Abstract
Background
Though South Asians experience cardiovascular disease (CVD) and risk factors at early age, distribution of CVD risks across the socioeconomic spectrum remains unclear.
Methods
We analysed 2011 Centre for cArdiometabolic Risk Reduction in South Asia survey data including 16,288 non-pregnant adults (≥20 years) that are representative of Chennai and Delhi, India, and Karachi, Pakistan. SES was defined by education (up to primary schooling, high/secondary schooling, ≥college graduate); wealth tertiles (low, middle, high), and occupation (not working outside home, semi/unskilled, skilled, white-collar work). We estimated age- and sex-standardized prevalence of behavioural (daily fruit/vegetables; tobacco use), weight (BMI; waist-to-height ratio), and metabolic risk factors (diabetes, hypertension, hypercholesterolemia; hypo-HDL; and hypertriglyceridemia) by each SES category.
Results
Across cities, 61.2% and 16.1% completed secondary and college educations, respectively; 52.8% reported not working, 22.9% were unskilled, 21.3% were skilled, and 3.1% were white-collar workers. Low fruit/vegetable intake, smoked, and smokeless tobacco use were more prevalent in lowest education, wealth, and occupation (for men only) groups compared to higher SES counterparts, while weight-related risks (BMI 25.0–29.9 and ≥30 kg/m2; WHtR ≥0.5) were more common in higher educated, wealthy groups, and technical/professional men. Higher prevalence of diabetes, hypertension, and dyslipidaemias were observed in more educated and affluent groups, with unclear patterns across occupation groups.
Conclusions
SES-CVD patterns are heterogeneous, suggesting customized interventions for different SES groups may be warranted. Different metabolic risk factor prevalence patterns across SES indicators may signal on-going epidemiological transition in South Asia.
Keywords: cardiovascular risk factors, South Asians, socioeconomic status, global cardiovascular health
Background
South Asia is one of the world’s most densely populated regions and is fast becoming an epicentre of atherosclerotic cardiovascular diseases (CVD). Between 1990 and 2010, healthy years lost due to ischemic heart disease and stroke increased by 73% and 54% in the region, outpacing global increases of 30% and 21%, respectively.[1] Moreover, South Asians (people from India, Pakistan, Bangladesh, Nepal) experience first myocardial infarction (MI) almost 10 years younger compared to people from other countries; this is largely explained by younger onset of preventable CVD risk factors.[2]
CVDs impose high direct (e.g., health expenditures) and indirect costs (e.g., lost productivity) on households, potentially stifling micro- and macro-economic development.[3] Since South Asia’s population pyramid is relatively young by world standards[4] and experiencing rapid economic transitions and burgeoning chronic disease burdens, this raises concerns about CVDs contributing to widening socioeconomic status (SES) differences.[5] While data from high-income countries now show consistent patterns (low socioeconomic groups experiencing higher CVD risk factors, events, and mortality),[6] these patterns were reversed in the first half of the 20th century.[7,8] Studies from South Asia in the most recent two decades show conflicting SES-CVD relationships, [9,10] which may indicate on-going epidemiological and socioeconomic transitions. Most studies examining these relationships in South Asia have used single SES measures, even though SES is multi-dimensional, reflecting human, social, and material capital; opportunities; and access to resources.[11] Furthermore, current literature is dominated by studies from India,[10,12–15] with few data originating in other South Asian countries; and few studies report data from representative populations.[14,16]
We examined relationships between different SES indicators and prevalence of objectively-measured (not just self-reported) CVD risk factors collected from representative population samples living in three mega-cities in South Asia: typical north Indian (Delhi), south Indian (Chennai), and Pakistani (Karachi) metropolises. These recent data will aid priority-setting and intervention development amidst rapid sociodemographic transitions in the region.
Methods
Study Population
We analysed cross-sectional survey data collected in 2011 from the baseline of the Centre for Cardio-metabolic Risk Reduction in South Asia (CARRS) cohort.[17] The study used multi-stage cluster random sampling to recruit 16,288 non-pregnant adults aged ≥20 years that are representative of Chennai and Delhi (India), and Karachi (Pakistan). All participants (or their next of kin or caregivers in the case of children or illiteracy) provided written informed consent prior to enrolment. Data were collected through household interviews in local languages and standardized clinical examinations and fasting blood sample collection either at home (Karachi) or at local camps (Chennai, Delhi). For the three cities together, response rates were 94.7% for questionnaire completion and 84.3% for bio-specimens. Missing observations for each CVD risk factor are reported in Appendix 1.
Data were collected by trained field teams using standardized techniques. All sites have accredited laboratories and participated in an external quality assurance scheme that standardized findings to a central laboratory (All India Institute of Medical Sciences [AIIMS]). Further details regarding study design, methods, and instruments are published separately.[17] The study received approval for human subjects’ research from the Ethics Committees of the Public Health Foundation of India and AIIMS (Delhi), Madras Diabetes Research Foundation (Chennai), Aga Khan University (Karachi), and Emory University (Atlanta).
Study Measures and Definitions
We used interview responses to classify participants’ age (20–44 years, 45–64years, ≥65years), and sex (male, female).
Exposures: SES indicators
Education level provides a characterization of human and social capital, while wealth reflects material household capital. Income is also a reflection of material worth, but literature suggests that wealth may be a more reliable indicator than self-reported income in some countries.[18] Occupation offers a composite reflection of one’s earning capacity and educational background.
Based on participant responses, we categorized highest education level attained (up to primary schooling, high or secondary schooling, college graduate and higher); monthly income (<Indian Rupees [INR] 10,000 [equivalent to US$200], INR10,000–20,000 [US$200–400], and >INR20,000 [US$400]); and occupation (not working outside home, semi- or unskilled, skilled, white-collar). To characterize household wealth, we used principal components analysis that weighted different household amenities (separate cooking room and toilet facilities) and assets (television, refrigerator, washing machine, microwave, mixer-grinder, mobile phone, DVD player, computer, car, motor cycle, bicycle). Components in wealth scores had a KMO statistic of 0.62;1 total scores were categorized in tertiles (lowest tertile representing poorest and highest tertile representing wealthiest). For each SES indicator, categories were considered mutually exclusive and collectively exhaustive, such that each person was only classified in a single category based on their highest reported SES level.
Outcomes: Cardiovascular Risk Factors
The INTERHEART and INTERSTROKE studies showed that 80–90% of all coronary and cerebrovascular events, respectively, could be attributed to tobacco use, hypertension, diabetes, dyslipidaemia, physical inactivity, excess body weight, alcohol, poor diet, and psychosocial stresses.[19,20] For this study, measurement and definitions of each behavioural (diet, tobacco use), weight-related (body mass index [BMI], central adiposity), and metabolic CVD risk factor (diabetes, hypertension, dyslipidaemias) are described below.
Behavioural risk factors were assessed by questionnaires. Using a modified food frequency questionnaire, we estimated proportions reporting low (<2 servings/day) fruit and vegetable consumption. Self-reported current tobacco use was classified as smoked (e.g., cigarettes, bidis) and smokeless tobacco (e.g., chewed).[21]
For weight-related risks, we calculated BMI (weight/height squared [kg/m2]) and reported Asia-specific and international overweight and obesity categories (23.0–24.9 [Asian overweight], 25.0–29.9 [international overweight], ≥30.0 kg/m2 [international obesity]). Central adiposity was defined as waist-to-height ratio (WHtR)≥0.5; this is considered a stronger predictor of CVD than waist circumference alone.[22]
Trained staff took two blood pressure (BP) readings after participants sat at rest for five minutes using an electronic sphygmomanometer (Omron Dalian Company, Liaoning, China). A third measurement was taken if the difference between the first two systolic or diastolic measurements was more than 10mmHg or 5mmHg, respectively. For analysis, we used the mean of the first two measurements, or the second and third measurements if a third measurement was obtained.. Hypertension was defined by self-report or measured BP≥140/90mmHg. Venous fasting plasma glucose (FPG) was estimated using hexokinase/kinetic methods while glycated haemoglobin (HbA1c) was estimated using high-performance liquid chromatography (NGSP standardized). Diabetes was defined by self-report, FPG≥126mg/dl, or HbA1c≥6.5%. Total cholesterol (TC: enzymatic colorimetric cholesterol oxidase peroxidase), high-density lipoprotein cholesterol (HDL; direct), and triglycerides (TG; enzymatic methods) were measured using Roche/Boehringer-Mannheim Diagnostics. Low-density lipoprotein (LDL) cholesterol was calculated using Friedewald’s formula.[23] Dyslipidaemia was defined separately: hypercholesterolemia (self-report, TC≥200mg/dl, or LDL≥130mg/dl); low HDL (<40mg/dl [men] or <50mg/dl [women]); and hypertriglyceridemia (≥150mg/dl).
Statistical analysis
We used Stata (version 12 SE, StataCorp, TX, USA) for data analysis with appropriate sampling weights. We used multiple imputation to account for missing outcomes (shown in Appendix 1). For all variables, separate data sheets were created using chained equations and missing CVD risk factors were predicted using all other available variables. Ten imputed datasets were generated and weighted estimates were pooled.
This analysis included 16,287 non-pregnant adults aged ≥20 years after excluding a single transgender participant. We described demographic and socioeconomic profiles of Chennai, Delhi, and Karachi residents, separately and cumulatively. To select SES exposures, we assessed relationships between different ordinal indicators of SES (education, occupation, income, wealth) using Spearman’s correlation and Wald chi-square tests. We noted highest correlation between income and wealth, moderate correlations between education and other SES measures, and lowest correlation between occupation and other SES measures (Appendix 2). Based on these findings and literature showing low reliability of self-reported income in the region,[18] we selected education, wealth, and occupation as SES exposures.
For each CVD risk factor by SES category, we calculated prevalence estimates which were age- and sex-standardized to the 2010 South Asia regional population. To achieve, this, we used five-year population projection tables (that account for age, sex, mortality, fertility, and migration) separately for India and Pakistan for 2010, provided by the World Bank.[4]
We estimated combined three-city CVD risk factor prevalence for education categories and asset tertiles. For occupation, an overwhelming majority of women (85%) accounted for those not working outside the home, which prompted us to stratify estimates by sex. To examine overall CVD risk, we estimated proportions of participants with 1, 2, or ≥3 CVD risk factors out of the following: tobacco exposure (smoked/smokeless), central adiposity (WHtR≥0.5), diabetes, hypertension, and hypercholesterolemia. We used F tests to assess whether CVD risk factor prevalence monotonically increased or decreased over ordered categories of SES..
We performed a number of sensitivity analyses. To assess influences of missing data, we compared imputed data with crude prevalence from complete case data. To assess how estimates from this young South Asian population compare with other global cities that have different population structures, we calculated prevalence estimates standardized to the 2010 world population.[4] Finally, we examined SES-CVD risk factor relationships by city to examine if patterns differ between cities surveyed.
Results
For the three cities combined, 47.7% of respondents were male and 59.1%, 34.0%, and 6.9% were in the 20–44, 45–64, and ≥65 year old age categories, respectively (Table 1). Of all respondents, 61.2% and 16.1% completed secondary schooling and college degrees, respectively. Majority of respondents (72.5%) reported household incomes <INR10,000 (US$200). Only 3.1% reported working in white-collar professions, 21.3% and 22.9% were in skilled or unskilled occupations, respectively, and 52.8% reported not working outside the home. Age, income, wealth, education, and occupational distributions were different across cities: compared to Chennai and Karachi, Delhi’s population had greater proportions of older, higher income, higher wealth, and skilled or white-collar workers.
Table 1.
Sociodemographic profile of Chennai, Delhi, and Karachi residents aged ≥20 years, CARRS (n=16,287).
| Chennai | Delhi | Karachi | Total | |
|---|---|---|---|---|
| N=6,906 | N=5,364 | N=4,017 | N=16,287 | |
| Age, % | ||||
| 20–44 years | 63.0 | 53.5 | 60.1 | 59.1 |
| 45–64 years | 31.8 | 38.1 | 32.2 | 34.0 |
| ≥65 years | 5.3 | 8.4 | 7.7 | 6.9 |
| Mean age (SE) | 41.7 (0.4) | 44.1 (1.0) | 41.7 (1.4) | 42.3 (0.6) |
| Sex, % | ||||
| Male | 46.1 | 50.1 | 47.1 | 47.7 |
| Female | 53.9 | 49.9 | 52.9 | 52.4 |
| Household Size, % | ||||
| ≤2 | 15.8 | 19.0 | 15.0 | 16.6 |
| 3 to 5 | 73.5 | 61.0 | 49.2 | 62.8 |
| ≥ 6 | 10.7 | 20.0 | 35.8 | 20.7 |
| Mean size (SE) | 4.0 (0.02) | 4.2 (0.03) | 4.9 (0.04) | 4.3 (0.02) |
| Education, % | ||||
| Up to Primary School | 18.7 | 21.5 | 30.9 | 22.6 |
| High school/Secondary | 70.4 | 54.5 | 54.8 | 61.2 |
| College graduate | 11.0 | 24.0 | 14.3 | 16.1 |
| Mean years completed | 7.5 (0.1) | 8.7 (0.3) | 7.1 (0.3) | 8.0 (0.1) |
| Household Income, % | ||||
| < INR 10,000 (US$200) | 83.5 | 51.3 | 82.1 | 72.5 |
| INR 10,000–20,000 | 12.5 | 21.5 | 15.1 | 16.1 |
| > INR 20,000 (US$400) | 4.0 | 27.2 | 2.8 | 11.4 |
| Assets Owned, % | ||||
| Low | 46.1 | 31.4 | 16.8 | 33.9 |
| Medium | 37.0 | 24.3 | 47.1 | 35.3 |
| High | 17.0 | 44.3 | 36.2 | 30.8 |
| Occupation | ||||
| Not working | 49.9 | 51.0 | 60.0 | 52.8 |
| Skilled worker | 20.6 | 24.1 | 18.9 | 21.3 |
| Semi- or unskilled | 28.4 | 19.8 | 17.7 | 22.9 |
| White collar | 1.2 | 5.2 | 3.4 | 3.1 |
Abbreviations: CARRS, Centre for cArdiometabolic Risk Reduction in South Asia; SE, standard error; INR, Indian Rupees; US$, United States dollars (exchange rate during period: US$1=INR50)
Behavioural risk factors were more common among lower educated and less-wealthy individuals (Table 2). From low to high education categories, low fruit/vegetable intake was less prevalent (primary: 68.0% [95%CI: 65.6, 70.2] vs. secondary: 60.3% [58.6, 62.0] vs. graduate: 48.0% [45.1, 50.9]; p-value <0.001), tobacco smoking was less prevalent (primary: 17.0% [15.5, 18.5] vs. secondary: 12.2% [11.3, 13.0] vs. graduate: 7.0% [5.8, 8.2]; p<0.001), and smokeless tobacco use was less prevalent (primary: 20.9% [18.7, 23.2] vs. secondary: 13.9% [12.8, 15.1] vs. graduate: 4.8% [3.8, 5.7]; p<0.001). Similarly, from low to high wealth, low fruit/vegetable intake (low: 68.0% [66.2, 69.8] vs. middle: 61.4% [59.3, 63.5] vs. high: 50.5% [48.4, 52.6]; p<0.001), tobacco smoking (low: 16.1% [14.9, 17.2] vs. middle: 11.9% [11.1, 12.7] vs. high: 9.3% [8.3, 10.4]; p<0.001), and smokeless tobacco use (low: 19.3% [17.6, 21.0] vs. middle: 14.3 [12.9, 15.7] vs. high: 6.8% [5.8, 7.8]; p<0.001) were all less common.
Table 2.
Age- and sex- standardized CVD risk factor prevalence* among Chennai, Delhi, and Karachi residents aged ≥20 years by education level and household assets, CARRS (n=16,287)
| Education Level | Asset Tertiles | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Up to Primary (n=3604) | High / Secondary (n=9924) | Graduate or higher (n=2759) | p-value | Low (n=5431) | Medium (n=5722) | High (n=5134) | p-value | |
| Low F&V Intake | 68.0 (65.6, 70.2) | 60.3 (58.6, 62.0) | 48.0 (45.1, 50.9) | <0.001 | 68.0 (66.2, 69.8) | 61.4 (59.3, 63.5) | 50.5 (48.4, 52.6) | <0.001 |
| Smoked Tobacco Use | 17.0 (15.5, 18.5) | 12.2 (11.3, 13.0) | 7.0 (5.8, 8.2) | <0.001 | 16.1 (14.9, 17.2) | 11.9 (11.1, 12.7) | 9.3 (8.3, 10.4) | <0.001 |
| Smokeless Tobacco | 20.9 (18.7, 23.2) | 13.9 (12.8, 15.1) | 4.8 (3.8, 5.7) | <0.001 | 19.3 (17.6, 21.0) | 14.3 (12.9, 15.7) | 6.8 (5.8, 7.8) | <0.001 |
|
| ||||||||
| BMI 23–24.9 kg/m2 | 14.2 (11.7, 16.6) | 14.8 (13.8, 15.9) | 17.8 (15.5, 20.2) | 0.038 | 14.5 (13.3, 15.7) | 15.1 (13.9, 16.4) | 16.0 (14.5, 17.5) | 0.130 |
| BMI 25–29.9 kg/m2 | 24.7 (22.4, 27.1) | 30.6 (29.3, 31.9) | 34.6 (31.9, 37.4) | <0.001 | 24.2 (22.6, 25.8) | 31.3 (29.8, 32.8) | 36.5 (33.4, 38.6) | <0.001 |
| BMI≥30 kg/m2 | 12.2 (10.3, 14.2) | 16.0 (15.1, 16.9) | 16.5 (14.7, 18.4) | 0.002 | 9.9 (8.6, 11.1) | 14.9 (13.7, 16.1) | 20.6 (19.1, 22.0) | <0.001 |
| WHtR≥0.5 | 59.6 (56.9, 62.3) | 65.6 (64.4, 66.8) | 69.7 (67.5, 72.0) | <0.001 | 56.7 (54.9, 58.4) | 66.2 (64.6, 67.7) | 73.9 (72.3, 75.5) | <0.001 |
|
| ||||||||
| Diabetes | 18.1 (16.6, 19.6) | 22.1 (21.1, 23.1) | 22.3 (20.6, 24.1) | <0.001 | 18.3 (16.8, 19.8) | 21.0 (19.7, 22.2) | 26.0 (24.5, 27.5) | <0.001 |
| Hypertension | 26.9 (25.1, 28.8) | 30.4 (29.3, 31.5) | 28.2 (25.9, 30.5) | 0.410 | 26.6 (25.0, 28.2) | 30.0 (28.6, 31.4) | 31.2 (29.8, 32.7) | <0.001 |
| Hypercholesterolemia | 40.0 (37.4, 42.7) | 41.4 (40.0, 42.8) | 44.3 (41.9, 46.7) | 0.025 | 40.7 (38.4, 43.2) | 40.3 (38.9, 41.8) | 44.5 (42.7,46.3) | 0.019 |
| Low HDL | 58.2 (55.5, 60.9) | 60.0 (58.6, 61.5) | 57.8 (55.1, 60.5) | 0.820 | 60.1 (58.1, 62.1) | 59.3 (57.4, 61.1) | 61.5 (59.4, 63.3) | 0.310 |
| Hypertriglyceridemia | 29.1 (26.6, 31.6) | 31.2 (30.0, 32.5) | 33.2 (30.8, 35.5) | 0.012 | 29.7 (27.7, 31.6) | 31.1 (29.5, 32.8) | 34.0 (32.3, 35.7) | 0.0002 |
Abbreviations: CVD, cardiovascular disease; F&V, fruit and vegetable; BMI, body mass index; WHtR, waist-to-height ratio; HDL, high-density lipoprotein cholesterol.
- Diabetes (self-report or fasting blood glucose ≥126mg/dl or glycated hemoglobin ≥6.5%);
- Hypertension (self-report or measured blood pressure ≥140/90mmHg)
- Hypercholesterolemia (self-report or total cholesterol ≥200mg/dl or low-density lipoprotein cholesterol ≥130mg/dl);
- Low HDL (<40mg/dl [males] and <50mg/dl [females]);
- Hypertriglyceridemia (≥150 mg/dl).
With 95% confidence intervals in parentheses
For weight-related risks, from low to high education levels, we noted higher prevalence of overweight ([BMI 25–29.9 kg/m2] primary: 24.7% [22.4, 27.1] vs. secondary: 30.6% [29.3, 31.9] vs. graduate: 34.6% [31.9, 37.4]; p<0.001) and obesity ([BMI≥30 kg/m2] primary: 12.2% [10.3, 14.2] vs. secondary: 16.0% [15.1, 16.9] vs. graduate: 16.5% [14.7, 18.4]; p=0.002) and central adiposity (primary: 59.6% [56.9, 62.3] vs. secondary: 65.6% [64.4, 66.8] vs. graduate: 69.7% [67.5, 72.0]; p<0.001). From low to high wealth, similarly, we noted higher prevalence of overweight (low: 24.2% [22.6, 25.8] vs. middle: 31.3% [29.8, 32.8] vs. high: 36.5% [33.4, 38.6]; p<0.001) and obesity (low: 9.9% [8.6, 11.1] vs. middle: 14.9% [13.7, 16.1] vs. high: 20.6% [19.1, 22.0]; p<0.001). Higher wealth was also associated with higher prevalence of central adiposity (low: 56.7% [54.9, 58.4] vs. middle: 66.2% [64.6, 67.7] vs. high: 73.9% [72.3, 75.5]; p<0.001).
With higher education, there were graded patterns of higher prevalence of diabetes (primary: 18.1% [16.6, 19.6] vs. secondary: 22.1% [21.1, 23.1] vs. graduate: 22.3% [20.6, 24.1]; p<0.001), hypercholesterolemia (primary: 40.0% [37.4, 42.7] vs. secondary: 41.4% [40.0, 42.8] vs. graduate: 44.3% [41.9, 46.7]; p=0.023), and hypertriglyceridemia (primary: 29.1% [26.6, 31.6] vs. secondary: 31.2% [30.0, 32.5] vs. graduate: 33.2% [30.8, 35.5]; p=0.012).. For wealth, similarly, there were clear graded relationships between higher assets and diabetes (low: 18.3% [16.8, 19.8] vs. middle: 21.0% [19.7, 22.2] vs. high: 26.0% [24.5, 27.5]; p<0.001), hypertension (low: 26.6% [25.0, 28.2] vs. middle: 30.0% [28.6, 31.4] vs. high: 31.2% [29.8, 32.7]; p<0.001), hypercholesterolemia (low: 40.7% [38.4, 43.2] vs. middle: 40.3% [38.9, 41.8] vs. high: 44.5% [42.7, 46.3]; p=0.019), and hypertriglyceridemia (low: 29.7% [27.7, 31.6] vs. middle: 31.1% [29.5, 32.8] vs. high: 34.0% [32.3, 35.7]; p<0.001).
Among men, from non-working to white-collar occupations (Table 3), we noted lower prevalence of low fruit and vegetable intake (p<0.001), smoked tobacco (p<0.001), and smokeless tobacco use (p=0.009), with the highest prevalence of each risk among low-skilled workers. The prevalence of overweight (p<0.001), obesity (p=0.019), and central adiposity (p<0.001) were all significantly higher among men in skilled and white-collar occupations. For metabolic risk factors among men in less-skilled to white-collar occupations, there were significant gradations of higher prevalence of hypercholesterolemia (p<0.001), and hypertriglyceridemia (p=0.006); but not for diabetes and hypertension.
Table 3.
Age- and sex-standardized CVD risk factor prevalence* among male and female Chennai, Delhi, and Karachi residents aged ≥ 20 years by occupation, CARRS (n=16,287)
| Male (N=7760) | p-value | Female ( N=8527) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Not Working N=1369 | Low skilled N=2791 | Skilled N=3129 | White Collar N=471 | Not Working N=7283 | Low skilled N=666 | Skilled N=508 | White Collar§ N=70 | |||
| Low F&V Intake | 59.9 (54.6, 65.2) | 62.4 (59.7, 65.2) | 53.3 (50.7, 55.9) | 47.6 (43.3,51.8) | <0.001 | 62.6 (60.4, 64.8) | 69.1 (64.3, 74.0) | 57.0 (52.4, 61.6) | 57.0 (47.2, 66.8) | 0.062 |
| Smoked Tobacco Use | 21.2 (17.2, 25.3) | 29.3 (27.1, 31.5) | 21.0 (19.1, 22.8) | 12.6 (8.5, 16.7) | <0.001 | 0.8 (0.5, 1.1) | 1.4 (0.6, 2.2) | 2.7 (0.0, 6.2) | ||
| Smokeless Tobacco | 19.0 (14.5, 23.4) | 28.0 (25.2, 30.8) | 16.6 (14.6, 18.7) | 13.5 (8.5, 18.5) | 0.009 | 6.6 (5.7, 7.4) | 9.5 (5.6, 13.4) | 3.2 (1.6, 4.7) | ||
|
| ||||||||||
| BMI 23–24.9 kg/m2 | 14.9 (11.3, 18.6) | 17.0 (14.9, 19.0) | 17.5 (15.5,19.5) | 15.4 (11.2, 19.5) | 0.840 | 12.5 (11.4, 13.6) | 14.9 (11.2, 18.5) | 16.7 (11.6, 21.7) | 14.6 (4.7, 24.4) | 0.613 |
| BMI 25–29.9 kg/m2 | 26.8 (21.7, 31.9) | 23.3 (21.2, 25.3) | 31.8 (29.3, 34.3) | 39.8 (32.6, 47.0) | <0.001 | 32.7 (31.3, 34.1) | 26.4 (21.7, 31.1) | 32.1 (25.9, 38.4) | 25.7 (13.1, 38.3) | 0.433 |
| BMI≥30 kg/m2 | 8.7 (5.3, 12.1) | 5.5 (4.5, 6.6) | 10.9 (9.4, 12.4) | 13.1 (9.4, 16.9) | 0.019 | 22.7 (21.2, 24.1) | 13.6 (10.8, 16.4) | 21.8 (17.2, 26.5) | 33.1 (22.1, 44.1) | 0.028 |
| WHtR≥0.5 | 58.6 (54.6, 62.7) | 54.4 (51.7, 57.0) | 64.8 (62.5, 67.1) | 71.7 (65.9, 77.5) | <0.001 | 70.5 (69.0, 72.1) | 57.8 (52.5, 63.1) | 70.8 (66.0, 75.6) | 65.4 (58.5, 72.2) | 0.820 |
|
| ||||||||||
| Diabetes | 22.9 (18.9, 26.9) | 17.3 (15.6, 18.9) | 22.8 (20.9, 24.6) | 22.6 (17.3, 28.0) | 0.650 | 23.3 (22.1, 24.5) | 18.7 (14.7, 22.7) | 20.6 (17.4, 23.7) | 20.7 (13.3, 28.1) | 0.580 |
| Hypertension | 35.5 (29.8, 41.2) | 27.7 (25.6, 29.8) | 31.5 (29.3, 33.7) | 31.5 (26.4, 36.6) | 0.500 | 28.8 (27.7, 30.0) | 25.5 (20.4, 30.8) | 22.1 (19.2, 25.0) | 24.5 (16.5, 32.6) | 0.200 |
| Hypercholesterolemia | 38.9 (35.7, 42.1) | 40.2 (37.6, 42.8) | 44.3 (41.8, 46.8) | 53.4 (48.4, 58.4) | <0.001 | 41.3 (39.6, 42.9) | 38.1 (32.9, 43.4) | 39.2 (34.9, 43.6) | 42.0 (32.4, 51.6) | 0.830 |
| Low HDL | 48.4 (42.7, 54.1) | 48.1 (44.9, 51.3) | 51.7 (48.7, 54.6) | 53.5 (45.1, 61.8) | 0.230 | 69.2 (67.5, 70.9) | 68.0 (61.8, 74.2) | 71.4 (66.8, 76.1) | 73.1 (59.1, 87.2) | 0.470 |
| Hypertriglyceridemia | 32.2 (26.4, 38.1) | 34.4 (31.9, 36.9) | 40.4 (38.2, 42.7) | 45.1 (36.6, 53.7) | 0.006 | 26.2 (24.9, 27.6) | 23.9 (19.0, 28.7) | 31.3 (26.9, 35.7) | 23.1 (12.6, 33.5) | 0.90 |
Abbreviations: CVD, cardiovascular disease; F&V, fruit and vegetable; BMI, body mass index; WHtR, waist-to-height ratio; HDL, high-density lipoprotein cholesterol.
- Diabetes (self-report or fasting blood glucose ≥126mg/dl or glycated hemoglobin ≥6.5%);
- Hypertension (self-report or measured blood pressure ≥140/90mmHg)
- Hypercholesterolemia (self-report or total cholesterol ≥200mg/dl or low-density lipoprotein cholesterol ≥130mg/dl);
- Low HDL (<40mg/dl [males] and <50mg/dl [females]);
- Hypertriglyceridemia (≥150 mg/dl).
With 95% confidence intervals in parentheses
Estimates likely unreliable due to small number of women in this occupation category
The vast majority of women reported not working outside the home. Also, the small numbers of women white-collar professionals meant those estimates were unstable. As such, the only significant difference observed was gradation of higher prevalence of obesity across non-working, low skilled, skilled, and white-collar women (p=0.028)(Table 3).
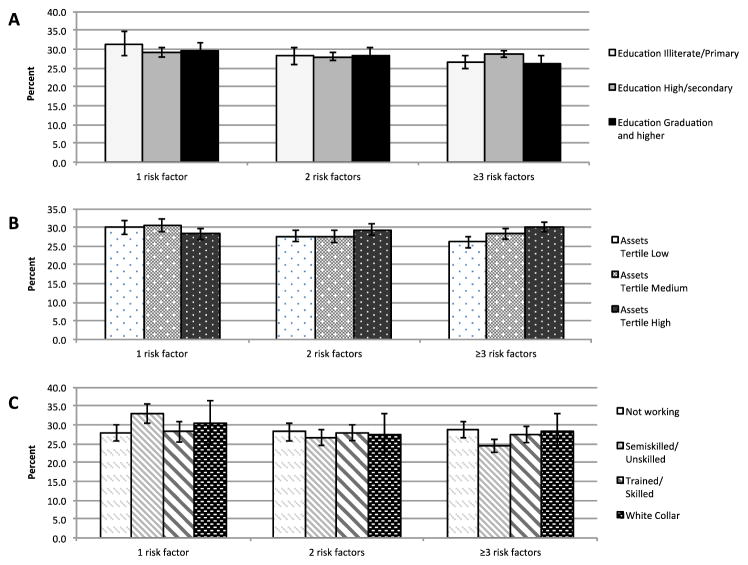
We noted 26–30% of participants across cities had 2 risk factors while 25–31% had ≥3 risk factors (Figure 1); there were no patterned differences in prevalence of 1, 2, or ≥3 risk factors across education, wealth, or occupation groups.
Figure 1.
Age- and sex-standardized prevalence (%)†* of multiple risk factors among Chennai, Delhi, and Karachi residents aged ≥20 years by education (A), assets accumulated (B), and occupation (C), CARRS (n=16,287)
† Proportion of adults with 1, 2, or ≥3 CVD risk factors (out of: current tobacco use [smoked or smokeless], central adiposity [waist-to-height ratio ≥0.5], diabetes, hypertension, or hypercholesterolemia)
* With 95% confidence intervals (error bars)
Wald tests comparing prevalence of 1, 2, and ≥3 CVD risk factors across education, asset, and occupation groups were statistically significant at p=0.008, p<0.001, and p<0.001, respectively.
Comparing our imputed analysis to complete case data (Table 4), there were some differences in estimates and almost all estimates using imputed data were lower than for the complete case analysis, except for low F&V intake (4 percentage points [ppt] higher) and hypercholesterolemia (8 ppt higher).. When standardized to the world’s age and sex distribution, prevalence of central adiposity, diabetes, hypertension, and ≥3 risk factors were all significantly higher –between 1 and 3ppt– than our non-standardized estimates.
Table 4.
Crude and standardized prevalence† of CVD risk factors using complete and imputed data for Chennai, Delhi, and Karachi residents aged ≥20 years, CARRS Surveillance study
| Complete case analysis (n=10,976) | Multiple Imputation analysis (n=16,287) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Crude Prevalence (%) | 95% CIs | Regional Standardized Prevalence (%) | 95% CIs | Global Standardized Prevalence (%) | 95% CIs | |
|
| ||||||
| Mean age (SD): 42.2 (13.3) | Mean age (SD): 42.2 (13.3) | Mean age (SD): 42.6 (15.9) | ||||
| Behavioural Risk Factors | ||||||
| Low F&V intake | 55.9 | (53.5, 58.3) | 59.7 | (58.3, 61.2) | 57.2 | (55.0, 59.4) |
| Tobacco Smoking | 12.9 | (11.4, 14.6) | 12.1 | (11.4, 12.7) | 12.5 | (11.8, 13.2) |
| Smokeless Tobacco Use | 12.1 | (10.7, 13.7) | 13.7 | (12.7, 14.6) | 12.7 | (11.7, 13.7) |
|
| ||||||
| Weight-related Risk Factors | ||||||
| BMI 23–24.9 kg/m2 | 15.3 | (14.5, 16.2) | 15.0 | (14.3, 15.8) | 15.3 | (14.5, 16.0) |
| BMI 25–29.9 kg/m2 | 33.7 | (32.6, 35.0) | 30.5 | (29.5, 31.6) | 31.6 | (30.4, 32.8) |
| BMI≥30 kg/m2 | 18.0 | (16.7, 19.3) | 15.3 | (14.5, 16.1) | 16.0 | (15.1, 16.9) |
| Waist height ratio≥0.5 | 72.2 | (70.6, 73.7) | 65.2 | (64.2, 66.3) | 67.8 | (66.7, 68.9) |
|
| ||||||
| Metabolic Risk Factors | ||||||
| Diabetes | 25.1 | (23.4, 26.9) | 21.7 | (21.0, 22.4) | 25.0 | (24.1, 25.8) |
| Hypertension | 32.3 | (28.9, 34.1) | 29.4 | (28.5, 30.3) | 32.6 | (31.6, 33.6) |
| Hypercholesterolemia | 33.7 | (32.0, 35.4) | 41.9 | (40.9, 43.0) | 43.5 | (42.3, 44.6) |
| HDL < 40(M) <50(F) | 62.6 | (60.8, 64.4) | 59.5 | (58.3, 60.8) | 59.9 | (58.4, 61.3) |
| Hypertriglyceridemia | 33.2 | (31.7, 34.8) | 31.5 | (30.4, 32.6) | 33.1 | (31.9, 34.3) |
|
| ||||||
| Number of Risk Factors | ||||||
| 1 Risk Factor | 29.6 | (28.3, 31.1) | 29.2 | (28.2, 30.2) | 27.3 | (26.3, 28.3) |
| 2 Risk Factors | 28.0 | (26.9, 29.0) | 27.8 | (27.0, 28.7) | 28.0 | (27.2, 28.9) |
| ≥3 Risk Factors | 29.3 | (27.4, 31.3) | 28.7 | (27.9, 29.4) | 32.0 | (31.2, 32.9) |
Prevalence data and 95% confidence intervals are age- and sex-standardized to the world’s population
Abbreviations: CVD, cardiovascular disease; F&V, fruit and vegetable; BMI, body mass index; WHtR, waist-to-height ratio; HDL, high-density lipoprotein cholesterol.
- Diabetes (self-report or fasting blood glucose ≥126mg/dl or glycated hemoglobin ≥6.5%);
- Hypertension (self-report or measured blood pressure ≥140/90mmHg)
- Hypercholesterolemia (self-report or total cholesterol ≥200mg/dl or low-density lipoprotein cholesterol ≥130mg/dl);
- Low HDL (<40mg/dl [males] and <50mg/dl [females]);
- Hypertriglyceridemia (≥150 mg/dl).
Examining data from each city (Appendices 3–6), the same pattern of low fruit/vegetable intake among lower educated was evident in Delhi and Karachi, and higher tobacco use was noted among Delhi and Chennai’s lower educated groups. The pattern of higher overweight, obesity, central obesity, and diabetes prevalence among highest educated was only evident in Delhi. The pattern of less behavioural risk factors and higher overweight, obesity, central obesity, diabetes, hypercholesterolemia, and hypertriglyceridemia among the wealthiest was evident in Delhi, but not other cities. There was no patterning of CVD risk across occupations in any city.
Discussion
Main findings
In this large representative urban South Asian population, smoked and smokeless tobacco use were 2–4 times more prevalent in low education and less-wealthy strata than their respective higher SES counterparts. Low fruit/vegetable intake was also more common in low education and wealth strata. The opposite was true for weight-related risks – prevalence of overweight, obesity, and central adiposity were 1.5 times as prevalent in higher than lower education or wealth groups. Prevalence estimates for diabetes and hypercholesterolemia were statistically higher among the most educated and wealthiest compared to their less-educated and less-wealthy counterparts, but not patterned across occupation groups. Hypertension was more prevalent among more affluent as well. The prevalence of multiple risk factors was not different across SES groups.
Comment
This mixed pattern of higher behavioural risk factors among the socioeconomically disadvantaged and higher weight profiles among advantaged groups are consistent with other reports from developing countries like Mexico,[24] Guatemala,[25] and previous reports from India.[15,16] At the individual and household level, lower SES and corresponding behaviours might reflect a preoccupation with food quantity over quality and low awareness of tobacco’s harms. With higher wealth, there is greater access to calorie-dense foods without corresponding increases in activity levels leading to a positive energy balance, or higher weight may be a socially desirable status symbol.
Our data show different patterns of association between different SES indicators and metabolic risks. This correctly reflects lack of correlation between occupation and other SES indicators in South Asia, as was observed in our analyses (Appendix 2).There were some differences in metabolic risks by education, and higher metabolic risks concentrated among the wealthiest and technical or professional occupations for men. This implies some epidemiological transition may be on going. At the very least, any previous higher prevalence among the educated, as was noted in a review of studies that collected data between 1975 and 2007,[26] is limited only to extreme ends of the SES spectrum. Also, these differences in prevalence of a few percentage points should be taken in the context, as 80% of our populations, which are representative of their respective cities, belong to lower-middle SES groups (only 17% completed tertiary education; 3% were white-collar occupations; and 12% earn ≥US$400/month). As such the absolute number of people in lower-middle SES that are affected by CVD risk is very high. These estimates become even more stark when one considers that India and Pakistan’s current combined urban population outnumbers 450 million; two-thirds of them have excess central adiposity and a quarter have ≥1 metabolic risk factor. Also, lower SES groups in our study had higher prevalence of behavioural risks, which foretells of impending metabolic and cardiovascular abnormalities. It appears CVD has cross-population impacts, no matter which SES group one belongs to.
Several hypotheses have been proposed [27,28] to explain these paradoxical social gradients in behavioural, weight-related, and metabolic risks. SES-CVD risk factor relationships may evolve as societies transition. Our data show that these large city populations are not in early phases of the CVD epidemic where risk-prone behaviours (e.g., tobacco use, poor diets) are confined to the affluent and educated; nor are these cities in advanced epidemiological phases where the advantaged exhibit healthier behaviours and metabolic profiles. Our data suggest that different SES groups have different risk profiles.
We noted that CVD risk factors were very prevalent, despite a fairly young average age across cities, and these estimates would be between 1 and 5 ppt higher when standardized to an older global population. This may reflect a period effect where South Asia’s young adults are exposed to economic and nutritional/lifestyle transitions. Also, this corroborates findings from the INTERHEART study showing higher prevalence of CVD risks in South Asians partly explained the younger age of first MI between South Asian and other countries.[2]
Examining SES-CVD relationships may have implications for societal action and policies as identifying modifiable exposure-outcome associations may help guide the design of appropriate interventions. For example, our findings suggest that identifying and implementing suitable and effective tobacco awareness, prevention, and cessation interventions for lower SES groups may have important benefits. Demand for tobacco has been shown to be especially price-elastic among lower SES groups.[29] Meanwhile, addressing metabolic risks appears to be necessary across SES groups.
Limitations and strengths
These cross-sectional urban data limit causal inferences and generalizations as South Asia has diverse countries, states, and cities. Furthermore, characterizing SES is challenging,[18,30] as is interpretation of SES indicators, especially when gender interacts (e.g., should wealthy homemakers get assigned to high or low-SES groups?). We used categorical exposure variables and covariates, which may reduce power and obscure patterns, especially if some groups have small sample sizes. SES is dynamic and we cannot account for how rapidly participants’ wealth was accrued, or for the considerable heterogeneity within each SES GROUP. Though caste, a hereditary class system in Hindu society, may be another important SES indicator in India, the concept doesn’t exist in Pakistan and was therefore not examined. There may have been slight misclassification of CVD risk factors as self-reported diabetes, hypertension, and dyslipidaemias and measured indicators may not have captured all those actively using medications. Lastly, use of non-overlapping confidence intervals is somewhat conservative.[31]
These concerns are counterbalanced by some important strengths. The cities surveyed are typical Indian and Pakistani metropolises. Compared to existing SES-CVD literature from South Asia, ours is one of a few studies[14,16] that used multiple SES indicators and examined sample populations that are representative of 35 million people from across the SES spectrum. A study of employees at ten Indian industries included a specific, homogenous population representing specific educational and occupation phenotypes.[13] The studies of 11 urban middle-class cities[16] and 1800 rural villages[15] both had response rates <50% and the latter relied on self-reported risk factors. Another study in 20 Andra Pradesh villages had 80% response, but data were self-reported.[32] The use of self-report is particularly prone to underestimation of chronic diseases, as was persuasively shown from 2007 data across six Indian states where differences between self-reported and measured prevalence of five chronic diseases were evident.[14] Data in our study were collected using uniform methods and tools across sites. We performed multiple imputation (adjusted for city-level differences) and sensitivity analyses to test for influences of missing data and city-specific variation.
Conclusions
SES-CVD relationships in South Asia are heterogeneous and still unravelling. The socioeconomically advantaged and disadvantaged in the region face some overlapping and some divergent risks. Addressing these will be challenging. Already, South Asians experience metabolic risks at younger ages and no SES group is spared. These recent data offer a helpful guide towards recognizing future risks as well as customizing interventions to address CVD in the region. Further surveillance is also needed to monitor these transitions and impacts of interventions.
Key Questions.
1. What is already known about this subject?
In South Asia, cardiovascular diseases (CVD) and risk factors are highly prevalent and disease events have been observed to occur at younger age.
Most epidemiological studies examining the distribution of cardiovascular risks across socioeconomic strata (SES) have been confined to a single locality or country, have not had representative populations, and have classified socioeconomic status on the basis of a single indicator (e.g., education or income)
Existing studies are mixed in terms of whether CVD risk factors are more or less common among the lowest SES groups.
2. What does this study add?
Recent data are representative of three major cities from two different countries in South Asia.
Use of multiple SES indicators to characterize the complexity of SES more comprehensively.
Use of multiple objectively collected CVD risk factors permits readers a more accurate and overarching view of CVD risk in urban South Asia.
Acknowledgments
Funding Sources
The CARRS Study and authors MKA, MMK, VM, NT, KMVN, and DP were funded in whole or in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health, Department of Health and Human Services (Contract No. HHSN268200900026C) and UnitedHealth Group (Minneapolis, MN, USA). Authors BB and RS were supported by grant number 1 D43 HD065249 from the Fogarty International Centre and the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the National Institutes of Health.
The authors would like to thank and recognize Drs. Bob Gerzoff and Kai M. Bullard (U.S. Centers for Disease Control and Prevention) for their help with statistical analyses, data management, and multiple imputation.
Footnotes
The Kaiser-Meyer-Olkin (KMO) statistic is a measure of sampling adequacy that compares correlations between variables of interest and reports values between 0 and 1: values>0.6 indicate that variables have enough in common to warrant being combined in a principal components analysis.
The authors have no conflicts of interest to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Contributorship Statement
- Planned, Designed, and Oversaw study
- Steering Committee: Dorairaj Prabhakaran, K. M. Venkat Narayan, K Srinath Reddy, Nikhil Tandon, V. Mohan, Muhammed M. Kadir, Mohammed K. Ali, Vamadevan S Ajay
Conduct and Operations:
- Dorairaj Prabhakaran, Nikhil Tandon, K. M. Venkat Narayan, Mohammed K Ali, S. Roopa, Imran Naeem, R. Pradeepa, M. Deepa
- Coordinating Centre (Delhi): Dorairaj Prabhakaran, Nikhil Tandon, S. Roopa, Vamadevan S Ajay, Deksha Kapoor
- Data management and statistical team: Dimple Kondal, Shivam Pandey, Praggya, Naveen
- Laboratory: Lakshmy Ramakrishnan, Ruby Gupta, Savita
- Authors Mohammed K Ali, Nikhil Tandon, K. M. Venkat Narayan, and Dorairaj Prabhakaran conceived of the analysis and study question, and wrote the manuscript.
- Authors Binukumar and S. Roopa conducted analyses. All other authors contributed to editing and revising of manuscript.
- Authors Mohammed K Ali, Nikhil Tandon, K. M. Venkat Narayan, and Dorairaj Prabhakaran are the guarantors of the data.
References
- 1.IHME. The Global Burden of Disease: Generating Evidence, Guiding Policy - South Asia Regional Edition. Seattle, Washington: Institute for Health Metrics and Evaluation, University of Washington and Human Development Network, The World Bank; 2013. [Google Scholar]
- 2.Joshi P, Islam S, Pais P, Reddy S, Dorairaj P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. Jama. 2007;297:286–294. doi: 10.1001/jama.297.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Stuckler D, Basu S, McKee M. Drivers of inequality in Millennium Development Goal progress: a statistical analysis. PLoS Med. 2010;7:e1000241. doi: 10.1371/journal.pmed.1000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Bank. HNP Stats. 2011. Population Projection Tables by Country and Group. [Google Scholar]
- 5.Alwan AD, Galea G, Stuckler D. Development at risk: addressing noncommunicable diseases at the United Nations high-level meeting. Bull World Health Organ. 2011;89:546–546A. doi: 10.2471/BLT.11.091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrie J, Martikainen P, Shipley M, Marmot M. Self-reported economic difficulties and coronary events in men: evidence from the Whitehall II study. International Journal of Epidemiology. 2005;34:640–648. doi: 10.1093/ije/dyi063. [DOI] [PubMed] [Google Scholar]
- 7.Marmot MG, Adelstein AM, Robinson N, Rose GA. Changing social-class distribution of heart disease. Br Med J. 1978;2:1109–1112. doi: 10.1136/bmj.2.6145.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marmot MG, Kogevinas M, Elston MA. Social/economic status and disease. Annu Rev Public Health. 1987;8:111–135. doi: 10.1146/annurev.pu.08.050187.000551. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KS, Perry CL, Stigler MH, Arora M. Differences in tobacco use among young people in urban India by sex, socioeconomic status, age, and school grade: assessment of baseline survey data. Lancet. 2006;367:589–594. doi: 10.1016/S0140-6736(06)68225-1. [DOI] [PubMed] [Google Scholar]
- 10.Deepa M, Anjana RM, Manjula D, Narayan KM, Mohan V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai Urban Population Study. J Diabetes Sci Technol. 2011;5:918–927. doi: 10.1177/193229681100500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Kaul V, Agrawal A, Guptha S, Gupta VP. Cardiovascular risk according to educational status in India. Prev Med. 2010;51:408–411. doi: 10.1016/j.ypmed.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Reddy KS, Prabhakaran D, Jeemon P, Thankappan KR, Joshi P, et al. Educational status and cardiovascular risk profile in Indians. Proc Natl Acad Sci U S A. 2007;104:16263–16268. doi: 10.1073/pnas.0700933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vellakkal S, Subramanian SV, Millett C, Basu S, Stuckler D, et al. Socioeconomic Inequalities in Non-Communicable Diseases Prevalence in India: Disparities between Self-Reported Diagnoses and Standardized Measures. PLoS One. 2013;8:e68219. doi: 10.1371/journal.pone.0068219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinra S, Bowen LJ, Lyngdoh T, Prabhakaran D, Reddy KS, et al. Sociodemographic patterning of non-communicable disease risk factors in rural India: a cross sectional study. BMJ. 2010;341:c4974. doi: 10.1136/bmj.c4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R, Deedwania PC, Sharma K, Gupta A, Guptha S, et al. Association of educational, occupational and socioeconomic status with cardiovascular risk factors in Asian Indians: a cross-sectional study. PLoS One. 2012;7:e44098. doi: 10.1371/journal.pone.0044098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 21.Nishida C, Uauy R, Kumanyika S, Shetty P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 2004;7:245–250. doi: 10.1079/phn2003592. [DOI] [PubMed] [Google Scholar]
- 22.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Fernald LC, Neufeld LM. Overweight with concurrent stunting in very young children from rural Mexico: prevalence and associated factors. Eur J Clin Nutr. 2007;61:623–632. doi: 10.1038/sj.ejcn.1602558. [DOI] [PubMed] [Google Scholar]
- 25.Martorell R, Khan LK, Hughes ML, Grummer-Strawn LM. Obesity in Latin American women and children. J Nutr. 1998;128:1464–1473. doi: 10.1093/jn/128.9.1464. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian S, Corsi DJ, Subramanyam MA, Davey Smith G. Jumping the gun: the problematic discourse on socioeconomic status and cardiovascular health in India. International Journal of Epidemiology. 2013;42:1410–1426. doi: 10.1093/ije/dyt017. [DOI] [PubMed] [Google Scholar]
- 27.Jeemon P, Reddy KS. Social determinants of cardiovascular disease outcomes in Indians. Indian J Med Res. 2010;132:617–622. doi: 10.4103/0971-5916.73415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hertzman C, Power C, Matthews S, Manor O. Using an interactive framework of society and lifecourse to explain self-rated health in early adulthood. Soc Sci Med. 2001;53:1575–1585. doi: 10.1016/s0277-9536(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 29.Jha P, Chaloupka FJ, Moore J, Gajalakshmi V, Gupta PC, et al. Tobacco Addiction. In: Jamison DTBJ, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. 2. Chapter 46. Washington D.C: World Bank; 2006. [PubMed] [Google Scholar]
- 30.Smith GD, Gordon D, Kelly M, Nandy S, Subramanian SV. Inequalities in Health in India: the methodological construction of indices and measures. UK Department of International Development; 2003. [Google Scholar]
- 31.Schenker N, Gentleman JF. On Judging the Significance of Differences by Examining the Overlap Between Confidence Intervals. The American Statistician. 2001;55:182–186. [Google Scholar]
- 32.Zaman MJ, Patel A, Jan S, Hillis GS, Raju PK, et al. Socio-economic distribution of cardiovascular risk factors and knowledge in rural India. Int J Epidemiol. 2012;41:1302–1314. doi: 10.1093/ije/dyr226. [DOI] [PubMed] [Google Scholar]