Abstract
We analyzed B cells infiltrating the Visceral Adipose Tissue (VAT) of young and old mice to identify contributors to the phenotypic and functional changes observed in aged B cells. We found higher percentages of pro-inflammatory (age-associated B cells, ABC) and less Follicular (FO) B cells in VAT as compared to spleen. VAT B cells express higher pro-adipogenic and inflammatory markers, including NF-kB, as compared to splenic B cells, and also secrete IgG2c antibodies, some of which have been shown to be autoimmune in other studies. Moreover, we found that in the presence of an adipocyte-conditioned medium FO B cells differentiated into ABC. Additional results showed production of pro-inflammatory chemokines by the adipocytes, suggesting a mechanism for B cell attraction by the VAT. These results are the first to show a direct effect of adipocytes on the generation of pro-inflammatory B cells.
Keywords: Aging, B cells, Obesity, Inflammation
1. Introduction
The ability to switch the heavy chain class (isotype) of immunoglobulin (Ig) is critical for proper effector functions of the Ig protein. Our laboratory has previously shown molecular and cellular mechanisms responsible for the decrease in Ig class switch recombination (CSR) in aged mice, including a decrease in the enzyme activation-induced cytidine deaminase (AID) and the transcription factor E47, which lowers IgG responses in vitro and in vivo in both aged BALB/c and C57BL/6 mice (Frasca et al., 2007a; Frasca et al., 2003; Frasca et al., 2007b; Frasca et al., 2010; Frasca et al., 2005; Frasca et al., 2004). We have also demonstrated (Frasca et al., 2012) that aging is characterized by higher serum TNF-α which increases TNF-α production by B cells and this significantly decreases their capacity to make protective antibodies in response to antigenic/mitogenic stimulation. Our hypothesis is that TNF-α is involved in the negative regulation of E47, as stimulation of B cells with TNF-α induces Tristetraprolin (Frasca et al., 2012).
More recently, our objectives were to discover underlying contributions to the inflammation causing decreased B lymphocyte response with age. In this report we present evidence that the adipose tissue is a major contributor to inflammation in aged mice. Low-grade inflammation in the adipose tissue increases with age and contributes to Insulin Resistance (IR) which also increases with age. Therefore, we hypothesized that the age-related increase in B cell inflammation may be associated with increased inflammation in the adipose tissue. The adipose tissue is a major immunologically active organ that contributes to serum inflammation (Grant and Dixit, 2015), and increases in size with aging in both mice (Wu et al., 2007) and humans (Tchkonia et al., 2010; van Harmelen et al., 2003). Adipose tissue inflammation is characterized by infiltration and activation of immune cells which secrete cytokines and chemokines, thus contributing to ongoing chronic inflammation that promotes degradation of metabolic pathways in obesity (Nikolajczyk, 2010; Nikolajczyk et al., 2011). Most of the studies conducted so far support a crucial role for an increase in pro-inflammatory T cells and macrophages promoting local inflammation in the visceral adipose tissue (VAT) leading to IR and a decrease in production of anti-inflammatory cytokines, which normally maintains insulin sensitivity (IS). Studies elucidating B cell function in obesity are limited. B cells have recently emerged as crucial players regulating inflammation in murine VAT and IR, to which they contribute by presenting antigens to T cells, secreting pro-inflammatory cytokines and producing pathogenic antibodies (Winer et al., 2011). B cells infiltrate the expanding adipose tissue in response to hyper-nutrition (Duffaut et al., 2009) but the mechanism for this was not established. B cells can be activated by products of altered lipolysis in the expanding adipose tissue to release pro-inflammatory cytokines and chemokines, thus contributing to local and systemic inflammation and to the recruitment of immune cells to the VAT (Nikolajczyk, 2010; Nikolajczyk et al., 2011). Moreover, B cells support T cell inflammation (DeFuria et al., 2013).
We have recently shown in humans (Frasca et al., 2016) that obesity is associated with attenuated in vivo and in vitro antibody responses in both young and elderly individuals and that the peripheral B cell pool of individuals with obesity is characterized by decreased percentages of anti-inflammatory B cell subsets (transitional B cells) and increased percentages of proinflammatory late/exhausted memory B cells. Moreover, total B cells from both young and elderly individuals with obesity, as compared to lean individuals, have impaired B cell function, and they secrete more pro-inflammatory (IL-6) and less anti-inflammatory (IL-10) cytokines in culture supernatants.
In this paper we have identified molecular mechanisms through which the adipose tissue leads to impaired B cell function in aging mice: increased size of the epididymal VAT, production of pro-inflammatory mediators by the adipocytes, increased inflammatory B cell recruitment into the VAT, and systemic inflammation. We propose that the age-related decline in B cell function, due to increased low level inflammation with refractory response to further antigenic stimulation, is at least in part due to the influence of the powerful feed-forward loop of inflammation by adipocytes on B lymphocytes. Our results identify a novel function for the adipocytes in the down-regulation of B cell function.
2. Materials and methods
2.1. Mice
Male young (3–4 months) and old (18–24 months) C57BL/6 mice were purchased from the National Institutes of Aging and maintained in our AAALAC-certified facility. Mice were acclimated for at least 7 days before sacrifice. Mice with evidence of disease were not used in these studies. In the experiments herein we used 8 pairs of young and old mice. All studies adhered to the principles of laboratory animal care guidelines and were IACUC approved.
2.2. Isolation of epididymal VAT
Epididymal VAT from young and old mice was collected, weighted, washed with 1× Hanks' Balanced salt Solution (HBSS), resuspended in Dulbecco's modified Eagle Medium (DMEM), minced into small pieces, passed through a 70 μm filter and digested with collagenase type I (SIGMA C-9263) for 1 h at 37 °C in a water bath. Digested cells were passed through a 300 μm filter, centrifuged at 300g in order to separate the floating adipocytes from the stromal vascular fraction (SVF) containing the immune cells. The cells floating on the top were transferred to a new tube as adipocytes. The cell pellet (SVF) on the bottom was resuspended in ACK for 3 min at RT (room temperature) to lyse the Red Blood Cells. Both adipocytes and SVF were washed 3 times with DMEM. B cells were isolated from the SVF as indicated immediately below. Adipocytes were sonicated for cell disruption in the presence of TRIzol, and then centrifuged at 1000 × g at 4 °C for 20 min to separate the soluble fraction from the lipid and cell debris. The soluble fraction was then used for RNA isolation.
2.3. B cell enrichment
B cells were isolated from the spleens or from the SVF of young and old mice after a 20 min incubation at 4°C with anti-CD19 Microbeads (Miltenyi Biotec), according to the MiniMacs protocol (20 μl Microbeads + 80 μl PBS, for 107 cells). Cells were then purified using magnetic columns. At the end of the purification procedure, cells were 90–95% CD19-positive by cytofluorimetric analysis. After the isolation procedure was ended, cells were maintained in PBS for 3 h at 4°C to minimize potential effects of anti-CD19 antibodies on B cell activation, then resuspended in TRIzol (Ambion-LifeTechnologies) for RNA extraction or in M-PER (Mammalian Protein Extraction Reagent, Thermo Scientific) for protein extraction.
2.4. B cell culture
Splenic B cells were cultured in complete medium (RPMI 1640, supplemented with 10% FCS, 10 μg/ml gentamicin, 2 × 10−5 M 2-ME, and 2 mM L-glutamine). FCS was certified to be endotoxin-free. B cells (106/ml) were stimulated in 24-well culture plates with 1 μg/ml of LPS (from E. coli, SIGMA L2880) for 7 days. VAT B cells were stimulated with the Toll-like Receptor 9 agonist CpG (ODN2006) (5 μg/106 cells). At the end of the stimulation time, B cells were counted in trypan blue to evaluate viability which was found comparable in cultures of young and old B cells.
2.5. Cell staining
Markers to identify splenic and VAT B2 cell subsets are: Follicular (FO, CD19+AA4.1/CD43-CD23+CD21int), Marginal Zone (MZ, CD19+AA4.1/CD43-CD23low/negCD21hi), Age-associated B cells (ABC, CD19+AA4.1/CD43-CD23-CD21-). Markers to identify splenic and VAT macrophages (MΦ) and T cells were F4/80 and CD3, respectively.
B2 cells were membrane stained for 20 min at RT with the following antibodies: APC-Cy7-conjugated anti-CD19 (BD 557655), PE-Cy7-conjugated anti-AA4.1 (eBioscience 25-5892-81), APC-conjugated anti-CD43 (BD 560663), FITC-conjugated anti-CD21/CD35 (BD 553818) and PE-conjugated anti-CD23 (BD 553139). Cells were then fixed with BD Cytofix (BD 554655).
For intracellular (ic) staining of IgG2c, after membrane staining with anti-CD19, AA4.1, CD43, CD21, CD23, cells were fixed, permeabilized with BD Cytofix and incubated with anti-IgG2c (Bethyl A90-136B). Up to 105 events in the lymphocyte gate were acquired on an LSR-Fortessa (BD) and analyzed using FlowJo 10.0.6 software.
2.6. Cell sorting
FO and ABC B cell subsets were sorted at the Sony SH800 cell sorter. After sorting, mRNA was extracted from B cell subsets using the μMACS mRNA isolation kit (Miltenyi), according to the manufacturer's protocol. The mRNA preparations were stored at −80 °C until use. Reverse Transcriptase reactions were performed in a Mastercycler Eppendorf machine to obtain cDNA.
2.7. Preparation of B cell cytoplasmic and nuclear protein extracts
Before protein extraction, B cells were counted using trypan blue and protein extracts were prepared from the same numbers of cells. Briefly, cells were centrifuged in a 5415C Eppendorf microfuge (2000 rpm, 5 min). The pellet was resuspended in 20 μl of solution A containing Hepes 10 mM, pH 7.9, KCl 10 mM, EDTA 1.0 mM, DTT 1 mM, MgCl2 1.5 mM, PMSF 1 mM, 1 tablet of protease inhibitor cocktail (Boeringer Manheim, Germany) (per 20 ml), 1 mM Na3VO4 and Nonidet P-40 (0.1%), briefly vortexed and centrifuged (8000 rpm, 5 min, 4°C). The supernatant containing the cytoplasmic extract was removed and stored at −80 °C.
To obtain nuclear protein extracts, the pellet containing the nuclei was resuspended in solution B containing Hepes 20 mM, pH 7.9, EDTA 0.1 mM, DTT 1 mM, MgCl2 1.5 mM, PMSF 2 mM, 1 tablet of protease inhibitor cocktail (per 20 ml), and glycerol 10%. The lysate was incubated on ice for 20 min, protein sonicated for a few seconds and centrifuged (14,000 rpm, 15 min, 4°C). Aliquots of the nuclear extract were stored at −80°C. Protein contents were determined by Bradford assay. The amount of protein extracted from the same number of B cells is highly reproducible (90%) from one experiment to another in both young and old mice.
2.8. Western blotting (WB)
For the evaluation of specific proteins, protein extracts at equal protein concentration were denatured and then electro-transferred onto nitrocellulose filters. Filters were incubated with the following primary antibodies in PBS-Tween 20 containing 5% milk: rabbit anti-mouse phospho HSL (Ser563) (1:1000 diluted, Cell Signaling 4139), mouse monoclonal anti-mouse SOD1 (1:1000 diluted, Cell Signaling 4266), goat polyclonal anti-mouse NF-kB (p50) (1:200 diluted, Santa Cruz sc-1190), mouse monoclonal anti-mouse GAPDH (1:5000 diluted, GeneTex GT239). After overnight incubation with the primary antibodies, immunoblots were incubated with the following secondary antibodies for 3 h at RT: goat polyclonal anti-rabbit IgG (1:50,000 diluted, Jackson ImmunoResearch Labs 111-035-003), donkey polyclonal anti-goat IgG (1:50,000 diluted, Jackson ImmunoResearch Labs 705-035-003), goat polyclonal anti-mouse IgG (1:50,000 diluted, Jackson ImmunoResearch Labs 115-035-003). Membranes were developed by enzyme chemiluminescence and exposed to CL-XPosure Film (Pierce). Films were scanned and analyzed using the AlphaImager Enhanced Resolution Gel Documentation & Analysis System (Alpha Innotech, San Leandro CA) and images were quantitated using the AlphaEaseFC 32-bit software.
2.9. RNA extraction and cDNA preparation
Total RNA was extracted from unstimulated B cells, as well as from adipocytes, and resuspended in TRIzol. The mRNA was extracted from stimulated B cells using the μMACS mRNA isolation kit (Miltenyi), as indicated above.
2.10. Quantitative PCR (qPCR)
Reactions were conducted in MicroAmp 96-well plates, and run in the ABI 7300 machine. Calculations were made with ABI software. Briefly, we determined the cycle number at which transcripts reached a significant threshold (Ct). A value for the amount of the target gene, relative to GAPDH, was calculated and expressed as ΔCt. Primers for PCR amplification were the following (all from Life Technologies): Mm00507774 (AID), Mm00443260 (TNF-α), Mm00446190 (IL-6), Mm00445235 (CXCL10), Mm00441242 (CCL2), Mm01302427 (CCL5), Mm9999054 (CXCR3), Mm99999051 (CCR2), Mm00515543 (CCR3), Prdm1 (Mm00476128), Tbx21 (Mm00450960), Kifc3 (Mm00516085), Stx3 (Mm01197689), IL-21 (Mm00517640), IFN-γ (Mm01168134), Mm99999915 (GAPDH).
2.11. Enzyme-linked immunosorbent assay (ELISA)
To measure IgG subclasses (IgG1/G2a/G2b/G2c/G3) in lysates of VAT lymphocytes, goat anti-mouse Southern Biotech purified anti-subclass antibodies were used for coating, at the concentration of 2 μg/ml. Detection antibody was a biotinylated goat anti-mouse IgG antibody (Jackson ImmunoResearch Labs 115-065-071), followed by streptavidin-HRP. Substrate was TMB substrate reagent set (BD OptEIA 555214).
2.12. Co-culture of adipocytes and splenocytes
The ratio between adipocytes and splenic lymphocytes in co-cultures was equal to that which we measured in ex vivo isolated VAT (ratio adipocytes:lymphocytes). In the transwells, cells were co-cultured by using inserts with a 0.4μm porous membrane (Corning) to separate adipocytes and splenic lymphocytes. Cells were left unstimulated. After 72 h, cells in the upper wells (splenic lymphocytes) were harvested, washed and stained to evaluate percentages and numbers of B2 B cell subsets.
2.13. Preparation of adipocyte-conditioned medium (ACM)
Freshly harvested epididymal fat pads from individual mice were cultured in complete RPMI for 24 h at the concentration of 1 g/100 μl to obtain ACM as previously described (Kratz et al., 2014). FO B cells were sorted as in 2.6 and were stimulated with adipocyte-conditioned medium for 48 h.
2.14. Statistical analyses
To examine differences between groups, Student's t-tests (two-tailed) were used. To examine the relationships between variables, bivariate Pearson's correlation analyses were performed, using GraphPad Prism 5 software.
3. Results
3.1. Expression of AID in stimulated splenic B cells is negatively correlated with epididymal fat size
The characteristics of the mice used in our study are shown in Table 1. Briefly, both mouse weight and the size of epididymal VAT increase with age and the increase in the size of epididymal VAT is positively correlated with the increase in mouse weight (r = 0.72, p = 0.0016). Also the number of nucleated immune cells increase with age in the SVF in the VAT (r = 0.81, p = 0.0001), as well as percentages of F4/80+ MΦ (r = 0.74, p = 0.0057), CD19+ (r = 0.69, p = 0.0063), CD3+ (r = 0.72, p = 0.0037), similar to what has already been shown (Amano et al., 2014; Winer et al., 2011).
Table 1.
Characteristics of the mice in this study.
| Young | Old | |
|---|---|---|
| Mouse weight (grams) | 27.5 ± 1.5 | 40.5 ± 1.9*** |
| Epididymal fat weight (grams) | 0.7 ± 0.1 | 2.6 ± 0.3**** |
| Number of nucleated cells in epididymal SVF(×103) | 763 ± 277 | 9173 ± 1122**** |
p<0.001.
p<0.0001.
We have previously shown that AID in LPS-stimulated B cells is a marker of optimal B cell function and is significantly decreased in B cells from aged as compared to young mice (Frasca et al., 2012). Here we show that AID is negatively associated with the size of epididymal VAT (Fig. 1). This novel observation suggests a role of the adipose tissue in the down-regulation of B cell function in aged mice.
Fig. 1.
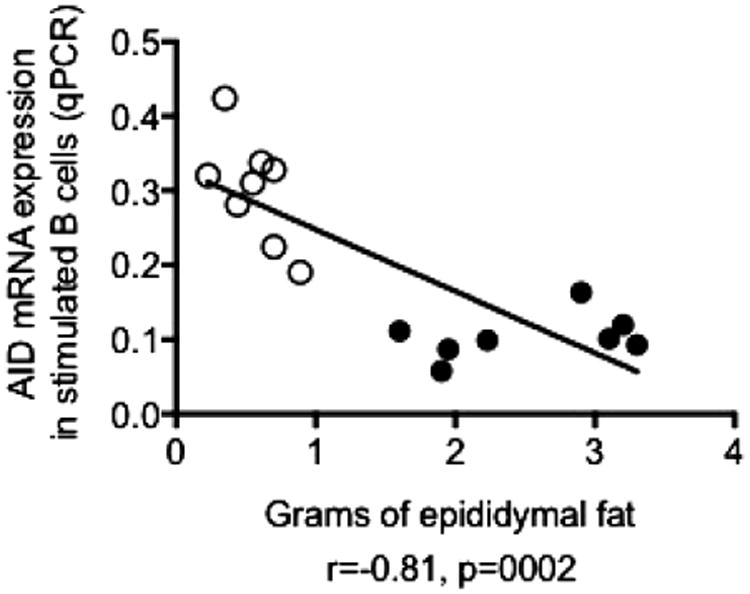
AID in stimulated splenic B cells is negatively correlated with the size of epididymal VAT. B cells were isolated by magnetic sorting from the spleens of 8 pairs of young (open symbols) and old (filled symbols) mice. B cells (106/ml) were stimulated with 1 μg/ml of LPS for 7 days, then mRNA was extracted and qPCR performed to evaluate mRNA expression of AID and GAPDH (control). Epididymal fat pads were isolated from the same young and old mice and AID values were correlated with the size of epididymal fat pads. Pearson's r and p values are at the bottom of the figure.
3.2. Age- and tissue-associated changes in the relative percentages of B2 B cell subsets in the spleen and the epididymal VAT
We measured the percentages of FO, ABC and MZ B2 B cell subsets in the spleens and epididymal VAT of young and old mice, as shown in Fig. 2A. Results show reduced percentages of the antiinflammatory FO subset in the spleen of old mice as compared to young mice and concomitant increased percentages of the proinflammatory ABC subset (Fig. 2B) as previously shown (Hao et al., 2011; Rubtsov et al., 2011) and (Frasca et al., 2012; Ratliff et al., 2013). No effects on MZ were observed. Percentages of FO were reduced (and percentages of ABC were increased) even more in VAT versus spleen (Fig. 2C). Numbers of cells showed similar differences (Fig. 2D, E).
Fig. 2.
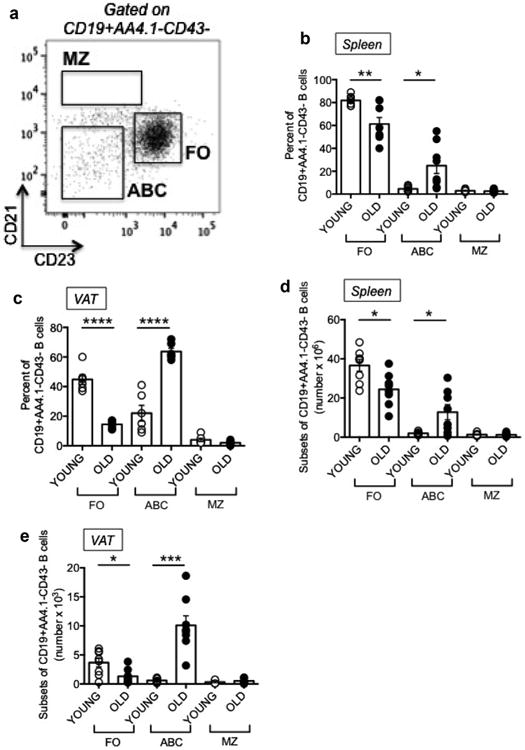
Age- and tissue-associated changes in the relative percentages of B2 B cell subsets in the spleen and the epididymal VAT. The spleens and VAT from 8 pairs of young and old mice, same as in Fig. 1, were stained and a representative dot plot of splenic B cells from a young mouse is shown in A. Percentages (B,C) and numbers (D,E) of the major B cell subsets, FO, ABC and MZ B cell subsets, were evaluated; cells were gated on CD19+AA4.1-CD43- cells to exclude transitional (AA4.1+) and B1 (CD43+) B cells. Mean comparisons between groups were performed by Student's t-test (two-tailed). *p<0.05, **p<0.01, ***p< 0.001, ****p< 0.0001.
B cells isolated from the VAT and spleen were compared for markers of lipolysis in cytoplasmic extracts, i.e. for phospho-HSL (Hormone-Sensitive Lipase), a key enzyme in the regulation of lipid stores which catalyzes the hydrolysis of triacylglycerol generating free fatty acids (FFAs) (Anthonsen et al., 1998; Degerman et al., 1990). This enzyme is crucial because the activation of the inflammatory process during obesity has been shown to be caused by the release of FFAs (Johnson et al., 2012). We also measured the anti-oxidant enzyme SOD-1 (superoxide dismutase-1) (McCord and Fridovich, 1988), which is anti-inflammatory and decreases reactive oxygen species and oxidative stress. These experiments were performed only on individual old mice, as young mice do not have a sufficient amount of VAT to allow the isolation of enough B cells to perform WB experiments. Results in Fig. 3 show that phospho-HSL is present in cytoplasmic extracts of VAT-isolated but not of spleen-isolated B cells, whereas SOD-1 is expressed in cytoplasmic extracts of splenic B cells only. We also evaluated NF-kB (p50) expression in nuclear extracts of the same VAT versus splenic B cells and found higher levels of expression in VAT-isolated B cells, as expected due to the higher inflammatory profile of the VAT versus the spleen.
Fig. 3.
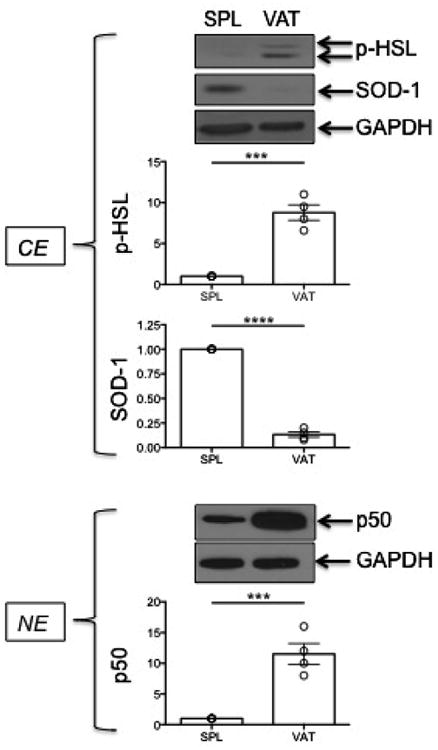
Markers of lipolysis and inflammation are expressed at higher levels in B cells from the VAT as compared to B cells from the spleen. Cytoplasmic (CE) and nuclear (NE) protein extracts (5 μg/lane) from B cells isolated from the spleens or VAT of 4 old mice (among those in Fig. 1) were blotted with the indicated antibodies. Percentages of ABC in these mice in spleen and VAT were, respectively: 48,32,55,29 and 58,62,60,72. Only old mice are shown as from young mice we did not extract enough proteins to run the experiment. Densitometric analyses (arbitrary units) of protein expression, normalized to GAPDH, are shown and referred to splenic B cell extracts (SPL), taken as 1. ***p < 0.001, ****p < 0.0001.
3.3. Induction of pro-inflammatory B cell subsets by co-cultures of splenic B cells with adipocytes
To evaluate the ability of adipocytes to promote inflammation and induce ABCs, we co-cultured in transwells adipocytes from epididymal fat pads of young and old mice with splenic B cells from the young mice. Results show that co-culture for 72 h of young splenic B cells with adipocytes from the same young mice, in the absence of any additional mitogenic stimulus, significantly changed the relative percentages of the B cell subsets, leading to an increase in the percentages of ABC, which was similar to what we have observed in the VAT, or even higher when splenic B cells were co-cultured with adipocytes from old mice (Fig. 4A). These results are the first to our knowledge to show a direct effect of the adipose tissue on the induction of pro-inflammatory B cell subsets. However, we did not know if in these experiments there was expansion of the ABC population, increased survival of ABC versus FO B cells, death of FO B cells, loss of cell markers, or a combination of these. To address this point we performed a new series of experiments in which we sorted splenic FO and ABC from the spleens of old mice and we measured by qPCR the expression of several markers described to be differentially expressed in these 2 subsets by a transcriptome analysis performed previously by the Marrack group (Rubtsov et al., 2011). We selected 4 markers among those most differentially expressed in FO versus ABC: Prdm1 (Blimp-1), Tbx21 (T-bet), Kifc3, Stx3. All these markers were found expressed at higher levels in ABC versus FO, as expected (Fig. 4B). Then, we cultured sorted splenic FO B cells from old mice in the presence of ACM and we measured if in these conditions FO B cells would up-regulate the expression of one of the markers in culture. Results (Fig. 4C) show that ACM induced significant increased expression of Prdm1 as compared to complete medium, suggesting that the differentiation of FO B cells into ABC may have occurred in these conditions. Experiments in progress in our laboratory are measuring the up-regulation of the other markers in these conditions.
Fig. 4.
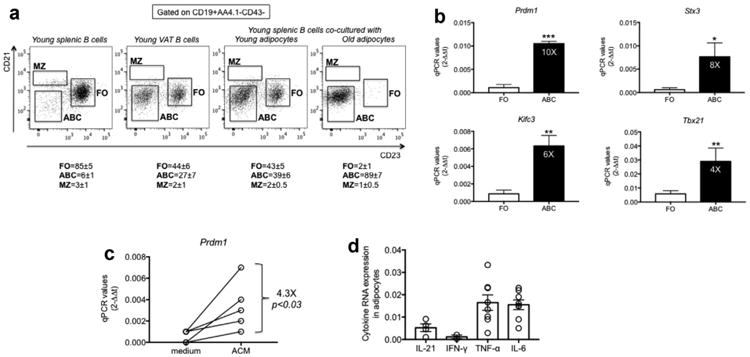
Induction of pro-inflammatory B cell subsets by co-culture of splenic B cells with adipocytes. A. B cells from the spleen and VAT of young mice were isolated and stained as in Fig. 2 to evaluate the relative percentages of FO, ABC, MZ. Adipocytes were isolated from the VAT of young and old mice and cultured for 72 h in transwells with splenic B cells from young mice. After this time, B cells were stained as indicated above and the percentages of the major B cell subsets measured by flow cytometry. Mice are different from those in previous Figs. Numbers below each graph represent percentage (means ± SE) from 4 independent experiments. Percentages of ABC in the VAT of old mice were 49,57,66,58. Splenocytes cultured for 72 h without adipocytes showed a profile similar to the left panel (not shown). B. FO and ABC were sorted from the spleens of old mice, mRNA extracted and qPCR performed. Results show qPCR values (2−ΔΔCt) of Prdm1, Tbx21, Kifc3, Stx3 mRNA expression. Mean comparisons between groups were performed by Student's t-test (two-tailed). *p<0.05, **p< 0.01, ***p< 0.001. C. Splenic FO B cells sorted as described in B were cultured for 48 h in the presence of complete RPMI or ACM at the concentration of 106/ml. The mRNA was harvested from the cultured cells, mRNA extracted and qPCR performed to evaluate mRNA expression of Prdm1. Results show qPCR values (2−ΔΔCt) of Prdm1 mRNA expression. Mean comparisons between groups were performed by Student's t-test (two-tailed). D. Adipocytes were isolated from the VAT of old mice. The mRNA was extracted and qPCR performed. Results, from 4 independent experiments (IL-21 and IFN-γ) or from 8 independent experiments (TNF-α and IL-6), show qPCR values (2−ΔΔCt) of cytokine mRNA expression.
In order to evaluate if the ACM contains factors which may be responsible for FO differentiation into ABC, as suggested by a paper recently published by Cancro's group (Naradikian et al., 2016b), we measured by qPCR production of IL-21 and IFN-γ by the adipocytes. Results in Fig. 4D show that adipocytes express mRNA for both cytokines. These results are consistent with the possibility that adipocytes may help to induce ABC in part be secreting IL-21 and IFN-γ. Lymphocytes in the SVF were negative for these cytokines (data not shown). In addition, we also show that adipocytes express mRNA for TNF-α and IL-6 which may contribute to a positive feedback loop in the induction of ABC in the VAT.
3.4. The adipocytes secrete chemokines which may recruit B cells to the VAT
We hypothesize that the adipocytes promote migration of B cells to the VAT through secretion of pro-inflammatory cytokines and chemokines and may regulate B cell phenotype and function in the VAT. Results in Fig. 5A show that the adipocytes from the VAT of both young and old mice express RNA for the chemokines CXCL10/CCL2/CCL5, with higher levels found in old mice for CCL2. The corresponding receptors are expressed by VAT B cells, with higher levels of expression found for CCR3, the receptor for CCL2 (Fig. 5B).
Fig. 5.
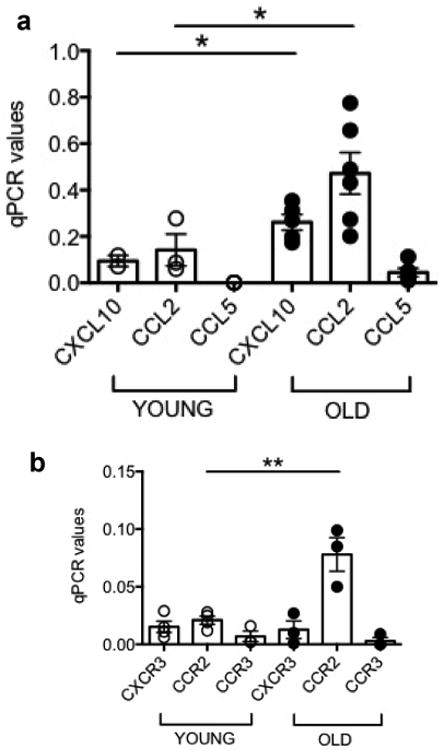
The adipocytes secrete chemokines which may recruit B cells to the VAT. A. Adipocytes were isolated from the VAT, sonicated for cell disruption in the presence of TRIzol to separate the soluble fraction (used for RNA isolation) from lipids and cell debris. Results show qPCR values (2−ΔΔCt) of CXCL10, CCL2, CCL5 RNA expression. Mean comparisons between groups were performed by Student's t-test (two-tailed). B. VAT B cells were isolated from the SVF and resuspended in TRIzol. Results show qPCR values (2−ΔΔCt) of CXCR2, CCR2, CCR3 RNA expression. Results in B are from those 3 mice among the 6 in A from which we had enough B cells. Mean comparisons between groups were performed by Student's t-test (two-tailed). *p<0.05, **p<0.01.
3.5. IgG2c is the major IgG subclass in the VAT
The ongoing apoptosis of cells in the VAT, due to increase in fat mass and consequent hypoxia, induces the release of “self” antigens and consequent production of class switched IgG “autoimmune” antibodies. Data previously published by Winer et al. have shown antibody staining in VAT localized inside crown-like structures (Winer et al., 2011), which represent aggregated mono- and multinucleate giant MΦ surrounding the adipocytes, which are typically associated with chronic inflammation (Murano et al., 2008). We therefore measured the different subclasses of IgG antibodies secreted by VAT lymphocytes and found a significant production of IgG2c antibodies, which was higher than that of other IgG subclasses (p < 0.05 versus IgG1, IgG2a, IgG2b, IgG3) (Fig. 6A). Because we found high percentages of ABC in the VAT, and because these cells have been shown at least in the spleen to produce IgG2c antibodies (Hao et al., 2011; Rubtsov et al., 2011), which are pathogenic (Winer et al., 2011) and autoimmune [reviewed in (Naradikian et al., 2016a)] antibodies, we measured the secretion of IgG2c antibodies by ic staining of ABC in the VAT (Fig. 6B) and in the spleen as control (Fig. 6C). We detectic IgG2c in splenic ABC after stimulation with CpG, and in VAT ABC even in the absence of stimulation. In these experiments we also tested icIgG2c production by splenic FO: MFI unstimulated was 115, MFI CpG-stimulated was 123 (data not shown). These data show that ABC in the VAT are already highly pre-activated and are refractory to further stimulation. When we correlated IgG2c antibodies (ratios of OD in IgG2c/OD in total IgG) in VAT lysates with the percentages of ABC in the VAT and in the spleen, we found significant positive associations in both cases (Fig. 6D and E), whereas no associations were found with the percentages of FO in the VAT and in the spleen (Fig. 6F and G). These results strongly suggest that ABC are the main producers of IgG2c antibodies in both tissues. These results also show for the first time higher IgG2c production by ABC in the VAT, as compared to splenic ABC.
Fig. 6.
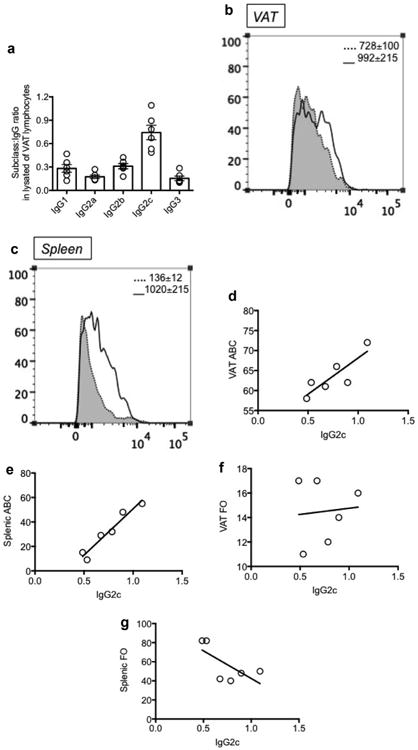
IgG2c is the major IgG subclass in the VAT. A. IgG subclasses in protein lysates of VAT lymphocytes were measured by Ig ELISA, after coating the plates with goat anti-mouse Southern Biotech purified anti-subclass antibodies at the concentration of 2 μg/ml. Detection antibody was a biotinylated goat anti-mouse IgG antibody, followed by streptavidin-HRP. Results are ratios of OD in subclasses/OD in total IgG. OD were measured with a spectrophotometer at 450 nm. B-C. ic staining of IgG2c in VAT ABC (B) and in splenic ABC (C) was performed after 48 h in culture in the absence (shadow, dotted line) or presence (solid line) of CpG. Mean Fluorescence Intensity from 3 independent experiments ± SE are shown in each quadrant. D-E. Correlation between IgG2c (ratios of OD in IgG2c/OD in total IgG) in VAT lysates and ABC percentages in VAT and spleen. Pearson's r = 0.85, p = 0.03 and r = 0.97, p = 0.002, respectively. F-G. Correlation between IgG2c (ratios of OD in IgG2c/OD in total IgG) in VAT lysates and FO percentages in VAT and spleen. Pearson's r = 0.09, p = 0.86 and r = −0.7, p = 0.13, respectively.
4. Discussion
Results in the present study show that the age-related decrease in B cell function is negatively associated with an increase in the VAT and that the adipose tissue directly impairs the function of B cells by changing the composition of the B cell pool and inducing pro-inflammatory B cell subsets. We also show reduced percentages and numbers of the anti-inflammatory FO subset in the spleen of old versus young mice and concomitant increased percentages of the pro-inflammatory ABC. Percentages and numbers of FO were reduced (and those of ABC were increased) even more in VAT versus spleen, suggesting higher local inflammation in the VAT as compared to the spleen. The VAT of old mice shows indeed a switch into a pro-inflammatory, tissue-remodeling, senescent-like state, as shown by higher mRNA expression of pro-inflammatory cytokine and chemokine mediators.
Studies in obese young mice have already shown that obesity induces B cell inflammation, production of pro-inflammatory cytokines and promote pro-inflammatory T cells and MΦ (DeFuria et al., 2013). Moreover, B cells produce pathogenic IgG antibodies as a consequence of increased fat mass, hypoxia and apoptosis in the VAT, leading to the release of self antigens which provoke an autoimmune response with the production of class switched IgG pathogenic antibodies which have been detected in serum (Winer et al., 2011). Transfer of these antibodies rapidly induces IR and glucose intolerance (Winer et al., 2011).
We show here for the first time that the VAT of old mice is also enriched in cells secreting IgG antibodies, including IgG2c, likely directed against self VAT antigens and we show here that the ABC subset in the VAT is the major B cell subset in which IgG2c antibodies are detected, similar to what has already been shown in the spleen (Hao et al., 2011; Rubtsov et al., 2011). Experiments to measure the specificity of these IgG2c antibodies are currently under investigation in our laboratory.
In addition to the secretion of factors which chemoattract the immune cells, the VAT is a source of lipotoxic danger signals that also induce inflammation. Among these, we evaluated the expression of p-HSL by B cells in the VAT as compared to B cells in the spleen. This enzyme catalyzes the hydrolysis of triacylglycerol generating FFAs (Anthonsen et al., 1998; Degerman et al., 1990), which have been shown to be the initiating cause of the inflammatory process during obesity (Johnson et al., 2012). We show here for the first time that p-HSL is expressed by VAT-B cells, more than in splenic B cells from old mice, suggesting that the activation of this enzyme in the VAT and consequent release of FFAs may be responsible for the high percentages of ABCs observed in the VAT as compared to the spleen. We also found higher levels of expression of NF-kB (p50) in nuclear extracts of VAT-isolated B cells, which indicate translocation from the cytoplasm and activation, which was expected due to the higher inflammatory profile of the VAT versus the spleen. Conversely, SOD-1 is present in splenic B cells, more than in VAT-B cells, indicating that anti-oxidant reactions are almost absent in VAT due to hypoxia, reduced amount of oxygen and oxygen radicals.
Our results altogether suggest that age-related increased inflammation in the VAT induces intrinsic inflammation in the adipocytes and a positive feed-forward loop, leading to the accumulation of infiltrating immune cells which feed-back and secrete pro-inflammatory cytokines and chemokines. This would contribute not only to local but also to systemic inflammation. Infiltrating B and T cells drawn to the VAT by the chemokines released by the adipocytes become more inflammatory and circulating to peripheral lymphoid organs, would generate suboptimal immune response in aged and obese mice and humans. This novel finding sheds light on causes and mechanisms leading to reduced responses to antigens and vaccines in individuals with obesity which are necessary to know in order to successfully treat disease conditions associated with chronic inflammation associated with aging. Our results have also identified the ABC as the major subset secreting IgG2c antibodies in the VAT and in the spleen. In a cohort of obese male individuals, it has been shown that IR is associated with a distinct profile of IgG autoantibodies (Winer et al., 2011). Additional work is needed to characterize the specificity of these antibodies. The characterization of pathogenic pathways involved in B cell-mediated immune responses within the VAT will open new avenues to modify obesity, control associated complications, and enhance B cell function.
Acknowledgments
Funding sources: This study was supported by NIH AG023717 (BBB), NIH AI096446 and AG042826 (BBB/DF).
Footnotes
Disclosures: No potential conflicts of interest relevant to this article are reported.
References
- Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degerman E, Smith CJ, Tornqvist H, Vasta V, Belfrage P, Manganiello VC. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci U S A. 1990;87:533–537. doi: 10.1073/pnas.87.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley RL, Blomberg BB. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J Immunol. 2003;170:719–726. doi: 10.4049/jimmunol.170.2.719. [DOI] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Landin AM, Gong D, Riley RL, Blomberg BB. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–6644. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Alvarez JP, Blackshear PJ, Riley RL, Blomberg BB. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007a;179:918–927. doi: 10.4049/jimmunol.179.2.918. [DOI] [PubMed] [Google Scholar]
- Frasca D, Riley RL, Blomberg BB. Aging murine B cells have decreased class switch induced by anti-CD40 or BAFF. Exp Gerontol. 2007b;42:192–203. doi: 10.1016/j.exger.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Romero M, Landin AM, Diaz A, Riley RL, Blomberg BB. Protein phosphatase 2A (PP2A) is increased in old murine B cells and mediates p38 MAPK/tristetraprolin dephosphorylation and E47 mRNA instability. Mech Ageing Dev. 2010;131:306–314. doi: 10.1016/j.mad.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Romero M, Diaz A, Alter-Wolf S, Ratliff M, Landin AM, Riley RL, Blomberg BB. A molecular mechanism for TNF-alpha-mediated downregulation of B cell responses. J Immunol. 2012;188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) 2016;24:615–625. doi: 10.1002/oby.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015;23:512–518. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968–1988) Free Radic Biol Med. 1988;5:363–369. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- Naradikian MS, Hao Y, Cancro MP. Age-associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol Rev. 2016a;269:118–129. doi: 10.1111/imr.12380. [DOI] [PubMed] [Google Scholar]
- Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, Bengsch B, Linderman SL, Stelekati E, Spolski R, Wherry EJ, Hunter C, Hensley SE, Leonard WJ, Cancro MP. Cutting edge: IL-4, IL-21, and IFN-gamma interact to govern T-bet and CD11c expression in TLR-activated B cells. J Immunol. 2016b;197:1023–1028. doi: 10.4049/jimmunol.1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–250. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajczyk BS. B cells as under-appreciated mediators of non-auto-immune inflammatory disease. Cytokine. 2010;50:234–242. doi: 10.1016/j.cyto.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFalpha and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- van Harmelen V, Skurk T, Rohrig K, Lee YM, Halbleib M, Aprath-Husmann I, Hauner H. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–895. doi: 10.1038/sj.ijo.0802314. [DOI] [PubMed] [Google Scholar]


