Abstract
We examined seizure-susceptibility in a Drosophila model of human epilepsy using optogenetic stimulation of ReaChR (red-activatable channelrhodopsin). Photostimulation of the seizure-sensitive mutant parabss1 causes behavioral paralysis that resembles paralysis caused by mechanical stimulation, in many aspects. Electrophysiology shows that photostimulation evokes abnormal seizure-like neuronal firing in parabss1 followed by a quiescent period resembling synaptic failure and apparently responsible for paralysis. The pattern of neuronal activity concludes with seizure-like activity just prior to recovery. We tentatively identify the mushroom body as one apparent locus of optogenetic seizure initiation. The α/β lobes may be primarily responsible for mushroom body seizure induction.
Keywords: sodium channel, epilepsy, seizure-suppression, red light activable channelrhodopsin
HUMAN seizure disorders are a substantial health problem because of the large number of people affected, the debilitating nature of seizure episodes, and limitations in treatment options. Recently, there has been considerable interest in using optogenetic approaches to study epilepsy, including investigations of basic seizure mechanisms and new treatment options (Tye and Deisseroth 2012; Paz and Huguenard 2015; Zhao et al. 2015). Optogenetics combines optical and genetic methodologies to control and manipulate neuronal excitability with high spatial and temporal resolution. For example, focal seizure-like afterdischarges are driven by photostimulation when excitatory channelrhodopsin2 (ChR2) is expressed in rat hippocampus dentate granule cells (Osawa et al. 2013). In the rat hippocampus, inhibitory halorhodopsin expression can reduce the severity of pilocarpine-induced seizures (Sukhotinsky et al. 2013).
Here, we use the opsin ReaChR (red-activatable channelrhodopsin) to examine seizure-sensitivity in a Drosophila model of human epilepsy. Drosophila bang-sensitive (BS) mutants are particularly sensitive to seizure; they are 5–10 more susceptible to seizure following electrical shock than wild-type flies (Kuebler and Tanouye 2000; Lee and Wu 2002). Three of the BS mutants examined here are parabss1, eas, and sda, whose genes encode a voltage-gated Na+ channel, ethanolamine kinase, and aminopeptidase, respectively. All of the mutations are severely seizure-sensitive, especially parabss1, suggested as a model for human intractable epilepsy, which it resembles in several aspects (Liao et al. 2010; Parker et al. 2011; Schwarz et al. 2016). ReaChR is a red-shifted variant of channelrhodopsin that has been shown to be especially effective for optogenetic activation of neurons in intact freely behaving Drosophila adult flies (Lin et al. 2013; Inagaki et al. 2014). This is thought to be due, in large part, to the higher penetrance of red light through the adult cuticle (5–10% penetrance) compared to blue light used for ChR2 activation (1% penetrance) (Inagaki et al. 2014). We show that activation of ReaChR in BS flies causes behavioral phenotypes of seizure and paralysis. ReaChR optogenetics allows us to examine large numbers of flies with a minimum of mechanical manipulation and under freely behaving conditions. Electrophysiological recording shows that the behavioral phenotypes are due to seizure-like firing of central nervous system neurons followed by synaptic failure. ReaChR under GAL4/UAS (upstream activating sequence) control may be used to localize sites of seizure activation. As an initial example, we show here that activation of mushroom body (MB) neurons alone is sufficient to drive seizure-like neuronal firing. Overall, our study shows that optogenetics can be a powerful method for the study of seizures in the Drosophila model, and implicates the αβ lobe of the (MB) as a locus of seizure initiation.
Materials and Methods
Fly cultures
Drosophila strains were maintained on standard cornmeal–molasses agar medium at room temperature (24°). For optogenetic experiments, newly eclosed flies were transferred into a vial containing food with 400 µM all-trans Retinal (ATR, Sigma [Sigma Chemical], St. Louis, MO). ATR food vials were prepared by heating standard vials to liquefy the food and mixing in the ATR to disperse it evenly. ATR food vials were placed in the dark throughout the preparation and during all genetic crosses. The paralytic (para) gene is located at map position 14E−15A9 and encodes a voltage-gated Na+ channel (Loughney et al. 1989; Ramaswami and Tanouye 1989). The allele used here is a BS paralytic mutation, parabss1, previously named bss1. It is the most seizure-sensitive of BS mutants, the most difficult to suppress by mutation and by drug, and is a model for human intractable epilepsy (Ganetzky and Wu 1982; Parker et al. 2011). The parabss1 allele is a gain-of-function mutation caused by a substitution (L1699F) of a highly conserved residue in the third membrane-spanning segment (S3b) of homology domain IV (Parker et al. 2011). The easily shocked (eas) gene is located at 14B on the cytological map and encodes an ethanolamine kinase (Pavlidis et al. 1994). The BS allele used in this study is easPC80, which is caused by a 2-bp deletion that introduces a frameshift; the resulting truncated protein lacks a kinase domain and abolishes all enzymatic activity (Pavlidis et al. 1994). The slamdance (sda) gene is located at 97D and encodes an aminopeptidase N. The allele used in this study is sdaiso7.8 caused by a 2-bp insertion in the 5′-untranslated region (Zhang et al. 2002). The fly lines for a307-GAL4, Gad1-GAL4, 104Y-GAL4, and c232-GAL4 were obtained from Rod Murphey (Florida Atlantic University), Gero Miesenbock (Yale University), and Roland Strauss (University of Wurzburg), respectively, and maintained as stocks in our laboratory. The fly lines for UAS-ReaChR (BL #53741), 117Y-GAL4 (BL #30814), 121Y-GAL4 (BL #30815), 7B-GAL4 (BL #7365), c305a-GAL4 (BL #30829), 1471-GAL4 (BL #9465), and Cha-GAL4 (BL #6798) were obtained from the Bloomington Drosophila Stock Center.
Behavioral testing for BS phenotypes
Flies were collected using CO2 anesthesia and 10 flies were placed in fresh food vials with foam plugs for testing the following day (Kuebler and Tanouye 2000). For testing, the food vial was stimulated mechanically with a vortex mixer (VWR International) at maximum speed for 10 sec. The number of flies displaying the phenotype of BS paralysis was tallied immediately after the vortex. Behavioral data for each genotype were pooled (n ≈ 50). All flies tested were 3 days posteclosion.
Electrophysiology of the giant fiber (GF) system
In vivo electrophysiological assays and recording of seizure-like activity in the dorsal longitudinal muscle (DLM) were performed as described previously (Kuebler and Tanouye 2000; Lee and Wu 2002). Unanesthetized flies were immobilized with dental wax on a glass microscope slide. Uninsulated tungsten electrodes were used for recordings and stimulation. Procedures for determining evoked seizure threshold were described previously (Kuebler and Tanouye 2000; Lee and Wu 2002). Seizure-like activity was evoked by electrical high-frequency stimuli (HFS) delivered to the brain (0.5-msec pulses at 200 Hz for 300 msec) and monitored by recording from the DLM. Evoked seizure-like activity is observed as uncontrolled, high-frequency (> 100 Hz) firing of DLM motorneurons. To assess GF neural circuit function, the GF was stimulated continuously with single-pulse electrical stimuli delivered to the brain (0.2-msec duration, 0.5 Hz). Recordings were obtained with Axoscope software and were analyzed with Axoscope and Stimfit software (Guzman et al. 2014). All flies tested were 2–3 days posteclosion.
Optogenetics
The experimental system for delivering high-intensity red light light-emitting diode (LED) stimulation with temporal control was adapted from previous descriptions (Pulver et al. 2011; De Vries and Clandinin 2013; Inagaki et al. 2014). The LED (627 nm Rebel, Luxeon Star LEDs) was used with optics (29.8° 10 mm Frosted optic, Carclo) and driven with a Buckpuck DC power converter (700 mA, Luxeon Star LEDs). Light intensity and duration were controlled with an electronic stimulator (S-900, Dagan). Heat sinks were utilized to dissipate excess heat (Luxeon Star LEDs: N50-25B). For behavioral experiments, flies were collected using CO2 anesthesia and 10 flies were placed in each well of a four-well cell culture plate used as a behavioral chamber. Flies were 3 days posteclosion at the time of testing. The behavioral chamber was covered by plastic food wrap to allow penetration of LED illumination and the plastic wrap was perforated to allow fly respiration. After preparation, behavioral chambers were placed in the dark for fly recovery (2 hr). For testing, the behavioral chamber was placed under high-power LEDs mounted on heat sinks. The distance between the behavioral chamber and LED was 1 cm and, during testing, red light illumination was applied continuously for 5 sec. For experiments utilizing photostimulation and electrical recording, flies were mounted for electrophysiology with recording and ground electrodes inserted in the DLMs and abdomen, respectively. The LED was placed 1.5 cm above the fly and triggered by the pulse generator for 500 msec. For stimulation frequency variation, the pulse generator triggered the LED at 1, 10, and 100 Hz frequencies at 50% duty cycle for 1 sec.
Data availability
Drosophila strains are available upon request. All data generated for this study are included in the main text and figures.
Results
ReaChR drives light-sensitive (LS) paralytic behavior in BS mutants
The pan-neuronal driver elavc155-GAL4 was used to drive UAS-ReaChR expression in all parabss1 neurons (genotype: elavc155 parabss1/Y; UAS-ReaChR/+). In the absence of mechanical or optical stimulation, flies double mutant for parabss1 and ReaChR showed no salient behavior abnormalities: feeding, grooming, mating, and locomotion appeared normal. The presence of ReaChR has no apparent effect on the parabss1 BS paralytic phenotype elicited by mechanical stimulation. A brief mechanical shock given to elavc155 parabss1/Y; UAS-ReaChR/+ flies continues to cause characteristic BS paralysis, with 100% of flies paralyzed. When stimulated with red light (5 sec), flies exhibit paralytic behavior (LS paralysis). This is due to ReaChR activation of parabss1 neurons because it is not observed in control flies lacking UAS-ReaChR (genotype: elavC155 parabss1/Y; Figure 1E). Also, LS paralysis did not occur in flies lacking parabss1 (genotype: elavc155/Y; UAS-ReaChR/+), flies that were not fed retinal, and flies using ChiEF as opsin illuminated with blue light (genotype: elavc155 parabss1/Y; UAS-ChiEF/+) (data not shown). Thus, the higher penetrance of red light for ReaChR activation and the parabss1 defect in neurons are both essential in the generation of LS paralytic behavior.
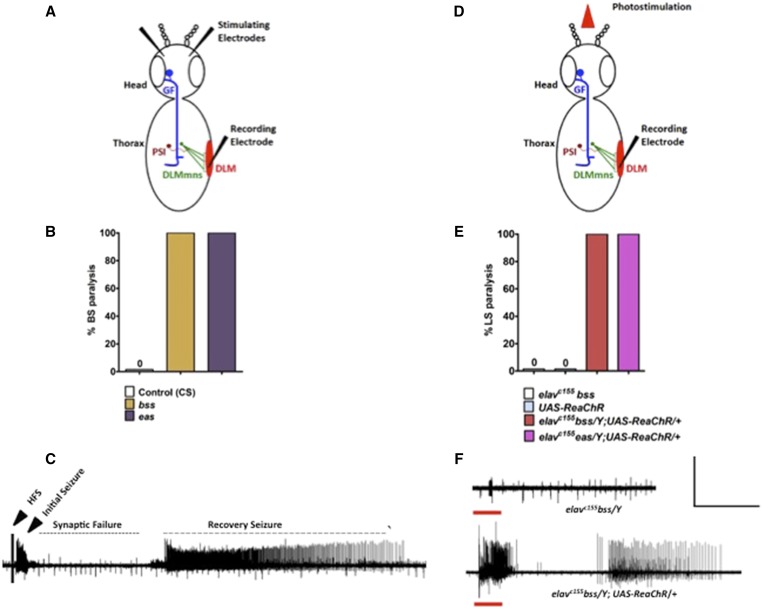
Figure 1.
Generation of SLA in BS mutants by optogenetics. (A) Schematic diagram of stimulation and recording model of the GF circuit in Drosophila. Stimulation of the brain by electrical HFS activates the GF, which electrically synapses with the PSI. The PSI forms chemical synapses with five mns of the contralateral DLMs, also known as indirect flight muscles. (B) Bang-sensitivity seen in parabss1 and eas BS mutants after mechanical shock. (C) Recording from a parabss1 BS mutant DLM fiber following delivery of a 4 V HFS stimulus that is effective in evoking SLA. (D) Schematic diagram of stimulation and recording model of the GF circuit. Only the recording electrode is inserted in the DLM. (E) LS paralysis behavior observed in elavc155parabss1/Y;;UAS-ReaChR/+ and elavc155 eas/Y;;UAS-ReaChR/+ double mutants. (F) Upper trace: photostimulation in control elavc155bss/Y flies did not evoke any SLA. Lower trace: light-induced seizure showed characteristic initial seizure, synaptic failure, and recovery seizure, similar to the seizure induced by HFS. The red bar represents the time duration of photostimulation. (C and F) Horizontal calibration is 10 sec and vertical calibration is 50 mV. BS, bang-sensitive; CS, control; DLMs, dorsal longitudinal muscles; GF, giant fiber; HFS, high-frequency stimuli; LS, light sensitive; mns, motorneurons; PSI, peripherally synapsing neurons; SLA, seizure-like activity.
LS paralysis in elavc155 parabss1/Y; UAS-ReaChR/+ flies resembles BS paralysis in most aspects. Optical stimulation causes 100% of flies to be paralyzed (n = 82, Figure 1, B and E). Paralysis occurs immediately following illumination, and flies remain immobile for 103 ± 4.04 sec (mean ± SEM, n = 40). LS paralysis is followed by behavioral seizure-like activity (recovery seizure), and flies recover shortly thereafter. These are prominent features of BS paralytic behavior in parabss1 mutants recapitulated by ReaChR optogenetics. However, there are some small differences in the details between LS paralysis for parabss1 observed here and BS paralysis reported previously (Parker et al. 2011). Mainly, initial BS paralysis in parabss1 homozygotes is followed by an extended period of tonic-clonic activity. During this period, the fly is usually quiescent (tonic phase), but this is broken up by multiple bouts of clonus-like activity. Tonic-clonic activity in parabss1 prolongs the time to recovery to ≈ 240 sec (Parker et al. 2011). For LS paralysis in parabss1, tonic-clonic activity is not observed and, thus, resembles parabss1/+ heterozygotes (≈ 50 sec recovery) and other BS mutants (≈ 80 and ≈ 40 sec recovery for eas and sda, respectively), which all lack clonus-like activity during BS paralysis (Parker et al. 2011).
ReaChR also drives LS paralysis in BS mutants other than parabss1, for example in an eas background (genotype: elavc155 eas/Y; UAS-ReaChR/+). Optical stimulation (5 sec) also caused complete LS paralysis in these flies (100% LS paralysis, n = 45, Figure 1E). Thus, optogenetics using ReaChR is an excellent method for studying behavioral abnormalities in freely moving BS flies. LS avoids the massive physical disturbance in these flies ordinarily generated by mechanical BS stimulation via a vortex mixer operating at maximum velocity.
ReaChR elicits seizure-like electrical activity in BS mutants
A prominent feature of Drosophila seizure-sensitivity is seizure-like neuronal firing evoked at low threshold by electrical HFS delivered to the brain of BS mutants (Kuebler and Tanouye 2000; Lee and Wu 2002; Parker et al. 2011). We show here that similar seizure-like activity is elicited by photostimulation of ReaChR in parabss1 neurons. Seizure-like LS activity was recorded from the DLM of elavc155 parabss1/Y; UAS-ReaChR/+ flies during optical stimulation (Figure 1F). Seizure-like activity following LS (5- sec light pulses) consisted of aberrant high-frequency DLM motorneuron firing (> 100 Hz) lasting 8–10 sec. This initial seizure-like activity resembles firing evoked by HFS previously called “initial seizure” or “initial discharge” (Figure 1, C and F and Figure 2; Kuebler and Tanouye 2000; Lee and Wu 2002; Parker et al. 2011).
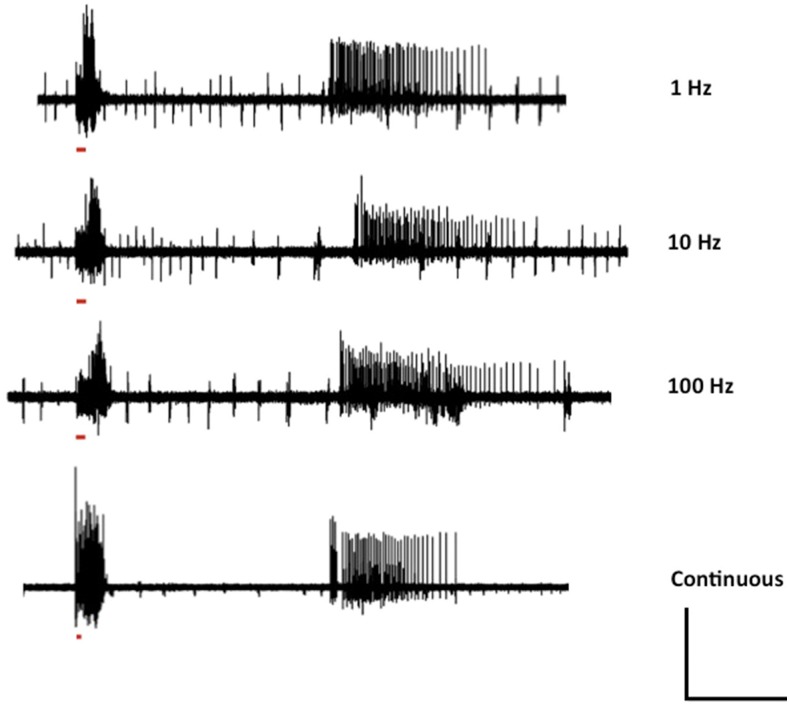
Figure 2.
Optimization of photostimulation frequency. Maximum intensity of red light for 500 msec with 50% duty cycle of frequency range from 1 to 100 Hz was delivered to elavc155 parabss1/y; UAS-ReaChR/+ double mutants. There was not any significant difference in seizure-like electrical activity in double mutants at different frequency compared to continuous red light photostimulation. The red bar represents the time duration of photostimulation. In the continuous photostimulation trace, the red bar is smaller than the other frequency traces as in this case we applied continuous photostimulation with 100% duty cycle. Horizontal calibration is 10 sec and vertical calibration is 100 mV.
After initial seizure, the next aspect of the LS phenotype is a quiescent period that lasts ≈ 25 sec (Figure 1, C and F and Figure 2). This quiescent period resembles a similar HFS phenotype due to transmission failure at many central synapses (Pavlidis and Tanouye 1995). The period of synaptic failure varies among flies of different genotype and age: for example, ≈ 38 sec for sda mutants and for young and old parabss1 mutants ≈ 45 and 75 sec, respectively (Parker et al. 2011). Synaptic failure is thought to be responsible for BS behavioral paralysis, and we infer a similar failure is responsible for LS paralysis (Pavlidis and Tanouye 1995). The final aspect of the patterned electrophysiology phenotype is a prominent recovery seizure observed after both HFS and LS stimulation (Figure 1, C and F and Figure 2; Parker et al. 2011).
The patterned DLM electrical activity described here for LS and HFS-induced seizures in parabss1 mutants differs from seizures seen in some other mutant flies. Most notably, seizure-like DLM activity is observed in shits and cacTS2 mutants following a shift to high temperatures (Salkoff and Kelly 1978; Rieckhof et al. 2003; Kroll et al. 2015; Saras and Tanouye 2016). However, for these mutants, the seizure-like activity does not appear to be patterned into distinct periods of initial seizure, quiescence, and recovery seizure. Temperature-induced seizure-like activity appears to manifest as continuous high-frequency firing of the DLM motorneurons, with occasional interruptions in firing. This appears to decrease in amplitude until complete failure of synaptic transmission at the neuromuscular junction (Siddiqui and Benzer 1976; Koenig and Ikeda 1989; Kawasaki et al. 2000).
Long light pulses induce seizure-like activity
Seizure-like activity evoked by electrical HFS shows frequency dependence in stimulus efficacy (Kuebler and Tanouye 2000). Single-pulse electrical stimulations (0.2-msec duration) are never effective in evoking seizure-like activity. Decreasing the stimulus pulse frequency within an HF wavetrain from 200 to 100 to 50 Hz decreased the likelihood of evoking a seizure event (Kuebler and Tanouye 2000). It was proposed previously that electrical stimulation in the HFS wavetrain drives populations of neurons synchronously and that temporal synaptic summation occurs in the underlying excitatory seizure circuits; this summation is not as effective at lower stimulation frequencies (Kuebler and Tanouye 2000).
We investigated the electrophysiology of seizure-like activity in elavc155 parabss1/Y; UAS-ReaChR/+ flies with different frequencies of red light stimulation. We tested 1, 10, and 100 Hz frequencies, along with continuous red light exposure. The elavc155 parabss1/Y; UAS-ReaChR/+ double mutants were exposed to continuous red light exposure to 1, 10, and 100 Hz light stimulation frequency. There were no apparent differences in seizure induction by any of these different stimulation frequency treatments (Figure 2). However, we found that a minimum light exposure was necessary to induce seizure-like activity; light pulses with durations shorter than 500 msec were not reliable at seizure induction. These optogenetic results presented here suggest that synchronous driving of neuronal populations may not be as important as previously believed (Kuebler and Tanouye 2000), although some form of summation may be occurring via UAS-ReaChR membrane depolarization.
A role for the Drosophila mushroom body in seizure induction
The use of UAS-ReaChR with GAL4 drivers of different spatial expression patterns provides a powerful way to identify brain regions contributing to initiation of Drosophila seizures. Here, we present an initial example of investigation: evidence for an MB role in seizure genesis. UAS-ReachR was driven using several brain-specific GAL4 drivers. The a307-GAL4 driver, specific for the adult GF system (Allen et al. 2006), did not elicit LS paralytic behavior via UAS-ReaChR (genotype: parabss1/Y; UAS-ReaChR/a307-GAL4). Thus, within the limitations of using a single GAL4 driver, it appears that activation of the GF system alone via UAS-ReaChR may not be capable of driving seizure-like activity. Two other GAL4 drivers also failed to elicit LS paralytic behavior via UAS-ReaChR in a parabss1 background: 104Y-GAL4 and c232-GAL4 that drive UAS expression in the fan-shaped body and the ellipsoid body of the central complex, respectively (Young and Armstrong 2009). UAS-ReaChR expression in excitatory interneurons by Cha-GAL4 (genotype: parabss1/Y; UAS-ReaChR/Cha-GAL4) was positive for eliciting LS behavioral paralysis by photostimulation. In contrast, UAS-ReaChR expression in inhibitory interneurons by Gad1-GAL4 failed to elicit LS behavioral paralysis.
Interestingly, when UAS-ReaChR was driven using the MB driver c739-GAL4 in a parabss1 background (genotype: parabss1/Y; UAS-ReaChR/c739-GAL4), photostimulation elicited LS paralytic behavior in 78% of animals tested (n = 36, Figure 3A). Electrophysiology recordings showed that photostimulation elicited seizure-like activity in all flies tested (n = 5, Figure 3B). A second MB driver, 117Y-GAL4, gave similar results (genotype: parabss1/Y; UAS-ReaChR/117Y-GAL4). LS paralytic behavior was observed in 90% of flies tested (n = 64, Figure 3A); electrophysiology showed seizure-like activity induced by photostimulation (n = 5). Taken together, these positive results utilizing two different MB GAL4 drivers indicate that the MB appears sufficient to initiate seizures in parabss1 mutants by ReaChR.
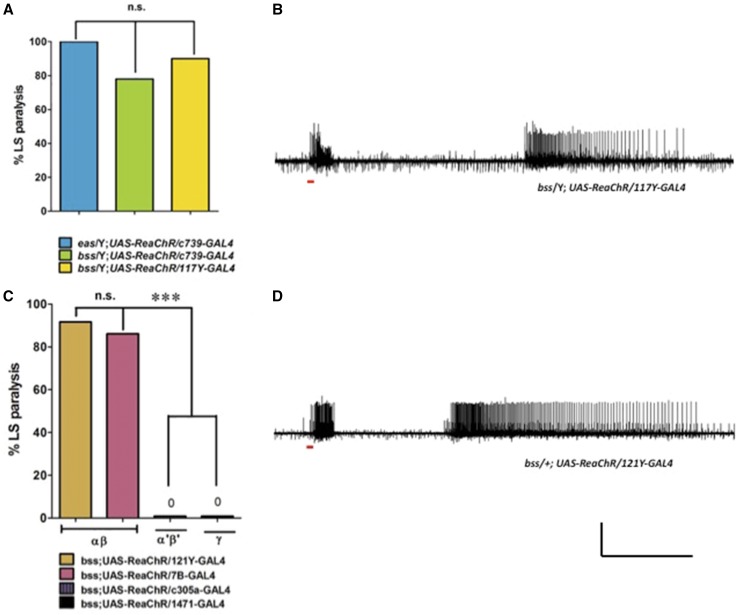
Figure 3.
Characterization of MB and its αβ lobe as a seizure initiation site in Drosophila brain. (A) Quantification of LS paralysis behavior elicited in MB by expression of UAS-ReaChR, using different MB-GAL4 drivers. (B) Expression of UAS-ReaChR in MB by 117Y-GAL4 driver and subsequent optical stimulation by red light-elicited seizures in BS mutants. (C) Quantification of LS paralysis behavior by different MB lobe GAL4 drivers. Neither α’β’ nor γ lobe GAL4 drivers produced any LS paralysis behavior. (D) Expression of UAS-ReaChR in MB αβ lobe using 121Y-GAL4 is sufficient to trigger seizures by light stimulation. (B and D) Horizontal calibration is 10 sec and vertical calibration is 50 mV. BS, bang-sensitive; LS, light-sensitive, MB, mushroom body.
Identification of αβ lobe as the locus for MB seizure induction
The MB is composed of different lobes: αβ, α’β’, and γ. Since different GAL4 drivers are capable of distinguishing these lobes, this allows the possibility of refining the MB localization for seizure initiation. Here, we find evidence for a MB αβ role in seizure genesis. UAS-ReaChR was driven with the MB αβ driver 7B-GAL4 in a parabss1 background (genotype: parabss1/Y; UAS-ReaChR/7B-GAL4). Photostimulation elicited LS paralytic behavior in 85% of flies tested (n = 41, Figure 3C). Electrophysiology recordings showed that photostimulation elicited seizure-like activity in all flies tested (n = 5, Figure 3D). A second MB αβ driver, 121Y-GAL4, gave similar results (genotype: parabss1/Y; UAS-ReaChR/121Y-GAL4). LS paralytic behavior was observed in 82% of flies tested (n = 46, Figure 3C). Electrophysiology recordings showed seizure-like activity induced by photostimulation (n = 5, Figure 3D). Taken together, these positive results utilizing two different MB αβ drivers indicate that the αβ lobe appears sufficient to initiate seizures in parabss1 mutants by ReaChR.
We tested two additional MB GAL4 drivers that did not induce LS paralysis behavior via UAS-ReaChR. LS paralytic behavior was not observed using c305a-GAL4 (genotype: parabss1/Y; UAS-ReaChR/c305a-GAL4, 0% LS paralysis, n = 44, Figure 3C) and 1471-GAL4 (genotype: parabss1/Y; UAS-ReaChR/1471-GAL4, 0% LS paralysis, n = 59, Figure 3C). Thus, we can conclude that not all MB GAL4 drivers are capable of driving LS paralytic behavior in parabss1 via ReaChR. However, from negative results, we are not able to make further conclusions. It may be that c305a-GAL4 and 1471-GAL4 drive lower ReaChR expression levels than the other drivers utilized in this study. It could be that the failure to drive seizures comes from differences in neuroanatomy or connectivity; c305a-GAL4 and 1471-GAL4 are specific for the MB α’β’ lobe and the γ lobe, respectively. Resolution of these issues awaits further analyses with additional drivers and/or positive findings.
Discussion
Channelrhodopsin2 (ChR2) has been used previously for optogenetic stimulation in Drosophila third instar larvae. (Schroll et al. 2006; Zhang et al. 2007; Pulver et al. 2009; Zimmermann et al. 2009). Blue light appears to penetrate the larval cuticle sufficiently for ChR2 activation. In adult flies, ChR2 use has been limited mainly to dissected nervous system preparations and the GF system (Lima and Miesenbock 2005; Zhang et al. 2007; Gordon and Scott 2009; Zimmermann et al. 2009; Pulver et al. 2011). Interestingly, a seizure-like response has been described for adult wild-type flies carrying four copies of UAS-ChR2, but not one or two copies (Zimmermann et al. 2009). We have previously been unsuccessful in generating seizure-like responses in BS mutants with UAS-ChR2 and UAS-Chief.
ReaChR is activated by red light that penetrates the Drosophila adult cuticle more readily than blue light (Suh et al. 2007; Lin et al. 2013; Inagaki et al. 2014). Inagaki et al. (2014) demonstrated the advantages of ReaChR in an elegant study of male courtship behavior in freely moving flies. In the present study of ReaChR in BS mutants, overhead LED illumination of the entire animal causes LS behavioral paralysis in 100% of flies. The red light is apparently penetrating the adult cuticle and causing extensive activation of neurons throughout the brain and thoracic ganglion. It is difficult to determine the actual extent of nervous system activation because the entire repertoire of LS behavioral phenotypes is observed with activation of only the MB; we expect that actual nervous system activation is more extensive. In future experiments, we will express ReaChR exclusively in the thoracic ganglion to determine if overhead illumination penetrates through the thoracic cuticle and indirect flight muscles to initiate seizure-like activity in the ventral nervous system. HFS electrical stimulation of the thoracic ganglion has previously been shown to evoke seizure-like activity (Kuebler and Tanouye 2000), but little is known about these thoracic seizures. An analysis would provide additional insight into mechanisms responsible for seizure initiation and spread from another locus in the Drosophila model.
Neurological dysfunction in BS mutants has been elicited in three ways: (1) mechanical BS stimulation, (2) HFS electrical stimulation, and now (3) ReaChR optogenetics. It had been presumed that electrophysiological phenotypes resembling those evoked by electrical HFS stimulation (initial seizure, synaptic failure, and recovery seizure) were responsible for the behavioral phenotypes caused by BS stimulation (seizure-like behavior, paralysis, and recovery seizure behavior) (Benzer 1971; Ganetzky and Wu 1982; Kuebler and Tanouye 2000; Lee and Wu 2002). This had been difficult to demonstrate directly because BS paralysis is a behavior seen in freely moving adult flies, whereas HFS electrical stimulation is on tethered flies mounted for electrophysiology. The relationship is made clearer in the present study using optogenetic stimulation. Remarkably, there are no salient differences among the phenotypes induced in the different ways. That is, BS paralytic behavior and LS paralytic behavior appear the same; both HFS and LS seizure-like electrophysiological activity appear nearly the same in their firing patterns. Taken together, these results confirm that seizure-like neuronal firing drives BS and LS paralytic behavioral phenotypes in BS flies.
We performed an initial analysis to map out brain locations where seizures initiate. UAS-ReaChR is an outstanding method for this type of mapping because specific spatial expression of opsin can be directed with different GAL4 drivers. We find that parabss1 MB αβ neurons are sufficient to initiate seizure-like activity when driven by ReaChR. The Drosophila MB is especially well-studied for its essential role in olfactory learning and memory (Heisenberg 2003; Keene and Waddell 2007; Berry et al. 2008; Waddell 2016). It is thought that the orderly arrangement of axons and neuropile of MB Kenyon cells facilitate learning and memory (Heisenberg 2003). In flies, this can provide in flies the type of anatomical substrate essential for seizures in mammals (Hauser and Hesdorfer 1990; Traub and Miles 1991). The MB was implicated previously in seizure initiation (Hekmat-Scafe et al. 2010). However, in another study, the MB was not markedly more seizure-sensitive than other brain regions (Rusan et al. 2014). Taking together from the combined results of these studies, we propose a model whereby the total amount of brain tissue that is BS mutant determines whether or not the nervous system is seizure-sensitive or not. For the seizure-sensitive brain, one site of seizure initiation is the αβ lobe of the MB.
The optogenetic approaches described here with UAS-ReaChR add a powerful new tool for investigating the circuitry responsible for seizures in Drosophila. For seizure-like behavior associated with BS mutants, there are several circuit questions that need to be resolved. What is the neurocircuitry responsible for seizure initiation? Following initiation, what circuitry is responsible for spreading seizure activity throughout the nervous system? And most interestingly, what is the circuitry responsible for terminating seizures? Future experiments using GAL4 drivers specific for different brain regions will aid substantially in resolving these issues. Another valuable use for optogenetic stimulation is the potential to be developed into an effective assay for antiepileptic drug screening. It is desirable to utilize minimally intrusive LS stimulation of MB αβ neurons in conducting screens, especially compared to the traumatic use of vortex mixing to provide mechanical BS stimulation. This will facilitate the testing of large numbers of compounds in high-throughput drug screens.
Acknowledgments
We thank the members of the Tanouye laboratory for helpful discussions throughout the project. This study was supported by awards from the McKnight Foundation and the National Institutes of Health (NS31231) to M.A.T.
Author contributions: A.S. and M.A.T. designed the study, experiments, and wrote the manuscript. A.S. performed behavior and electrophysiology experiments, analyzed data, and performed statistical analysis. V.V.W. and H.J.B. performed genetic crosses and behavioral experiments. All authors edited the manuscript.
Footnotes
Communicating editor: H. J. Bellen
Literature Cited
- Allen M. J., Godenschwege T. A., Tanouye M. A., Phelan P., 2006. Making an escape: development and function of the Drosophila giant fibre system. Semin. Cell Dev. Biol. 17: 31–41. [DOI] [PubMed] [Google Scholar]
- Benzer S., 1971. From the gene to behavior. JAMA 218: 1015–1022. [PubMed] [Google Scholar]
- Berry J., Krause W. C., Davis R., 2008. Olfactory memory traces in Drosophila. Prog. Brain Res. 169: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries S. E., Clandinin T., 2013. Optogenetic stimulation of escape behavior in Drosophila melanogaster. J. Vis. Exp. 71: e50192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F., 1982. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 100: 597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. D., Scott K., 2009. Motor control in a Drosophila taste circuit. Neuron 61: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman S. J., Schlogl A., Schmidt-Hieber C., 2014. Stimfit: quantifying electrophysiological data with Python. Front. Neuroinform. 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W. A., Hesdorfer D. C., 1990. Epilepsy: Frequency, Causes and Consequences. Demos, New York. [Google Scholar]
- Heisenberg M., 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4: 266–275. [DOI] [PubMed] [Google Scholar]
- Hekmat-Scafe D. S., Mercado A., Fajilan A. A., Lee A. W., Hsu R., et al. , 2010. Seizure sensitivity is ameliorated by targeted expression of K+-Cl- cotransporter function in the mushroom body of the Drosophila brain. Genetics 184: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H. K., Jung Y., Hoopfer E. D., Wong A. M., Mishra N., et al. , 2014. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F., Felling R., Ordway R. W., 2000. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J. Neurosci. 20: 4885–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene A. C., Waddell S., 2007. Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8: 341–354. [DOI] [PubMed] [Google Scholar]
- Koenig J. H., Ikeda K., 1989. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 9: 3844–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. R., Wong K. G., Siddiqui F. M., Tanouye M. A., 2015. Disruption of endocytosis with the dynamin mutant shibirets1 suppresses seizures in Drosophila. Genetics 201: 1087–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler D., Tanouye M. A., 2000. Modifications of seizure susceptibility in Drosophila. J. Neurophysiol. 83: 998–1009. [DOI] [PubMed] [Google Scholar]
- Lee J., Wu C. F., 2002. Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang sensitive mutants. J. Neurosci. 22: 11065–11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Anttonen A. K., Liukkonen E., Gaily E., Maljevic S., et al. , 2010. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology 75: 1454–1458. [DOI] [PubMed] [Google Scholar]
- Lima S. Q., Miesenbock G., 2005. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121: 141–152. [DOI] [PubMed] [Google Scholar]
- Lin J. Y., Knusten P. M., Kleinfeld D., Tsien R. Y., 2013. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16: 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K., Kreber R., Ganetzky B., 1989. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58: 1143–1154. [DOI] [PubMed] [Google Scholar]
- Osawa S. I., Iwasaki M., Hosaka R., Matsuzaka Y., Tomita H., et al. , 2013. Optogenetically induced seizure and the longitudinal hippocampal network dynamics. PLoS One 8: e60928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L., Padilla M., Du Y., Dong K., Tanouye M. A., 2011. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics 187: 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P., Tanouye M. A., 1995. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J. Neurosci. 15: 5810–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P., Ramaswami M., Tanouye M. A., 1994. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell 79: 1–20. [DOI] [PubMed] [Google Scholar]
- Paz J. T., Huguenard J. R., 2015. Optogenetics and epilepsy: past, present and future. Epilepsy Curr. 15: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver S. R., Pashkovki S. L., Hornstein N. J., Garrity P. A., Griffith L. C., 2009. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol. 101: 3075–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver S. R., Hornstein N. J., Land B. L., Johnson B. R., 2011. Optogenetics in the teaching laboratory: using channelrhodopsin-2 to study the neural basis of behavior and synaptic physiology in Drosophila. Adv. Physiol. Educ. 35: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M., Tanouye M. A., 1989. Two sodium channel genes in Drosophila: implications for channel diversity. Proc. Natl. Acad. Sci. USA 86: 2079–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhof G. E., Yoshihara M., Guan Z., Littleton J. T., 2003. Presynaptic N-type calcium channels regulate synaptic growth. J. Biol. Chem. 278: 41099–41108. [DOI] [PubMed] [Google Scholar]
- Rusan Z. M., Kingsford O. A., Tanouye M. A., 2014. Modeling glial contributions to seizures and epileptogenesis: cation-chloride cotransporters in Drosophila melanogaster. PLoS One 9: e101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L., Kelly L., 1978. Temperature-induced seizure and frequency-dependent neuromuscular block in a ts mutant of Drosophila. Nature 273: 156–158. [DOI] [PubMed] [Google Scholar]
- Saras A., Tanouye M. A., 2016. Mutations of the calcium channel gene cacophony suppress seizures in Drosophila. PLoS Genet. 12: e1005784 10.1371/journal.pgen.1005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C., Riemensperger T., Bucher D., Ehmer J., Voller T., et al. , 2006. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16: 1741–1747. [DOI] [PubMed] [Google Scholar]
- Schwarz N., Hahn A., Bast T., Muller S., Loffler H., et al. , 2016. Mutations in the sodium channel gene SCN2A cause neonatal epilepsy with late-onset episodic ataxia. J. Neurol. 263: 334–343. [DOI] [PubMed] [Google Scholar]
- Siddiqui O., Benzer S., 1976. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 73: 3253–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh G. S., Ben-Tabou De Leon S., Tanimoto H., Fiala A., Benzer S., et al. , 2007. Light activation of an innate olfactory avoidance response in Drosophila. Curr. Biol. 17: 905–908. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I., Chan A. M., Ahmed O. J., Rao V. R., Gradinaru V., et al. , 2013. Optogenetic delay of status epilepticus onset in an in vivo rodent epilepsy model. PLoS One 8: e62013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Miles R., 1991. Neuronal Networks of the Hippocampus. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Tye K. M., Deisseroth K., 2012. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 13: 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S., 2016. Neural plasticity: dopamine tunes the mushroom body output network. Curr. Biol. 26: R109–R112. [DOI] [PubMed] [Google Scholar]
- Young J. M., Armstrong J. D., 2009. Structure of the adult central complex in Drosophila: organization of distinct neuronal subsets. J. Comp. Neurol. 518: 1500–1524. [DOI] [PubMed] [Google Scholar]
- Zhang H., Tan J., Reynolds E., Kuebler D., Faulhaber S., et al. , 2002. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics 162: 1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Ge W., Wang Z. A., 2007. Toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur. J. Neurosci. 26: 2405–2416. [DOI] [PubMed] [Google Scholar]
- Zhao M., Alleva R., Ma H., Daniel A. G. S., Schwartz T. H., 2015. Optogenetic tools for modulating and probing the epileptic network. Epilepsy Res. 116: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G., Wang L.-p., Vaughan A. G., Manoli D. S., Zhang F., et al. , 2009. Manipulation of an innate escape response in Drosophila: photoexcitation of acj6 neurons induces the escape response. PLoS ONE 4: e5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Drosophila strains are available upon request. All data generated for this study are included in the main text and figures.