Abstract
Background
This study aimed to examine the association between renal recovery status at hospital discharge after acute kidney injury (AKI) and long-term mortality following transcatheter aortic valve replacement (TAVR).
Methods
We screened all adult patients who survived to hospital discharge after TAVR for aortic stenosis at a quaternary referral medical center from January 1, 2008, through June 30, 2014. An AKI was defined as an increase in serum creatinine level of 0.3 mg/dL or a relative increase of 50% from baseline. Renal outcome at the time of discharge was evaluated by comparing the discharge serum creatinine level to the baseline level. Complete renal recovery was defined as no AKI at discharge, whereas partial renal recovery was defined as AKI without a need for renal replacement therapy at discharge. No renal recovery was defined as a need for renal replacement therapy at discharge.
Results
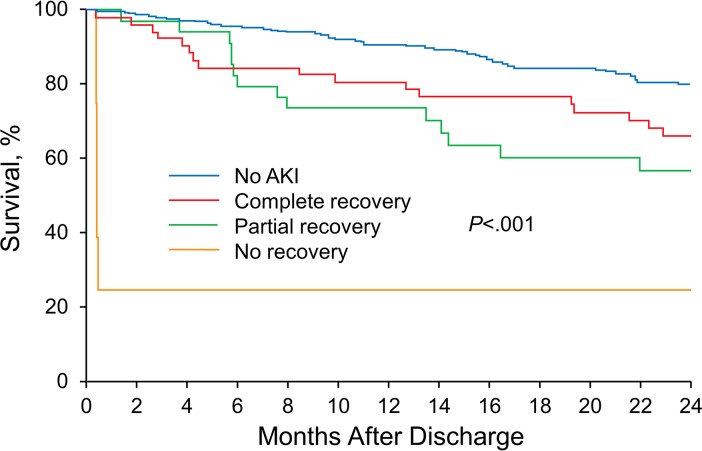
The study included 374 patients. Ninty-eight (26%) patients developed AKI during hospitalization: 55 (56%) had complete recovery; 39 (40%), partial recovery; and 4 (4%), no recovery. AKI development was significantly associated with increased risk of 2-year mortality (hazard ratio [HR], 2.20 [95% CI, 1.37–3.49]). For patients with AKI, the 2-year mortality rate for complete recovery was 34%; for partial recovery, 43%; and for no recovery, 75%; compared with 20% for patients without AKI (P < .001). In adjusted analysis, complete recovery (HR, 1.87 [95% CI, 1.03–3.23]); partial recovery (HR, 2.65 [95% CI, 1.40–4.71]) and no recovery (HR, 10.95 [95% CI, 2.59–31.49]) after AKI vs no AKI were significantly associated with increased risk of 2-year mortality.
Conclusion
The mortality rate increased for all patients with AKI undergoing TAVR. A reverse correlation existed for progressively higher risk of death and the extent of AKI recovery.
Introduction
Transcatheter aortic valve replacement (TAVR) is a revolutionary, relatively new catheter-based technique for treating patients with severe aortic stenosis who are at high risk for a surgical aortic valve replacement [1]. In addition, recent studies have also suggested the potential use of TAVR for patients at intermediate [2,3] and low risk [4], as well as for a subset of patients whose aortic stenosis was more technically challenging to repair surgically [5,6]. Although TAVR is considered less invasive than surgical repair, patients undergoing TAVR usually have more comorbidities [7]. Nearly 25% of patients die within the first year after the procedure, despite the recent advances and success of TAVR technology [1,8–10].
Acute kidney injury (AKI) after TAVR is common (reported for up to 57% of patients) [7,11,12] and is independently associated with a higher risk of mortality [12,13]. Recently, the impact of renal recovery after AKI on patient outcomes has been comprehensively described [14–16]. Recovery status has been reported to affect long-term outcomes of critically ill patients [15], and patients who have complete renal recovery have significantly better survival rates than patients who have only partial renal recovery or no recovery [15,17,18]. Poor baseline renal function and AKI have been shown to predict outcomes after TAVR [11,19]; however, little is known about the impact of renal recovery following AKI. Therefore, we aimed to examine the association between renal recovery status at hospital discharge after AKI and long-term mortality following TAVR.
Materials and methods
Patient population
A retrospective cohort study was conducted at Mayo Clinic Hospital in Rochester, Minnesota, from January 1, 2008, through June 30, 2014. Adult patients (≥18 years) were included who survived to hospital discharge after having a TAVR. Patients were excluded if they had dialysis within 14 days before the procedure or they did not provide research authorization. This study was approved by the Mayo Clinic Institutional Review Board and informed consent was waived for patients who provided research authorization.
Data collection
Manual and automated retrieval of institutional electronic health records were performed to collect demographic, clinical, laboratory, echocardiographic, procedural, and postprocedural data. The estimated percentage for 30-day mortality was determined by using the Society of Thoracic Surgeons risk score, which calculates risk via a model that uses patient demographic characteristics, preoperative clinical characteristics, and type of procedure being performed [20–22]. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate the estimated glomerular filtration rate (eGFR) [23].
Definition of AKI and renal recovery
AKI was defined according to the criteria of the Valve Academic Research Consortium-2 (VARC-2), ie, an increase in serum creatinine of at least 0.3 mg/dL within 48 hours or a relative increase of at least 50% within 7 days from baseline [24]. The most recent outpatient value from 7 to 180 days before the procedure was used as baseline. If that value was not available, the lowest serum creatinine value within 5 days before the procedure was used.
Renal outcome was assessed at the time of hospital discharge by comparing the serum creatinine at discharge to the serum creatinine at baseline. Complete renal recovery was defined as no AKI at patient discharge, whereas partial renal recovery was defined as AKI without the need for renal replacement therapy at discharge. No renal recovery was defined as a need for renal replacement therapy at discharge.
Clinical outcome
The primary outcome was all-cause mortality 2 years after hospital discharge. We reviewed the Mayo Clinic electronic health records and the Social Security Death Index to determine patient deaths [25]. The secondary outcomes included the change in eGFR at 2 years after discharge and initiation of dialysis.
Statistical analysis
Continuous variables were described as mean±standard deviation and were compared using analysis of variance. Categorical variables were reported as counts with percentages and were compared using the χ2 test. Kaplan-Meier analysis was used to generate curves for patient survival after hospital discharge, which were compared using the the log-rank test. Cox proportional hazards regression analysis was used to assess the association between degree of renal recovery and 2-year mortality after hospital discharge, adjusting for a priori defined covariates, Society of Thoracic Surgeons risk score, age, eGFR, and arterial approach. Results of 2-sided tests with a P value less than .05 were considered statistically significant. All analyses were performed with JMP statistical software (SAS Institute Inc, Cary, NC).
Results
We screened 390 patients who underwent TAVR for aortic stenosis during the study period. Sixteen patients were excluded: 11 died in the hospital, 3 received dialysis before their procedure, and 2 did not provide research authorization. Therefore, 374 patients were included in the study.
The baseline characteristics of the study cohort are shown in Table 1. Their mean age was 81±8 years, and 56% were men. The mean Society of Thoracic Surgeons risk score was 8.5±6.2. The mean eGFR was 55±21 mL/min/1.73m2. The approach to TAVR was transfemoral in 50% of patients, transapical in 45%, and transaortic in 5%. Table 1 also includes the clinical characteristics of the patients according to the degree of renal recovery.
Table 1. Baseline characteristicsa.
| Characteristics | Complete Recovery (n = 55) | Partial Recovery (n = 39) | No Recovery (n = 4) | No AKI (n = 276) | P Value |
|---|---|---|---|---|---|
| STS risk score | 10.1±6.3 | 8.6±5.2 | 11.2±3.7 | 8.1±6.3 | .12 |
| Age, y | 82±8 | 81±7 | 82±4 | 81±8 | .77 |
| Male sex | 27 (49) | 26 (67) | 3 (75) | 152 (55) | .32 |
| White | 51 (93) | 39 (100) | 3 (75) | 269 (97) | .01 |
| BMI (kg/m2) | 30.7±7.3 | 30.0±6.6 | 34.8±9.8 | 30.3±7.6 | .65 |
| eGFR (mL/min/1.73 m2) | 47±20 | 50±26 | 32±14 | 58±19 | < .001 |
| NYHA class III-IV | 51 (93) | 34 (87) | 4 (100) | 234 (85) | .37 |
| Comorbidity | |||||
| Diabetes mellitus | 22 (40) | 24 (62) | 3 (75) | 104 (38) | .02 |
| Hypertension | 51 (93) | 37 (95) | 4 (100) | 246 (89) | .54 |
| Dyslipidemia | 48 (87) | 38 (97) | 4 (100) | 245 (89) | .31 |
| Myocardial infarction | 15 (27) | 18 (46) | 3 (75) | 98 (36) | .10 |
| Congestive heart failure | 36 (65) | 21 (54) | 4 (100) | 152 (55) | .16 |
| Stroke | 15 (27) | 14 (36) | 1 (25) | 79 (29) | .80 |
| Peripheral vascular disease | 35 (64) | 23 (59) | 4 (100) | 157 (57) | .29 |
| Anemia | 2 (4) | 1 (3) | 1 (25) | 6 (2) | .04 |
| Chronic lung disease | 33 (60) | 25 (64) | 2 (50) | 174 (63) | .92 |
| Smoking within 1 y | 1 (2) | 2 (5) | 0 (0) | 8 (3) | .80 |
| Prior cardiac intervention | |||||
| Percutaneous coronary intervention | 25 (45) | 28 (72) | 4 (100) | 135 (49) | .01 |
| Cardiac surgery | 21 (38) | 16 (41) | 2 (50) | 135 (49) | .45 |
| CABG | 21 (38) | 15 (38) | 2 (50) | 122 (44) | .78 |
| Valve surgery | 14 (25) | 11 (28) | 1 (25) | 56 (20) | .63 |
| Aortic valve surgery | 1 (2) | 0 (0) | 0 (0) | 9 (3) | .64 |
| Echocardiographic finding | |||||
| Ejection fraction | 56±13 | 55±13 | 39±5 | 57±14 | .05 |
| Aortic valve gradient | 46±16 | 48±12 | 40±9 | 49±14 | .27 |
| Aortic valve insufficiency | 33 (60) | 19 (49) | 2 (50) | 149 (54) | .74 |
| Mitral valve dysfunction | 45 (82) | 27 (69) | 3 (75) | 216 (78) | .53 |
| Preoperative medication | |||||
| ACE inhibitor/AR blocker | 22 (40) | 15 (38) | 1 (25) | 115 (42) | .90 |
| β-Blocker | 33 (60) | 29 (74) | 4 (100) | 192 (70) | .22 |
| Statin | 38 (69) | 29 (74) | 3 (75) | 205 (74) | .88 |
| Aspirin | 35 (64) | 28 (72) | 4 (100) | 212 (77) | .13 |
| Normal sinus rhythm | 34 (62) | 28 (72) | 2 (50) | 205 (74) | .21 |
| Elective surgery | 50 (91) | 38 (97) | 3 (75) | 266 (96) | .06 |
| Arterial approach | .003 | ||||
| Transfemoral | 17 (31) | 13 (33) | 2 (50) | 156 (57) | |
| Transapical | 36 (65) | 24 (62) | 2 (50) | 105 (38) | |
| Transaortic | 2 (4) | 2 (5) | 0 (0) | 15 (5) | |
| Surgery duration, min | 132±68 | 125±48 | 113±42 | 126±47 | .81 |
| RBC transfusion | 21 (38) | 19 (49) | 4 (100) | 78 (28) | .001 |
| Intra-aortic balloon pump | 1 (2) | 0 (0) | 1 (25) | 1 (0.3) | < .001 |
Abbreviations: ACE, angiotensin-converting enzyme; AR, angiotensin II receptor; BMI, body mass index; CABG, coronary artery bypass graft surgery; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; RBC, red blood cell; STS, Society of Thoracic Surgeons.
a Continuous variables are reported as mean ± SD, categorical variables as count (percentage).
During the study period, 98 (26%) patients developed AKI, of whom 55 (56%) had a complete renal recovery, 39 (40%) had partial renal recovery, and 4 (4%) had no renal recovery. The development of AKI was significantly associated with increased risk of 2-year mortality (hazard ratio [HR], 2.20 [95% CI, 1.37–3.49]). The 2-year mortality rate for patients with AKI was 34% for patients who had complete recovery, 43% for patients with a partial recovery, and 75% for patients with no renal recovery vs 20% for patients without AKI (P < .001) (Fig 1). In an adjusted analysis, complete recovery (HR, 1.87 [95% CI, 1.03–3.23]), partial recovery (HR, 2.65 [95% CI, 1.40–4.71]), and no recovery (HR, 10.95 [95% CI, 2.59–31.49]) after AKI were significantly associated with increased risk of 2-year mortality compared with complete recovery for patients who did not have AKI (Table 2). A subgroup analysis based on status of chronic kidney disease (GFR <60 mL/min/m2) and arterial approach showed similar results (Tables 3 and 4).
Fig 1. Kaplan-Meier curve showing 2-year follow-up stratified by recovery status after acute kidney injury (AKI).
Table 2. Hazard ratios of renal recovery status for 2-year mortality.
| Renal Recovery Status | 2-Year Mortality, % | Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratioa (95% CI) | P Value |
|---|---|---|---|---|---|
| No acute kidney injury | 20 | 1 (ref) | 1 (ref) | ||
| Complete recovery | 34 | 1.90 (1.06–3.22) | .03 | 1.87 (1.03–3.23) | .04 |
| Partial recovery | 43 | 2.59 (1.38–4.55) | .004 | 2.65 (1.40–4.71) | .004 |
| No recovery | 75 | 12.26 (2.98–33.45) | .002 | 10.95 (2.59–31.49) | .003 |
a Adjusted for Society of Thoracic Surgeons risk score, age, estimated glomerular filtration rate, and arterial approach.
Table 3. Hazard ratios of renal recovery status for 2-year mortality: Subgroup analysis based on GFR.
| Renal Recovery Status | 2-Year Mortality, % | Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratioa (95% CI) | P Value |
|---|---|---|---|---|---|
| GFR <60 mL/min/m2 (n = 223) | |||||
| No acute kidney injury | 20 | 1 (ref) | 1 (ref) | ||
| Complete recovery | 34 | 2.02 (1.01–3.83) | .047 | 1.84 (0.89–3.59) | .10 |
| Partial recovery | 48 | 2.97 (1.41–5.83) | .006 | 2.97 (1.36–6.11) | .01 |
| No recovery | 75 | 12.13 (2.89–34.58) | .003 | 9.81 (2.27–29.63) | .005 |
| GFR ≥60 mL/min/m2 (n = 151) | |||||
| No acute kidney injury | 21 | 1 (ref) | 1 (ref) | ||
| Complete recovery | 31 | 1.68 (0.49–4.36) | .37 | 1.48 (0.33–4.56) | .56 |
| Partial recovery | 32 | 1.78 (0.42–5.11) | .38 | 1.86 (0.54–4.93) | .29 |
| No recovery | … | … | … | … | … |
Abbreviation: GFR, glomerular filtration rate.
a Adjusted for Society of Thoracic Surgeons risk score, age, estimated glomerular filtration rate, and arterial approach.
Table 4. Hazard ratios of renal recovery status for 2-year mortality: Subgroup analysis based on arterial approach.
| Renal Recovery Status | 2-Year Mortality, % | Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratioa (95% CI) | P Value |
|---|---|---|---|---|---|
| Femoral Approach (n = 188) | |||||
| No acute kidney injury | 19 | 1 (ref) | 1 (ref) | ||
| Complete recovery | 25 | 1.47 (0.43–3.76) | .49 | 1.22 (0.35–3.24) | .72 |
| Partial recovery | 33 | 2.24 (0.66–5.74) | .17 | 2.41 (0.71–6.21) | .14 |
| No recovery | 100 | 265.89 (24.62–5851.46) | < .001 | 227.87 (20.28–5130.88) | < .001 |
| Nonfemoral Approach (n = 186) | |||||
| No acute kidney injury | 22 | 1 (ref) | 1 (ref) | ||
| Complete recovery | 39 | 1.97 (0.97–3.84) | 0.06 | 2.20 (1.06–4.38) | .04 |
| Partial recovery | 48 | 2.64 (1.20–5.41) | 0.02 | 3.13 (1.37–6.71) | .008 |
| No recovery | 50 | 3.96 (0.22–18.79) | 0.27 | 4.33 (0.24–22.24) | .25 |
a Adjusted for Society of Thoracic Surgeons risk score, age, estimated glomerular filtration rate
At 2 years after discharge, the eGFR of patients had changed as follows: no AKI, −2.7±13.9 mL/min/1.73 m2; complete recovery, −3.6±11.0 mL/min/1.73 m2; and partial recovery, −7.5±15.2 mL/min/1.73 m2. No significant differences were found in eGFR changes between groups (P = .18). No patients without AKI needed dialysis after discharge, whereas 2 patients who had complete or partial recovery required dialysis after discharge.
Discussion
To our knowledge, this is the first report of rates of different renal recovery patterns after TAVR-associated AKI. Although most patients (56%) who developed AKI after TAVR completely recovered, 44% of patients did not have full recovery of their renal function (40%, partial recovery; 4%, no recovery) at hospital discharge. Long-term outcomes after TAVR were associated with development of AKI and subsequent recovery status at hospital discharge. Patients who did not develop AKI after TAVR had an 80% survival rate at 2 years. Of patients who developed AKI after TAVR, those who had a complete recovery had a 66% survival rate at 2 years, whereas the survival rate for those who did not recover from AKI was only 25%.
The Acute Disease Quality Initiative 16 Workgroup recently published a consensus report that emphasized the importance of renal recovery after AKI [14]. Recovery of renal function after AKI has been shown to be an independent determinant of morbidity and mortality in patients who are hospitalized, including in an intensive care unit, or who had cardiac surgery [17,26–28]. Studies done in intensive care units have shown that renal function did not completely recover in 8% to 26% of patients who had AKI by the time of hospital discharge [15,29–31]. In our study, which evaluated renal recovery in patients undergoing TAVR, we found that 44% of patients who had AKI following TAVR did not completely recover. This number is also higher than the previously reported nonrecovery rates among critically ill patients or patients undergoing cardiac surgery, which ranged from 9% to 39% (15, 28–31,32). However, patients who undergo TAVR have many comorbidities [7] that may affect the recovery of kidney function after AKI [31].
Previous studies showed that approximately 20% of survivors of AKI develop long-term complications characterized by chronic kidney disease, cardiovascular complications, physical limitations, disabilities, and greater mortality [32–35]. In our current study, survivors of AKI whose kidney function did not recover had an approximately 11-fold increased risk of 2-year mortality than those who did not have AKI. Our findings underscore the urgent need for better strategies to prevent AKI and to improve the care of patients with AKI who do not recover or only partially recover their kidney function. Because AKI after TAVR is multifactorial and related to pre-, intra-, and postoperative factors (such as patients’ comorbidities, baseline renal function, and catheter-based techniques), a multidisciplinary approach with careful risk stratification of patients and multiple targeted interventions should be incorporated into potential strategies to prevent TAVR-related AKI [36].
Our study has several limitations. The study design was retrospective and observational, which can create selection biases. In addition, the cohort was predominantly white, and a urine output criterion was not used for AKI diagnosis. Oliguria and increased creatinine levels are more common in patients with AKI who do not completely recover renal function [37]. Urinary output data were not used to identify AKI because the data were unavailable for most patients, and a substantial number of patients received diuretics after their procedure. A prospective, multicenter investigation is needed to address these limitations. Finally, the study had the disadvantage of our basing definitions of renal recovery on serum creatinine and/or eGFR because serum creatinine and true GFR have a nonlinear relation. Definitions of renal recovery based on serum creatinine might also be confounding [38].
Conclusion
After TAVR, patients with AKI had an increased risk for mortality, and a reverse correlation existed between progressively higher risk of death and the extent of AKI recovery. Future studies are needed to identify better strategies to improve care for AKI survivors.
Abbreviations
- AKI
acute kidney injury
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- TAVR
transcatheter aortic valve replacement
- VARC-2
Valve Academic Research Consortium-2
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no funding for this work.
References
- 1.Hinterbuchner L, Strohmer B, Hammerer M, Prinz E, Hoppe UC, Schernthaner C. Frailty scoring in transcatheter aortic valve replacement patients. Eur J Cardiovasc Nurs. 2016. October;15(6):384–97. Epub 2015 Jul 27. doi: 10.1177/1474515115596640 [DOI] [PubMed] [Google Scholar]
- 2.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. ; SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017. April 6;376(14):1321–31. Epub 2017 Mar 17. doi: 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. ; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016. April 28;374(17):1609–20. Epub 2016 Apr 2. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 4.Arora S, Strassle PD, Ramm CJ, Rhodes JA, Vaidya SR, Caranasos TG, et al. Transcatheter versus surgical aortic valve replacement in patients with lower surgical risk scores: a systematic review and meta-analysis of early outcomes. Heart Lung Circ. 2017. August;26(8):840–845. Epub 2017 Jan 24. doi: 10.1016/j.hlc.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Harjai KJ, Grines CL, Paradis JM, Kodali S. Transcatheter aortic valve replacement: the year in review 2016. J Interv Cardiol. 2017. April;30(2):105–13. Epub 2017 Mar 2. doi: 10.1111/joic.12372 [DOI] [PubMed] [Google Scholar]
- 6.Enezate TH, Kumar A, Fadel MA, Patel M, Al Dadah A, Omran J. Transcatheter versus surgical aortic valve replacement in patients with non-high surgical risk severe aortic stenosis: a systematic review. Cardiovasc Revasc Med. 2017. February 20. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Thongprayoon C, Cheungpasitporn W, Srivali N, Harrison AM, Gunderson TM, Kittanamongkolchai W, et al. AKI after transcatheter or surgical aortic valve replacement. J Am Soc Nephrol. 2016. June;27(6):1854–60. Epub 2015 Oct 20. doi: 10.1681/ASN.2015050577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. ; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012. May 3;366(18):1686–95. Epub 2012 Mar 26. doi: 10.1056/NEJMoa1200384 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds MR, Hong JC. What we are learning from transcatheter aortic valve replacement risk prediction models. J Am Coll Cardiol. 2016. October 25;68(17):1878–80. doi: 10.1016/j.jacc.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 10.Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, et al. ; FRANCE 2 Investigators. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012. May 3;366(18):1705–15. doi: 10.1056/NEJMoa1114705 [DOI] [PubMed] [Google Scholar]
- 11.Cheungpasitporn W, Thongprayoon C, Kashani K. Transcatheter aortic valve replacement: a kidney’s perspective. J Renal Inj Prev. 2016. January 18;5(1):1–7. doi: 10.15171/jrip.2016.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thongprayoon C, Cheungpasitporn W, Srivali N, Ungprasert P, Kittanamongkolchai W, Greason KL, et al. Acute kidney injury after transcatheter aortic valve replacement: a systematic review and meta-analysis. Am J Nephrol. 2015;41(4–5):372–82. Epub 2015 Jun 19. doi: 10.1159/000431337 [DOI] [PubMed] [Google Scholar]
- 13.Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, et al. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis. 2014. February;107(2):133–9. Epub 2014 Feb 17. doi: 10.1016/j.acvd.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 14.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. ; Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017. April;13(4):241–57. Epub 2017 Feb 27. doi: 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
- 15.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017. March 15;195(6):784–91. doi: 10.1164/rccm.201604-0799OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellum JA. Persistent acute kidney injury. Crit Care Med. 2015. August;43(8):1785–6. doi: 10.1097/CCM.0000000000001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F, et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015. August;43(8):e269–75. doi: 10.1097/CCM.0000000000001077 [DOI] [PubMed] [Google Scholar]
- 18.Ronco C, Ferrari F, Ricci Z. Recovery after acute kidney injury: a new prognostic dimension of the syndrome. Am J Respir Crit Care Med. 2017. March 15;195(6):711–4. doi: 10.1164/rccm.201610-1971ED [DOI] [PubMed] [Google Scholar]
- 19.Edwards FH, Cohen DJ, O’Brien SM, Peterson ED, Mack MJ, Shahian DM, et al. ; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016. April 1;1(1):46–52. doi: 10.1001/jamacardio.2015.0326 [DOI] [PubMed] [Google Scholar]
- 20.Shahian DM, Edwards FH. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: introduction. Ann Thorac Surg. 2009. July;88(1 Suppl):S1 doi: 10.1016/j.athoracsur.2009.05.054 [DOI] [PubMed] [Google Scholar]
- 21.O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. ; Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2: isolated valve surgery. Ann Thorac Surg. 2009. July;88(1 Suppl):S23–42. doi: 10.1016/j.athoracsur.2009.05.056 [DOI] [PubMed] [Google Scholar]
- 22.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. ; Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3: valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009. July;88(1 Suppl):S43–62. doi: 10.1016/j.athoracsur.2009.05.055 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009. May 5;150(9):604–12. Erratum in: Ann Intern Med. 2011 Sep 20;155(6):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. ; Valve Academic Research Consortium (VARC)-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg. 2012. November;42(5):S45–60. Epub 2012 Oct 1. doi: 10.1093/ejcts/ezs533 [DOI] [PubMed] [Google Scholar]
- 25.Wentworth DN, Neaton JD, Rasmussen WL. An evaluation of the Social Security Administration master beneficiary record file and the National Death Index in the ascertainment of vital status. Am J Public Health. 1983. November;73(11):1270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagshaw SM. Epidemiology of renal recovery after acute renal failure. Curr Opin Crit Care. 2006. December;12(6):544–50. doi: 10.1097/01.ccx.0000247444.63758.0b [DOI] [PubMed] [Google Scholar]
- 27.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013. February;8(2):194–202. Epub 2012 Nov 2. doi: 10.2215/CJN.06480612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swaminathan M, Hudson CC, Phillips-Bute BG, Patel UD, Mathew JP, Newman MF, et al. Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg. 2010. April;89(4):1098–104. doi: 10.1016/j.athoracsur.2009.12.018 [DOI] [PubMed] [Google Scholar]
- 29.Bell M, Granath F, Schon S, Ekbom A, Martling CR; SWING. Continuous renal replacement therapy is associated with less chronic renal failure than intermittent haemodialysis after acute renal failure. Intensive Care Med. 2007. May;33(5):773–80. Epub 2007 Mar 16. doi: 10.1007/s00134-007-0590-6 [DOI] [PubMed] [Google Scholar]
- 30.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005. August 17;294(7):813–8. doi: 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 31.Schiffl H, Fischer R. Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant. 2008. July;23(7):2235–41. Epub 2008 Apr 11. doi: 10.1093/ndt/gfn182 [DOI] [PubMed] [Google Scholar]
- 32.Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015. January 6;5(1):e006497 Erratum in: BMJ Open. 2015;5(1):e006497corr1. doi: 10.1136/bmjopen-2014-006497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palant CE, Amdur RL, Chawla LS. The acute kidney injury to chronic kidney disease transition: a potential opportunity to improve care in acute kidney injury. Contrib Nephrol. 2016;187:55–72. Epub 2016 Feb 8. doi: 10.1159/000442365 [DOI] [PubMed] [Google Scholar]
- 34.Zhao XJ, Zhu FX, Li S, Zhang HB, An YZ. Acute kidney injury is an independent risk factor for myocardial injury after noncardiac surgery in critical patients. J Crit Care. 2017. June;39:225–31. Epub 2017 Jan 26. doi: 10.1016/j.jcrc.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 35.Villeneuve PM, Clark EG, Sikora L, Sood MM, Bagshaw SM. Health-related quality-of-life among survivors of acute kidney injury in the intensive care unit: a systematic review. Intensive Care Med. 2016. February;42(2):137–46. Epub 2015 Dec 1. doi: 10.1007/s00134-015-4151-0 [DOI] [PubMed] [Google Scholar]
- 36.Villablanca PA, Ramakrishna H. The renal frontier in TAVR. J Cardiothorac Vasc Anesth. 2017. June;31(3):800–803. Epub 2017 Feb 6. doi: 10.1053/j.jvca.2017.02.030 [DOI] [PubMed] [Google Scholar]
- 37.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015. September;26(9):2231–8. Epub 2015 Jan 7. doi: 10.1681/ASN.2014070724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srisawat N, Murugan R, Wen X, Singbartl K, Clermont G, Eiam-Ong S, et al. Recovery from acute kidney injury: determinants and predictors. Contrib Nephrol. 2010;165:284–91. Epub 2010 Apr 20. doi: 10.1159/000313768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.