Abstract
Ovarian function is directly correlated with survival of the primordial follicle reserve. Women diagnosed with cancer have a primary imperative of treating the cancer, but since the resting oocytes are hypersensitive to the DNA-damaging modalities of certain chemo- and radiotherapeutic regimens, such patients face the collateral outcome of premature loss of fertility and ovarian endocrine function. Current options for fertility preservation primarily include the collection and cryopreservation of oocytes or in vitro-fertilized oocytes, but this necessitates a delay in cancer treatment and additional assisted reproductive technology procedures. Here, we evaluated the potential of pharmacological preservation of ovarian function by inhibiting a key element of the oocyte DNA damage checkpoint response, checkpoint kinase 2 (CHK2; CHEK2). Whereas nonlethal doses of ionizing radiation (IR) eradicate immature oocytes in wild-type mice, irradiated Chk2−/− mice retain their oocytes and, thus, fertility. Using an ovarian culture system, we show that transient administration of the CHK2 inhibitor 2-(4-(4-chlorophenoxy)phenyl)-1H-benzimidazole-5-carboxamide-hydrate (“CHK2iII”) blocked activation of the CHK2 targets TRP53 and TRP63 in response to sterilizing doses of IR, and preserved oocyte viability. After transfer into sterilized host females, these ovaries proved functional and readily yielded normal offspring. These results provide experimental evidence that chemical inhibition of CHK2 is a potentially effective treatment for preserving the fertility and ovarian endocrine function of women exposed to DNA-damaging cancer therapies such as IR.
Keywords: fertility, primordial follicles, premature ovarian failure, oncofertility
IT is of paramount importance that organisms minimize the transmission of deleterious mutations to their offspring. Accordingly, sensitive mechanisms have evolved to eliminate germ cells that have sustained certain threshold amounts of DNA damage (Heyer et al. 2000; Suh et al. 2006; Bolcun-Filas et al. 2014; Pacheco et al. 2015). Under normal circumstances, this is desirable. However, because women are born with a finite number of oocytes, environmental factors that cause DNA damage to oocytes can result in primary ovarian insufficiency (POI), sterility, and ovarian failure. This is a crucial issue for cancer patients undergoing certain types of chemotherapy or radiation therapy (Woodruff 2007). For example, POI occurs in nearly 40% of all female breast cancer survivors (Oktay et al. 2006). The resulting premature ovarian failure has a major impact on women’s’ lives, both physiologically and emotionally. As the life expectancy of cancer survivors increases, so does the need to address the adverse outcomes to fertility. Therefore, the ability to inhibit oocyte death and preserve fertility, in both prepubertal cancer patients and premenopausal women, would have a major impact on survivors’ lives.
At present, cancer patients have few options regarding fertility preservation (“oncofertility”) before treatment, and most involve invasive surgical procedures such as extraction of oocytes or ovarian tissue for cryopreservation, or in vitro fertilization followed by embryo cryopreservation (Redig et al. 2011; Salama and Mallmann 2015; Kim et al. 2016). Not only are these procedures invasive, but also they necessitate a delay in cancer treatment. An alternative is to coadminister drugs that protect oocytes from chemotherapy at the time of treatment. Based upon the knowledge that activation of the “TA” isoform of the DNA damage checkpoint gene Trp63 (TP63 in humans, also known as p63; the TA isoform of the protein will be referred to as TAp63) occurs via phosphorylation, the use of kinase inhibitors was suggested as a means to prevent radiation-induced oocyte loss in mice (Suh et al. 2006). It was reported (Gonfloni et al. 2009), but later challenged (Kerr et al. 2012) and counter argued (Maiani et al. 2012), that the tyrosine kinase inhibitor imatinib (Gleevec) is effective in protecting oocytes. Even if imatinib proves to have such activity, it is a relatively promiscuous kinase inhibitor that blocks, among other targets, the receptor tyrosine kinase KIT that functions in germline stem cells (Lee and Wang 2009).
We previously reported that mouse checkpoint kinase 2 (CHK2) is a key component of the meiotic DNA damage checkpoint, and that deletion of Chk2 prevented irradiation-induced killing of postnatal oocytes (Bolcun-Filas et al. 2014). We also showed that the CHK2 kinase phosphorylates both p53 (formally TRP53; TP53 in humans) and TAp63 in oocytes to activate these proteins (and stabilize p53). Therefore, the deletion of Chk2 effectively impairs the activation of these two downstream effectors, which are both needed to trigger efficient oocyte elimination (Bolcun-Filas et al. 2014). More importantly, damaged oocytes that survived in the absence of CHK2 produced healthy pups, suggesting that the inflicted DNA damage was repaired (Bolcun-Filas et al. 2014). The resistance of Chk2−/− oocytes to otherwise lethal levels of ionizing radiation (IR) prompted us to explore whether chemical inhibition of CHK2 would be effective at preventing radiation-induced oocyte death, and thus constitute a potential option for preserving ovarian function in women undergoing cancer therapy. Here, we show that transient chemical inhibition of CHK2 suppresses follicle loss and allows for the production of healthy offspring.
Materials and Methods
Mice
Mice were obtained from The Jackson Laboratory, strains C3HeB/FeJ, stock # 000658[(agouti mice, homozygous dominant for coat color (A/A)] and C3FeLe.B6-a/J, stock # 000198 [black mice, recessive for coat color, (a/a)]. Cornell’s Animal Care and Use Committee approved all animal usage, under protocol 2004-0038 awarded to J. C. Schimenti.
Organ culture
Ovaries were cultured using an adaptation of a published method (Livera et al. 2008). Briefly, ovaries were collected from 5-day postpartum (dpp) C3FeLe.B6-a/J mice and, following removal from the bursa, placed into cell culture inserts (Millicell; pore size, 8 µm; diameter, 12 mm) presoaked in ovary culture media (minimal essential media supplemented with 10% fetal bovine serum, 25 mM HEPES pH = 7.0, 100 units/ml penicillin, 100 µg/ml streptomycin, 0.25 µg/ml Fungizone, 1% DMSO, and CHK2 inhibitor). The inserts were placed into 24-well plates (Model MD24; Thermo Fisher) with carriers for the inserts. Sufficient media was added to keep organs moist, but not completely submerged. Organs were incubated at 37°, 5% CO2, and atmospheric O2.
Drug treatment
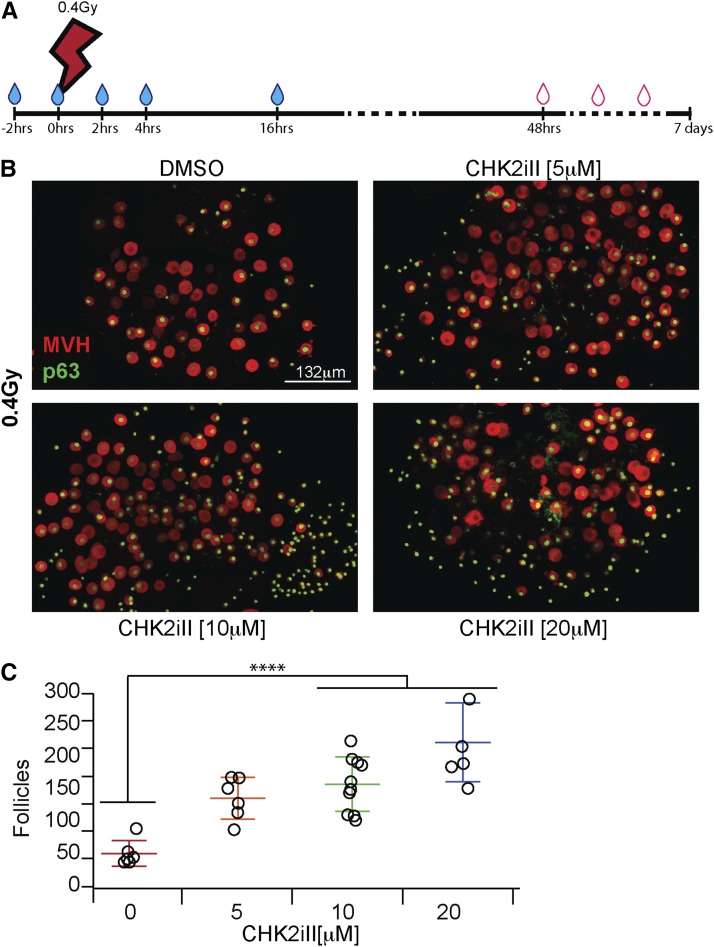
2-(4-(4-chlorophenoxy)phenyl)-1H-benzimidazole-5-carboxamide-hydrate (designated “Chk2 inhibitor II” by the manufacturer, referred to hereafter as CHK2iII) (220486; Calbiochem, San Diego, CA; La Jolla, CA) was prepared as 1 and 2 mM stock solutions in DMSO and kept frozen at −20°. Media containing the desired concentration of inhibitor was prepared shortly before use, assuring that the DMSO concentration was constant (1% DMSO) throughout the different conditions. Explanted ovaries were preincubated for 2 hr in warm (37°) media containing the desired concentration of inhibitor or 1% DMSO alone before being subjected to IR in a 137Cs irradiator with a rotating turntable. Figure 2A presents the media change regimen, with the first replacement being immediately after irradiation. The ovaries were cultured for either 3 hr before being processed for western blot analysis (to detect DNA damage responses), or 7 days followed by either fixation and immunostaining (to quantify oocyte survival) or ovary transplant surgery into sterile agouti females.
Figure 2.
Concentration-dependent protection of irradiated oocytes by CHK2iII. (A) Schematic of CHK2iII treatment regimen, beginning with placing explanted 5 dpp ovaries into culture. Blue droplets denote times at which fresh media containing CHK2iII was added/replaced. Red outlined droplets indicate changes with drug-free media. (B) Maximum intensity projections of immunostained ovary whole ovaries. For 3D visualization, see Movie M1 in File S1, Movie M2 in File S2, and Movie M3 in File S3. The ovaries were cultured according to the timeline in (A) in the presence of the indicated concentrations of CHK2iII. DMSO corresponds to diluent control. MVH is a cytoplasmic germ cell protein, and p63 labels oocyte nuclei. Note that growing follicles (oocytes with larger MVH-stained cytoplasm) are relatively refractory to IR. (C) Quantification of follicles. Data points represent total follicle counts derived from one ovary. Horizontal hashes represent mean and SD. Colors correspond to the different concentrations of inhibitors. Asterisks indicate P-value ≤ 0.0001 (Tukey’s HSD). CHK2, checkpoint kinase 2; CHK2iII, 2-(4-(4-chlorophenoxy)phenyl)-1H-benzimidazole-5-carboxamide-hydrate; dpp, days postpartum; hrs, hours; HSD, honest significant difference; IR, ionizing radiation; MVH, .
Western blot analyses and antibodies
Ovary protein lysates, immunoblotting, probing, and detection were conducted as described (Bolcun-Filas et al. 2014). Primary antibodies and dilutions used were: mouse anti-p63 (1:500, 4A4; Novus Biologicals); rabbit anti-p53 (1:300, #9282; Cell Signaling); mouse anti-β-actin (1:5000; Sigma [Sigma Chemical], St Louis, MO) and rabbit anti-MVH (Mouse Vasa homolog) (1:1000; Abcam). Secondary antibodies used were: Immuno Pure goat anti-mouse IgG(H+L) peroxidase conjugate (1:5000; Thermo Fisher) and goat anti-rabbit IgG HRP (Horseradish peroxidase)-linked antibody (1:5000; Cell Signaling).
Immunofluorescence
Cultured ovaries were fixed in 4% paraformaldehyde (PFA)/PBS at 4° overnight (ON), and then washed and stored in 70% ethanol. Ovaries were either embedded in paraffin and sectioned at 5 μm for immunostaining or subjected to whole-mount immunostaining and clearing. For the standard immunofluorescence, slides were deparaffinized and rehydrated prior to antigen retrieval using sodium citrate buffer. Slides were blocked with 5% goat serum (PBS/Tween 20), incubated at 4° ON with the aforementioned primary antibodies (anti-p63 at 1:500 dilution and anti-MVH 1:1000), and subsequently incubated with Alexa Fluor secondary antibodies for 1 hr and Hoechst dye for 5 min. Slides were mounted with ProLong Antifade (Thermo Fisher) and imaged.
Ovary transfer surgery and postmortems
Agouti females were sterilized with 0.5 Gy of IR at 1 week of age. At 8 weeks of age, females were housed with males known to be fertile. Eight weeks later, and 2 days prior to ovary transfer surgery, the males were removed. Three ovaries, either treated or not with CHK2iII, were placed in the intrabursal space of each ovary (a total of six ovaries per recipient female). The females were allowed a recovery period of 6 weeks, then housed with males. Three to four months later, upon euthanasia, dissection was performed for visual inspection of the transplantation sites.
Whole-organ immunofluorescence
Ovaries cultured for 7 days were fixed in freshly prepared 4% PFA/PBS at 4° ON. Afterward, tissues were washed and stored in 70% ethanol at 4° until further processing.
Fixed ovaries were washed and left to equilibrate for a minimum of 4 hr in PBS before initializing the whole-mount immunostaining protocol. To facilitate handling and tissue integrity, ovaries were kept in the culture insert throughout the whole procedure. The ovaries were treated for 4 hr in permeabilization solution [PBS, 0.2% polyvynal alcohol (PVA), 0.1% NaBH4-solution (Sigma), and 1.5% Triton X-100], then incubated for 24 hr in blocking solution (PBS, 0.1% Triton X-100, 0.15% glycine pH 7.4, 10% normal goat serum, 3% BSA, 0.2% sodium azide, 100 units/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml Fungizone). All the immunostaining and clearing was performed at room temperature with gentle rocking. Antibodies were diluted to appropriate concentrations in the blocking solution. Primary antibodies [mouse anti-p63 (1:500, 4A4; Novus Biologicals) and rabbit anti-MVH (1:600; Abcam)] were incubated for 4 days. Afterward, ovaries were incubated with washing solution (PBS, 0.2% PVA, and 0.15% Triton X-100) for 10 hr then two times for 2 hr each. Secondary antibodies (1:1000 Alexa Fluor secondary antibodies) were incubated for 3 days in vials protected from light. Ovaries were washed with washing solution for three times of 12 hr (if needed, DAPI 50 ng/ml was added to the first wash).
Clearing, imaging, and oocyte quantification
Immunostained ovaries were cleared with modified, freshly-prepared ScaleS4(0) reagent [40% D-(-)-sorbitol (w/v), 10% glycerol, 4 M urea, and 20% DMSO, pH 8.1 (Hama et al. 2015), gently mixed by inversion at 50° for 30 min, and degassed prior to use]. Solution was refreshed twice daily until tissue became transparent (usually 2 days). The insert was placed on top of a glass slide, and the membrane containing the cultured ovaries was carefully removed with a fine tip scalpel and placed on the slide. Slides were imaged on an upright laser-scanning Zeiss LSM880 (Zeiss [Carl Zeiss], Thornwood, CA) confocal/multiphoton microscope, using a 10 × NA 0.45 water immersion objective. For proper image stitching, the adjacent images (tiles) were overlapped by 20%. The z-steps were set for 5 μm between optical sections. Images were reconstructed, visualized, and analyzed using Fiji-ImageJ (Schindelin et al. 2012).
Movies were made using the 3D project feature of Fiji-ImageJ (Schindelin et al. 2012). Oocyte quantification was performed in flattened maximum intensity projections of the Z-stacks image series, using the “analyze particle” feature of Fiji-ImageJ.
Statistical analysis
Statistical analyses were done using JMP Pro12 software (version 12.0.1; SAS Institute, Cary, NC). Fertility was analyzed using a mixed model with mother as random effect and ovary treatment as fixed effect. Least square (LS) means difference between litter sizes derived from treated vs. nontreated ovaries was performed using the Student’s t-test. LS mean differences between follicle counts from the different treatment groups were tested using Tukey’s honest significant difference (HSD).
Data availability
Reagents used are commercially available, as is the mouse strain used. The supplemental movies provided in the “.mov” format can be provided in the “.avi” format upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results and Discussion
Irradiation of ovaries induces CHK2-dependent phosphorylation of TAp63 in oocytes, and this phosphorylation is essential for triggering their death (Suh et al. 2006; Bolcun-Filas et al. 2014). CHK2 is a key component of the DNA damage response pathway that responds primarily to DNA double-strand breaks (DSBs), lying downstream of the apical kinase ATM (ataxia telangiectasia mutated). Members of the ATM > CHK2 > p53/p63 pathway have been implicated as potential anticancer drug targets sensitizing cancer cells to genotoxic therapies, and chemical inhibitors have been developed against CHK2 (Garrett and Collins 2011), which, when deleted in mice, causes only minor phenotypic consequences (Takai et al. 2002). Therefore, we tested whether a well-characterized and highly specific (Arienti et al. 2005; Garrett and Collins 2011) CHK2 inhibitor (CHK2iII) could mimic the oocyte-protective effect of genetic Chk2 deletion, and if it could do so in a nontoxic manner.
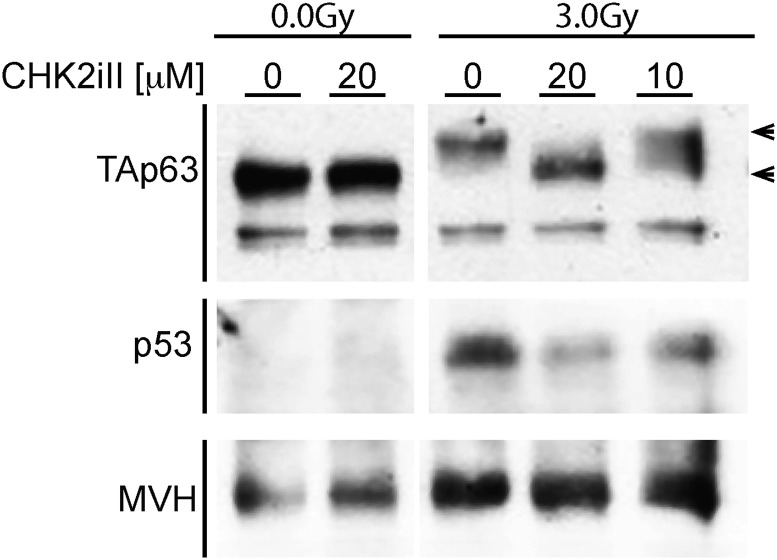
We employed an organ culture paradigm to control concentrations, penetration, and timing of drug delivery to the ovary. Using dose ranges based upon published data (Arienti et al. 2005) and the manufacturer’s recommendations, we first tested the ability of CHK2iII to block phosphorylation of TAp63 in irradiated ovaries, and to block the stabilization of p53, which is normally rapidly degraded in cells unless stabilized by DNA-damage-induced phosphorylation by proteins including CHK2 (Chehab et al. 2000; Hirao et al. 2000). We used ovaries from 5-dpp females to ensure that oocytes were in the dictyate arrest stage of meiosis, residing within primordial follicles. Explanted ovaries were cultured for 2 hr in the presence of 0, 10, or 20 µM CHK2iII, then subjected (or not) to 3 Gy of IR, a level that not only kills oocytes, but also causes extensive p53 stabilization and TAp63 phosphorylation (Suh et al. 2006; Bolcun-Filas et al. 2014). Ovaries were harvested 3 hr later for protein extraction and western blot analysis. In nonirradiated ovaries, TAp63 remained unphosphorylated and p53 was undetectable (Figure 1). Irradiation in the absence of inhibitor led to robust p53 stabilization, and all TAp63 was shifted to a higher mobility, which is known to be due to phosphorylation (Suh et al. 2006; Livera et al. 2008). Addition of 10 and 20 µM CHK2iII led to partial and complete inhibition of TAp63 phosphorylation, respectively, and also progressively decreased p53 levels (Figure 1). This confirms that CHK2iII treatment rapidly acts to prevent the activation of two proapoptotic factors in the ovary.
Figure 1.
Inhibition of radiation-induced phosphorylation of p53 and TAp63 by CHK2iII. Western blot analysis of protein extracted from 5 dpp ovaries. Ovaries were incubated with the indicated concentrations of CHK2 inhibitor II (see Materials and Methods), and exposed or not to 3 Gy of γ-radiation. The immunoblot membrane was cut into two parts, one containing proteins > 60 kDa and the other < 60 kDa, and probed with anti-p63 and anti-p53, respectively. The > 60 kDa portion was stripped and reprobed for the germ cell marker MVH. Arrowheads indicated the expected molecular weight of the phosphorylated form of TAp63 (upper), and nonphosphorylated TAp63 (lower). CHK2, checkpoint kinase 2; CHK2iII, 2-(4-(4-chlorophenoxy)phenyl)-1H-benzimidazole-5-carboxamide-hydrate; dpp, days postpartum; MVH, .
Next, we tested whether CHK2iII could permanently protect oocytes from a lower dose of radiation (0.4 Gy) that normally kills all oocytes within 2 days (Bolcun-Filas et al. 2014) but is far below levels (> 5 Gy) that are lethal to whole animals. Ovaries were cultured in media supplemented with 0, 5, 10, or 20 µM of inhibitor for 2 hr before irradiation. Following IR exposure, ovaries were cultured for two more days with media changes as delineated in Figure 2A, after which the drug was removed from the medium. This protocol of media changes with drug replenishment was optimized for oocyte survival. Seven days after irradiation (and 5 days after removal of CHK2iII), oocyte survival was assessed by co-immunolabeling of histological sections (Supplemental Material, Figure S1) and of whole mounts, with the cytoplasmic germ cell marker MVH and oocyte nuclear marker p63 (Figure 2B, Figure S2A, Movie M1 in File S1, Movie M2 in File S2, and Movie M3 in File S3). Under these conditions, the inhibitor was well tolerated and oocyte survival in unirradiated ovaries was not compromised (Figure S2B, Movie M4 in File S4, and Movie M5 in File S5).
Remarkably, though 10 μM CHK2iII only partially inhibited TAp63 phosphorylation induced by 3 Gy of IR (Figure 1), it dramatically improved oocyte survival in ovaries exposed to 0.4 Gy of IR, a level sufficient to trigger TAp63 phosphorylation and eliminate nearly all primordial follicles in ovaries (Figure 2, B and C and Figure S3) (Bolcun-Filas et al. 2014). A small but significant protective effect was also observed with 5 μM CHK2iII (P = 0.004, Figure 2, B and C).
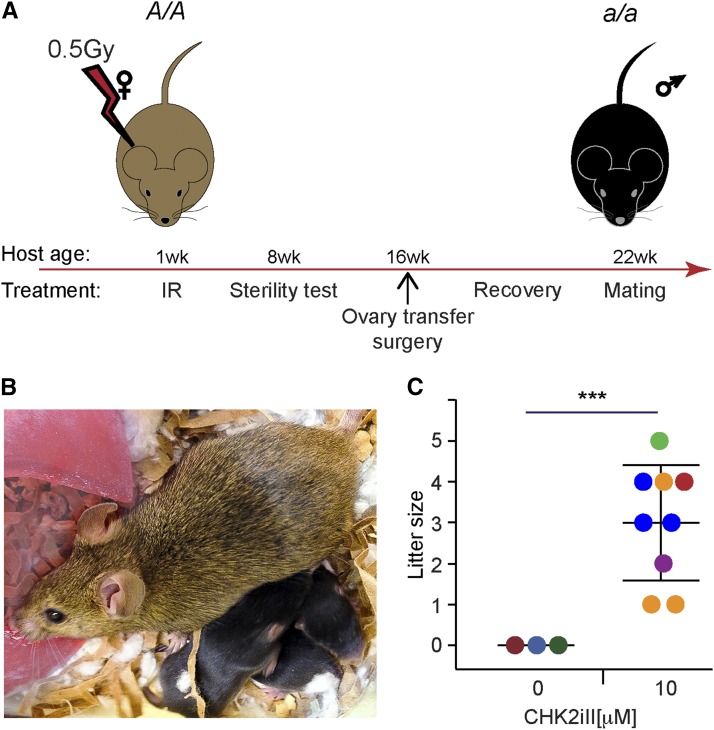
To assess if oocytes protected by CHK2iII from otherwise lethal levels of irradiation were capable of ovulation, fertilization, and subsequent embryonic development, we performed intrabursal transfers of irradiated ovaries (0.4 Gy) into histocompatible (strain C3H) agouti females. These recipient females were first sterilized at 7 dpp by exposure to 0.5 Gy of IR. Once females were 8 weeks old, sterility was verified by housing them with fertile males for at least 8 weeks. The IR-induced oocyte death led to premature ovarian failure, yielding sufficient intrabursal space without the need to physically remove the vestigial ovaries before ovary transfer. These recipients were 16 weeks of age at the time of surgery. Three cultured ovaries, derived from black female animals also of strain C3H (see Materials and Methods for additional information), were placed into each bursa (total six ovaries per animal). Figure 3A summarizes the experimental timeline. A total of eight successful embryo transfer surgeries were completed. Three females received mock-treated (cultured in media containing 1% DMSO alone), irradiated ovaries (0.4 Gy), and five females received irradiated ovaries (also 0.4 Gy) treated with 10 μM CHK2iII in media containing 1% DMSO.
Figure 3.
CHK2iII-rescued ovaries are fertile. (A) Experimental timeline. Agouti females (A/A) were sterilized with 0.5 Gy of IR at 1 week of age. The transplanted ovaries were from black donor females (a/a) treated as outlined in Figure 2A. (B) Agouti host females gave birth to black offspring (a/a) exclusively; thus, the ovulated eggs produced were from the donor ovaries. (C) Litter sizes of mock-treated and treated ovaries. Each circle represents a litter and the circle’s color represents the female that generated that litter. The combined average litter size produced by all host females was three. CHK2iII, 2-(4-(4-chlorophenoxy)phenyl)-1H-benzimidazole-5-carboxamide-hydrate; IR, ionizing radiation; wk, week.
Once the transferred donor ovary reached 8 weeks of age (with respect to the time at which it was explanted), the recipient females were mated to proven fertile C3H black (a/a) males for 3 months, and monitored for litters and the coat colors of offspring. Only females that received CHK2iII-treated ovaries delivered progeny, all of which were black, confirming that they were produced from fertilization of oocytes ovulated from donor ovaries (Figure 3, B and C). All offspring had no visible abnormalities that would suggest the inheritance of gross chromosomal abnormalities (Bolcun-Filas et al. 2014). The viability of these animals indicated that even though oocytes have sensitive checkpoint mechanisms rendering them vulnerable to low levels of DNA damage, they are capable of repairing damaged DNA in a manner compatible with normal embryogenesis. Consistent with the IR-induced oocyte death in ovaries not treated with CHK2iII, and also the absence of progeny from females receiving such ovaries, postmortem inspection revealed only residual ovaries in these recipients compared to those mice that received the CHK2iII-treated ovaries (Figure S4).
Our results provide proof-in-principle for the strategy of targeting the CHK2-dependent DNA damage checkpoint pathway for preventing loss of the ovarian reserve, and thus ovarian failure, in cancer patients undergoing therapies that are toxic to oocytes. Importantly, checkpoint inhibitors have already been explored as potential anticancer therapies, thus substantial information is already available on members of this drug class (Antoni et al. 2007; Garrett and Collins 2011). Nevertheless, it remains to be seen whether systemic administration of CHK2iII or other CHK2 inhibitors can achieve similar oocyte-protective efficacy against IR- or drug-induced DSBs in vivo, and whether they will be effective for both prepubertal and adult females. Additionally, it will be important to conduct more thorough studies of potential genetic risks associated with oocytes rescued from DNA damage-induced death by checkpoint inhibition.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.203455/-/DC1.
Acknowledgments
We thank C. Abratte, Jordana Bloom, Johanna DelaCruz, and Rebecca Williams for technical assistance. This work was supported by National Institutes of Health grants S10-OD018516 (to Cornell’s Imaging facility) and R01-GM45415 to J.C.S.
Footnotes
Communicating editor: S. K. Sharan
Literature Cited
- Antoni L., Sodha N., Collins I., Garrett M. D., 2007. CHK2 kinase: cancer susceptibility and cancer therapy - two sides of the same coin? Nat. Rev. Cancer 7: 925–936. [DOI] [PubMed] [Google Scholar]
- Arienti K. L., Brunmark A., Axe F. U., McClure K., Lee A., et al. , 2005. Checkpoint kinase inhibitors: SAR and radioprotective properties of a series of 2-arylbenzimidazoles. J. Med. Chem. 48: 1873–1885. [DOI] [PubMed] [Google Scholar]
- Bolcun-Filas E., Rinaldi V. D., White M. E., Schimenti J. C., 2014. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 343: 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab N. H., Malikzay A., Appel M., Halazonetis T. D., 2000. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 14: 278–288. [PMC free article] [PubMed] [Google Scholar]
- Garrett M. D., Collins I., 2011. Anticancer therapy with checkpoint inhibitors: what, where and when? Trends Pharmacol. Sci. 32: 308–316. [DOI] [PubMed] [Google Scholar]
- Gonfloni S., Di T. L., Caldarola S., Cannata S. M., Klinger F. G., et al. , 2009. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 15: 1179–1185. [DOI] [PubMed] [Google Scholar]
- Hama H., Hioki H., Namiki K., Hoshida T., Kurokawa H., et al. , 2015. ScaleS: an optical clearing palette for biological imaging. Nat. Neurosci. 18: 1518–1529. [DOI] [PubMed] [Google Scholar]
- Heyer B. S., MacAuley A., Behrendtsen O., Werb Z., 2000. Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Genes Dev. 14: 2072–2084. [PMC free article] [PubMed] [Google Scholar]
- Hirao A., Kong Y. Y., Matsuoka S., Wakeham A., Ruland J., et al. , 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287: 1824–1827. [DOI] [PubMed] [Google Scholar]
- Kerr J. B., Hutt K. J., Cook M., Speed T. P., Strasser A., et al. , 2012. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat. Med. 18: 1170–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Kim S. K., Lee J. R., Woodruff T. K., 2016. Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women. J. Gynecol. Oncol. 27: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Wang J. Y., 2009. Exploiting the promiscuity of imatinib. J. Biol. 8: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livera G., Petre-Lazar B., Guerquin M. J., Trautmann E., Coffigny H., et al. , 2008. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 135: 3–12. [DOI] [PubMed] [Google Scholar]
- Maiani E., Di B. C., Klinger F. G., Cannata S. M., Bernardini S., et al. , 2012. Reply to: cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat. Med. 18: 1172–1174. [DOI] [PubMed] [Google Scholar]
- Oktay K., Oktem O., Reh A., Vahdat L., 2006. Measuring the impact of chemotherapy on fertility in women with breast cancer. J. Clin. Oncol. 24: 4044–4046. [DOI] [PubMed] [Google Scholar]
- Pacheco S., Marcet-Ortega M., Lange J., Jasin M., Keeney S., et al. , 2015. The ATM signaling cascade promotes recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 11: e1005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redig A. J., Brannigan R., Stryker S. J., Woodruff T. K., Jeruss J. S., 2011. Incorporating fertility preservation into the care of young oncology patients. Cancer 117: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M., Mallmann P., 2015. Emergency fertility preservation for female patients with cancer: clinical perspectives. Anticancer Res. 35: 3117–3127. [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., et al. , 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E. K., Yang A., Kettenbach A., Bamberger C., Michaelis A. H., et al. , 2006. p63 protects the female germ line during meiotic arrest. Nature 444: 624–628. [DOI] [PubMed] [Google Scholar]
- Takai H., Naka K., Okada Y., Watanabe M., Harada N., et al. , 2002. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21: 5195–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T. K., 2007. The emergence of a new interdiscipline: oncofertility. Cancer Treat. Res. 138: 3–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Reagents used are commercially available, as is the mouse strain used. The supplemental movies provided in the “.mov” format can be provided in the “.avi” format upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.