impact on melatonin levels.60,61,64 Simple reaction times are also shorter, but more complex forms of performance are usually affected to a lesser extent because many other factors (e.g., learning) affect performance than being alert.
Moreover, in support of the idea that melatonin does not need to be suppressed to increase alertness, recent studies conducted during the day when melatonin concentrations are low showed that narrowband blue (40 lux a the cornea of a 470-nm light) and red (40 lux at the cornea of a 640-nm light) light as well as white (200 lux at the cornea of a 2700 K) light, can also be effective at increasing brain activities, reducing subjective feelings of sleepiness and reducing reaction times during the day.62,63 For nightshift work this insight might be quite useful for enhancing alertness without affecting melatonin rhythms because red light will not suppress the hormone melatonin.
Circadian Entrainment in Infants
The NICU is usually illuminated without regard to minimizing circadian disruption of its occupants, including the infant. Several research studies have shown, however, that compared to infants who remained in constant light or constant darkness, infants exposed to cycled light (bright during the day and dim at night) spent more time sleeping while in the NICU and after discharge, had a greater rate of weight gain while in the NICU and after discharge, were able to be fed orally sooner, spent fewer days on the ventilator, and displayed enhanced motor coordination.6-9 In another study, cycled light significantly reduced fussing and crying behavior, and there was a trend toward higher motor activity during daytime for those infants exposed to cycled light, compared with infants cared for in dim light conditions.2 In that study there was no significant difference between groups observed for sleep behavior, but there was a trend to improve daily weight gain during neonatal care compared with those who remained in continuous dim light.
The hypothesized mechanism for these beneficial effects of cycled light was that fetal circadian rhythms in the third trimester of pregnancy are imposed exogenously by the mother via the hormone melatonin which is detected in the placenta and via changes in the mother's core body temperature. If exogenous rhythms promote circadian rhythm expression in a fetus, then environmental lighting in the NICU could serve as this exogenous factor, facilitating circadian rhythms in premature infants. Tenreiro et al. examined whether circadian rhythmicity originates from the neonate's own body clock, or if it was imposed by external factors such as lighting.5 Heart rate and skin temperature were used as measures of circadian rhythms. The infants who received cycled lighting showed improved circadian rhythms by these measures. The authors concluded that neonates seem to benefit from a rhythmic environment, recommending further investigation of neonates in an NICU environment with strong circadian cues.
In terms of rest-activity rhythms, Rivkees et al. analyzed the number of movements made per day and assessed whether there was a diurnal variation in movement and showed that while distinct day-night differences in activity were not seen in control subjects over the first 10 days at home, intervention group subjects were more active during the day than at night.4 Mirmiran et al. investigated the effect of intermediate nursery illumination on circadian rhythm and sleep development of preterm infants and found that circadian rhythms and sleep showed significant development with age, but there was no environmental lighting effect; however the authors noted there was no measurement of light exposure in the NICU prior to intervention.3
Psychological Message Requirements
Lighting is also psychological. Families entering an NICU with light bulbs hanging from a ceiling might have second thoughts about the care provided to the infant by the hospital even though those light bulbs provide adequate light for visual performance and alertness. In this context, lighting is not really independent of the furnishings and architecture. A “good” lighting system illuminating dirt and stains on the walls will certainly send the wrong message to families. Moreover the psychological factors associated with lighting are not completely divorced from those factors affecting the visual and non-visual systems. Glare from the lighting system can also connote a poor commitment by hospital administrators to creating a comforting environment.
Windows play an ever-growing role in the NICU. They obviously provide light for the visual and non-visual systems, but a view to the outside environment is highly desirable in any building,65 including the NICU. As noted before, however, window treatments need to be part of the decision to include windows in the NICU because direct sunlight can reduce visibility and may even harm the developing retina of the infant.
Finally, architectural tastes change. A hospital concerned with message needs to be sensitive to these changing expectations and regularly review the “message” that lighting can send to occupants.
Putting It Together for Design
In a discussion of current trends in NICU design, Rizzo et al.1 suggested new lighting design practices in modern NICUs, evaluating the effect of lighting on newborns, families, and health care professionals. Modern NICUs are being designed as a series of single-family rooms, rather than a large ward housing multiple infants, which requires a re-evaluation of lighting. Engineering recommendations for hospital ward lighting rose from 200-300 lux in 1947 to 1000 lux in 1970, and it had been hypothesized that higher ambient light levels could cause medical problems for both infants at risk of retinopathy of prematurity (ROP) and nightshift workers whose circadian rhythms are disrupted by bright light. It is important to point out that while ROP was believed to be associated with ambient lighting in earlier studies, a direct causal relationship between ambient light exposure and ROP has never been established. For nightshift workers, while a direct link between light at night exposure in the work environment and suppression of the hormone melatonin has not been established mainly due to lack of sufficient studies measuring personal light exposures and melatonin levels, it is recommended that ambient lighting conditions during the nightshift be reduced to avoid melatonin suppression in workers. Task lights should be used to increase light levels on the workplane and on specific, critical tasks, such as insertion of an IV.
After the ambient, white lighting has been dimmed to < 30 lux of white light at the cornea to minimize circadian disruption, arrays of light around computer screens or light goggles delivering saturated red light (40-60 lux at the eye of narrowband light sources peaking at 640 nm) can be used intermittently throughout the shift to enhance alertness.64 Exposure to these supplemental red light sources enhances alertness, just like a cup of coffee. However, while red light has been shown to increase alertness in laboratory studies, these lighting solutions designed to maintain alertness at night without disrupting circadian rhythms of workers need to be tested in the field. Finally, it is essential to point out that bright illumination of critical or demanding visual tasks during emergencies should still be available to the professional staff through local, task lights or through a manual override of the ambient lighting system.
Rizzo et al. recommend using dynamic ambient lighting that would change in color and light level over the course of the day to support the circadian system, dedicated task lighting for critical visual tasks, and simple, intuitive lighting controls; for example, a preset wall control for ambient lighting, labeled “Daytime,” “Nighttime” and “Off” would suffice.1
Many NICUs are redesigning their wards based on recommendations similar to those proposed by Rizzo et al. These recommendations for lighting in the NICU correspond with the Recommended Standards for Newborn ICU Design published by the Eighth Consensus Conference on Newborn ICU Design in 2012.11 These standards encourage hospitals to re-envision their current NICU to follow standards that have been determined to improve care for infants, and provide more space for caregivers and families. Studies should be conducted specifically evaluating the efficacy of these new designs on neonates.
For entrainment of the infants, White and colleagues developed a “baby blanket” (Lumi-nate; Figure 4) that can be placed on top of the incubator to deliver various lighting schemes based on specific needs. Lumi-nate is a multi-functional examination light source for NICUs that can control the five main characteristics of the illumination it provides: intensity, distribution, spectrum, duration, and timing. Lumi-nate is intended to provide illumination adaptable to multiple scenarios, including in-room examination, remote observation via digital sensing cameras, direct skin examination, vein detection for catheter and needle administration, administration of critical procedures, and circadian entrainment. It delivers light for circadian entrainment of the infant and meets all of the recommended lighting standards (see below) for good color rendering and minimum flicker. Future field studies need to be performed to test the effectiveness of this lighting system in maintaining entrainment and improving visibility of visual tasks in the NICU.
Figure 4.

The prototype Lumi-nate illumination system for incubators. The lighting system is designed to fit on top of an infant incubator and under a blanket that is often used to cover the incubator. When used as a procedural light, the Lumi-nate system provides diffuse white illumination of 2000 lux to minimize shadows. When positioned under a blanket the light is programmed to provided “cool” illumination of 600 lux during the morning and “warm” illumination of 6 lux at night to promote circadian entrainment for the infant. The Lumi-nate system always provides flicker-free illumination of excellent color rendering. The Wi-Fi enabled camera imaging system can display remotely on a tablet.
Recommended Lighting Standards
The impact of the NICU environment on the development and well-being of infants was first formally recognized in 1976.10 Since the early 1990s, design guidelines for the NICU have been developed and periodically updated through a large, dedicated and multidisciplinary professional committee. Their recommendations have become the de facto standard among, professional staff, architects and building engineers. The latest standard, Recommended Standards for Newborn ICU Design (http://www3.nd.edu/∼nicudes), was published in 2012.11
The standard reflects well-established principles of good lighting but in addition reflects the concepts generated by recent published research. The current standard provides five recommendations directly or indirectly related to lighting:
- Standard 14: Ambient lighting in Infant Care Area
- 10 to 600 lux with adjustable, manual controls
- CRI > 80 and 80 < GAI < 100
- Standard 15: Procedure lighting in Infant Care Area
- 2000 lux for critical areas while protecting the infant's developing retinae
- Standard 16: Illumination of Support Areas
- 300 lux
- Standard 17: Daylighting
- Access to daylight, with controls to limit direct sunlight
- Standard 18: Floor surfaces
- Gloss factor less than 30 (semi-gloss paint); maximum diffuse reflectance from floor of 40%
A thorough discussion of these standards is provided by White et al.10 and will not be repeated here except for two revisions that have been implemented in the 2012 recommendations.
The color rendering requirements in 2012 only specify limits on two metrics, CRI and GAI whereby CRI must be greater than 80 and GAI must be greater than 80 and less than 100.
Flicker had essentially been eliminated in the 1990s with the widespread adoption of high frequency ballasts to operate fluorescent lamps. With the advent of SSL, however, the potential for flicker has increased. For this reason, the current 2012 standard sets a limit on flicker. The standard requires flicker to be no more than that produced by an incandescent lamp operated on alternating current, that is, no more than 13% modulation at 120 Hz.
Also, as discussed in this document, there are few specifics with regard to regulating non-visual effects of infants and professional staff. Since the data are clear that 24-hour cycled light improves developmental and health outcomes in infants, professional staff should go beyond the standard. Bright light during the day and dim light at night is a simple recipe for both infants and dayshift workers. Thus with regard to the infant, blankets should not be used to cover incubators during the day, but probably should during the night. Minimizing circadian disruption of nightshift workers is more challenging, but a delayed lighting schedule with red supplemental lighting, as discussed in the present paper, should be considered for implementation for the nightshift workers.64
Acknowledgments
Rebekah Mullaney and Dennis Guyon of the Lighting Research Center are acknowledged for their technical and editorial assistance.
Appendix: A brief guide for managing light exposure for dayshift and nightshift workers
Retinal exposure to a regular, 24-hour light-dark pattern is essential for circadian entrainment and thus, for good sleep, performance and well-being. Dayshift workers should receive high levels of illumination during the day (at least 200-300 lux at the cornea of a white light source), particularly during the morning after awakening, and minimize bright light during the evening and at night. In the NICU, morning exposure to at least 200-300 lux at the cornea from a white light should ensure entrainment as long as light levels are below about 30 lux at the cornea several hours before bedtime. It should be noted that luminous tablets can provide levels higher than this, so dimming these items is also important for maintaining entrainment.
Generally, both dayshift and nightshift workers have hormonal rhythms consistent with a dayshift lifestyle. This is because nightshift workers still follow many daytime social activities like supporting their children's school schedule, conducting commerce, and engaging in recreational activities. Attempting to shift nightshift workers to a nocturnal lifestyle is impractical and undesirable for most people. Therefore the following suggestions are offered as a compromise. Assuming a 7:00 pm to 7:00 am nightshift, provide bright, white light in the NICU (200-300 lux at the eye) until 9:00 or 10:00 pm followed by dim white light (30 lux at the eye) in the NICU until the end of the shift. During the dim light period, professional staff should be provided access to saturated red light (640-nm light) of at least 40-60 lux in rest areas or at their workspaces. Exposure to these lights can be used intermittently throughout the night, similar to drinking a cup of coffee. Red light exposure will not affect circadian rhythms, but will provide an alerting stimulus.64
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rizzo P, Rea M, White R. Lighting for Today's Neonatal Intensive Care Unit. Newborn and Infant Nursing Reviews. 2010;10(2):107–113. doi: 10.1053/j.nainr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyer C, Huber R, Fontijn J, et al. Cycled light exposure reduces fussing and crying in very preterm infants. Pediatrics. 2012;130(1):e145–151. doi: 10.1542/peds.2011-2671. [DOI] [PubMed] [Google Scholar]
- 3.Mirmiran M, Baldwin RB, Ariagno RL. Circadian and sleep development in preterm infants occurs independently from the influences of environmental lighting. Pediatr Res. 2003;53(6):933–938. doi: 10.1203/01.PDR.0000061541.94620.12. [DOI] [PubMed] [Google Scholar]
- 4.Rivkees SA, Mayes L, Jacobs H, Gross I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. 2004;113(4):833–839. doi: 10.1542/peds.113.4.833. [DOI] [PubMed] [Google Scholar]
- 5.Tenreiro S, Dowse HB, D'Souza S, et al. The development of ultradian and circadian rhythms in premature babies maintained in constant conditions. Early Hum Dev. 1991;27(1-2):33–52. doi: 10.1016/0378-3782(91)90026-y. [DOI] [PubMed] [Google Scholar]
- 6.Mann NP, Haddow R, Stokes L, Goodley S, Rutter N. Effect of night and day on preterm infants in a newborn nursery: randomised trial. Br Med J (Clin Res Ed) 1986;293(6557):1265–1267. doi: 10.1136/bmj.293.6557.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boo NY, Chee SC, Rohana J. Randomized controlled study of the effects of different durations of light exposure on weight gain by preterm infants in a neonatal intensive care unit. Acta Paediatr. 2002;91(6):674–679. doi: 10.1080/080352502760069106. [DOI] [PubMed] [Google Scholar]
- 8.Miller CL, White R, Whitman TL, O'Callaghan MF, Maxwell SE. The effects of cycled versus noncycled lighting on growth and development in preterm infants. Infant Behavior and Development. 1995;18(1):87–95. [Google Scholar]
- 9.Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks' gestation have improved growth in cycled light compared with continuous near darkness. J Pediatr. 2002;140(2):192–199. doi: 10.1067/mpd.2002.121932. [DOI] [PubMed] [Google Scholar]
- 10.White RD. Recommended standards for the newborn ICU. J Perinatol. 2007;27:S4–S19. doi: 10.1038/sj.jp.7211837. [DOI] [PubMed] [Google Scholar]
- 11.Recommended Standards for Newborn ICU Design. Clearwater Beach, FL: Committee to Establish Recommended Standards for Newborn ICU Design; 2012. Eighth Census Conference on Newborn ICU Design. [Google Scholar]
- 12.Rea MS, editor. IESNA Lighting Handbook: Reference and Application. 9th. New York, NY: Illuminating Engineering Society of North America; 2000. [Google Scholar]
- 13.Rea MS, Ouellette MJ. Relative visual performance: A basis for application. Light Res Technol. 1991;23(3):135–144. [Google Scholar]
- 14.Rea MS. Some Basic Concepts and Field Applications for Lighting, Color, and Vision. In: Nadler M, Miller D, Nadler D, editors. Glare and Contrast Sensitivity for Clinicians. New York, NY: Springer-Verlag; 1990. pp. 120–138. [Google Scholar]
- 15.Bullough JD, Radetsky LC. Roadway lighting, relative visual performance and safety. Illuminating Engineering Society Annual Conference; November 2-4, 2014; Pittsburgh, PA. [Google Scholar]
- 16.Bullough JD, Donnell ET, Rea MS. To illuminate or not to illuminate: Roadway lighting as it affects traffic safety at intersections. Accid Anal Prev. 2013;53:65–77. doi: 10.1016/j.aap.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Fotios SA. Lamp colour properties and apparent brightness: a review. Light Res Technol. 2001;33(3):163–178. [Google Scholar]
- 18.Bullough JD, Radetsky LC, Besenecker UC, Rea MS. Influence of spectral power distribution on scene brightness at different light levels. LEUKOS. 2014;10(1):3–9. [Google Scholar]
- 19.Rea MS, Radetsky LC, Bullough JD. Toward a model of outdoor lighting scene brightness. Light Res Technol. 2011;43(1):7–30. [Google Scholar]
- 20.Bullough JD, Radetsky LC, Rea MS. Testing a provisional model of scene brightness with and without objects of different colours. Light Res Technol. 2011;43(2):173–184. [Google Scholar]
- 21.Rea M, Mou X, Bullough J. Scene brightness of illuminated interiors. Light Res Technol. 2015:1477153515581412. [Google Scholar]
- 22.Rea MS, Freyssinier JP. Color rendering: Beyond pride and prejudice. Color Research & Application. 2010;35(6):401–409. [Google Scholar]
- 23.Rea MS, Freyssinier-Nova JP. Color rendering: A tale of two metrics. Color Research & Application. 2008;33(3):192–202. [Google Scholar]
- 24.Rea MS. Value Metrics for Better Lighting. Bellingham, WA: SPIE; 2013. [Google Scholar]
- 25.Judd DB. A flattery index for artificial illuminants. Illuminating Engineering. 1967;62:593–598. [Google Scholar]
- 26.Nickerson D. Light sources and color rendering. JOSA. 1960;50(1):57–68. [Google Scholar]
- 27.Rea MS, Freyssinier JP. White lighting. Color Research & Application. 2011;38(2):82–92. [Google Scholar]
- 28.Bullough JD. Luminance versus luminous intensity as a metric for discomfort glare. Warrendale, PA: 2011. [Google Scholar]
- 29.Dowling JE. The site of visual adaptation. Science. 1967;155(3760):273–279. doi: 10.1126/science.155.3760.273. [DOI] [PubMed] [Google Scholar]
- 30.Bullough J, Hickcox KS, Klein T, Lok A, Narendran N. Detection and acceptability of stroboscopic effects from flicker. Light Res Technol. 2012;44(4):477–483. [Google Scholar]
- 31.Bullough J, Hickcox KS, Klein T, Narendran N. Effects of flicker characteristics from solid-state lighting on detection, acceptability and comfort. Light Res Technol. 2011;43(3):337–348. [Google Scholar]
- 32.Wilkins A, Nimmo-Smith I, Slater A, Bedocs L. Fluorescent lighting, headaches and eyestrain. Light Res Technol. 1989;21(1):11–18. [Google Scholar]
- 33.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 34.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50(2):213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Rea MS, Figueiro MG, Bierman A, Hamner R. Modelling the spectral sensitivity of the human circadian system. Light Res Technol. 2012;44(4):386–396. [Google Scholar]
- 37.Figueiro MG, Rea MS, Bullough JD. Circadian effectiveness of two polychromatic lights in suppressing human nocturnal melatonin. Neurosci Lett. 2006;406:293–297. doi: 10.1016/j.neulet.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 38.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45(4):421–434. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueiro MG, Hunter CM, Higgins PA, et al. Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep Health. 2015;1(4):322–330. doi: 10.1016/j.sleh.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Figueiro MG, Plitnick B, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression and agitation in persons with Alzheimer's disease and related dementia living in long-term care facilities. Clinical Interventions in Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueiro M, Plitnick B, Rea M. Research Note: A self-luminous light table for persons with Alzheimer's disease. Light Res Technol. 2015:1477153515603881. doi: 10.1177/1477153515603881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueiro M, Rea M. Office lighting and personal light exposures in two seasons: Impact on sleep and mood. Light Res Technol. 2014:1477153514564098. [Google Scholar]
- 43.Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon. 2013;44(2):237–240. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(1):S23–28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nurminen T. Shift work and reproductive health. Scand J Work Environ Health. 1998:28–34. [PubMed] [Google Scholar]
- 47.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I. Night-shift work and risk of colorectal cancer in the Nurses' Health Study. J Natl Cancer Inst. 2003;95:825–888. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 48.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses' Health Study. J Natl Cancer Inst. 2001;93(20):1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 49.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization (WHO) International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization (WHO); 2010. [Google Scholar]
- 51.Figueiro MG, White RD. Health consequences of shift work and implications for structural design. J Perinatol. 2013;33:S17–S23. doi: 10.1038/jp.2013.7. [DOI] [PubMed] [Google Scholar]
- 52.Dumont M, Benhaberou-Brun D, Paquet J. Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms. 2001;16:502–511. doi: 10.1177/074873001129002178. [DOI] [PubMed] [Google Scholar]
- 53.Dumont M, Lanctot V, Cadieux-Viau R, Paquet J. Melatonin production and light exposure of rotating night workers. Chronobiol Int. 2012;29(2):203–210. doi: 10.3109/07420528.2011.647177. [DOI] [PubMed] [Google Scholar]
- 54.Miller D, Figueiro MG, Bierman A, Schernhammer E, Rea MS. Ecological measurements of light exposure, activity and circadian disruption. Light Res Technol. 2010;42:271–284. doi: 10.1177/1477153510367977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith MR, Fogg LF, Eastman CI. A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. Sleep. 2009;32(11):1481–1489. doi: 10.1093/sleep/32.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Figueiro MG, Plitnick B, Rea MS. The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Medicine. 2014;15(12):1554–1564. doi: 10.1016/j.sleep.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50(3):583–588. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 58.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115(1):75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 59.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11(6):453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Figueiro MG, Bierman A, Plitnick B, Rea MS. Preliminary evidence that both blue and red light can induce alertness at night. BMC Neurosci. 2009;10:105. doi: 10.1186/1471-2202-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plitnick B, Figueiro MG, Wood B, Rea MS. The effects of red and blue light on alertness and mood at night. Light Res Technol. 2010;42(4):449–458. [Google Scholar]
- 62.Sahin L, Figueiro MG. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol Behav. 2013;116-117:1–7. doi: 10.1016/j.physbeh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Sahin L, Wood B, Plitnick B, Figueiro MG. Daytime light exposure: Effects on biomarkers, measures of alertness, and performance. Behav Brain Res. 2014;274:176–185. doi: 10.1016/j.bbr.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Figueiro MG, Sahin L, Wood B, Plitnick B. Light at night and measures of alertness and performance: Implications for shift workers. Biological Research for Nursing. 2016;18(1):90–100. doi: 10.1177/1099800415572873. [DOI] [PubMed] [Google Scholar]
- 65.Heerwagen J, Heerwagen D. Lighting and psychological comfort. Lighting Design and Application. 1986;6:47–51. [Google Scholar]


