Abstract
Introduction
Presence of chronic low grade inflammation has often been implicated in the etiology of atrial fibrillation (AF). Whether pre-existing inflammatory state promotes AF or initiation of AF activates inflammation is a dilemma among clinicians. This study investigates the role of high sensitive C reactive protein (hs-CRP) and interleukin 6 (IL-6) in AF with rheumatic mitral stenosis (Rh-MS) as markers of chronic inflammation.
Methods
This case control cohort included sixty five (n = 65) Rh-MS patients having other valve lesions as trivial to mild. Out of them twenty nine (n = 29; group C) had baseline AF and rest were normal sinus rhythm (NSR). A 24 h holter recording was done in NSR patients to diagnose paroxysmal AF/tachyarrhythmia forming group B (n = 12) and not having any tachyarrhythmia were designated as NSR; group A (n = 24).
Results
hs-CRP and IL6 showed statistically significant increase in group C (permanent AF) compared to group A (95% CI: 4.2–0.9, p = 0.007; 95% CI: 1.2–0.89; p = 0.05 respectively), while it was non significant between group A and group B (p > 0.05). A weak positive correlation was observed with hs-CRP and left atrial volume index (LAVi) (r = 0.45, p = 0.06) in AF group as compared to NSR group. 68.2% of patients in AF group (27/41) had moderate to severe spontaneous echo contrast (SEC) as compared to 37.5% (10/24) in NSR group.
Conclusion
Increased hs-CRP and IL-6 levels in the paroxysmal and permanent AF group may favour the hypothesis that low grade chronic inflammation could be the cause of atrial fibrillation than a consequence.
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; ECG, electrocardiography; ELISA, enzyme linked immunosorbent assay; hs-CRP, high sensitive C reactive protein; IL, interleukin; LA, left atrium; LAVi, left atrial volume index; LVEF, left ventricular ejection fraction; MMP, matrix metalloproteinases; MR, mitral regurgitation; MVA, mitral valve area; NSR, normal sinus rhythm; PHT, pressure half time; RHD, rheumatic heart disease; Rh MS, rheumatic mitral stenosis; ROC, receiver operating curve; RVSP, right ventricular systolic pressure; SD, standard deviation; SEC, spontaneous echo contrast; SVE, supraventricular ectopics
Keywords: Rheumatic mitral stenosis, Atrial fibrillation, Hs-CRP & IL6
1. Introduction
The alarming prevalence to 2.2/1000 population of rheumatic heart disease (RHD) continues to be a serious health burden in developing countries like India,1 fuelling interest in clinicians and researchers to study its epidemiology, pathogenesis and prevention.2 The clinical events leading to atrial fibrillation (AF) are multiple which include hemodynamic, electrophysiological and metabolic abnormalities, in addendum with genetic factors.3, 4 The dogma of inflammation is in quest since a decade whether initiation of AF activates inflammatory effects or the presence of pre-existing inflammatory state promotes persistence of atrial arrhythmias. It has been cited that low grade inflammation is not only a response to the underlying arrhythmic process but also an integral part of it .5, 6
Biomarkers act as surrogate markers in understanding the biological, pathogenesis, clinical states of a disease in response to an intervention or identifying patients at high risk.7 Inflammatory biomarkers could potentially refine clinical risk stratification for stroke and thromboembolism. The association of inflammation with non rheumatic AF has been demonstrated in recent studies with significantly raised high sensitive C reactive protein (hs-CRP) and interleukin (IL)6 in both paroxysmal and permanent AF with marked inflammatory infiltrates and myocyte necrosis in atrial biopsies.8 It was observed that CRP was two-fold higher in AF patients when compared with control group having no history of atrial arrhythmia.9
The role of biomarkers in chronic low grade inflammation in atrial fibrillation in rheumatic mitral stenosis (Rh-MS) has not been addressed. The mechanism of this chronic process is debatable and is thought to be due to a continuing low-grade rheumatic process or due to hemodynamic stresses on the damaged valve.10, 11 The objectives of this research were a) to investigate the role of inflammatory cytokines– hs-CRP and IL-6 in isolated Rh-MS and their association with atrial fibrillation; b) to detect episodes of atrial arrhythmias in patients of Rh-MS in normal sinus rhythm (NSR) and their association with chronic inflammation.
2. Methods
This prospective observational case control study included patients with isolated Rh-MS with other valve lesions as trivial to mild (except tricuspid regurgitation without organic involvement). Patients of either sex, between 18 and 45 years were recruited from the cardiology clinics of the institute over a period of one year. Age matched patient’s relatives or local hospital and community dwellers formed healthy controls. Patients with known supraventricular tachyarrhythmias with left ventricular ejection fraction (LVEF) <50%, coronary artery disease, recent cerebro-vascular accident within 3months), hyperthyroidism, diabetes mellitus, fasting blood sugar >126 mg/dl or on drugs/insulin), obesity – body mas index (BMI) >30, acute or chronic infections, malignancy, liver dysfunction, major surgical procedure in the last 3 months and renal failure (creatinine > 2.5 or on dialysis) were excluded. The study had ethics committee approval and written informed consent was obtained from all eligible patients prior to recruitment. Baseline demographic data, prior medications, clinically relevant history and examination was performed in all patients. All patients underwent routine hematological, biochemical and cardiac investigations like echocardiography (ECG) and doppler.
2.1. Echocardiographic evaluation
Echocardiography was performed on Philips IE 33(Philips Ultrasound, Bothell, WA) machine. Mitral valve area (MVA) was calculated by pressure half time (PHT) as well as by 2D planimetry. Lowest of the two was taken as actual MVA. Patients with mitral valve area <1 cm2 were classified as severe MS, between 1.0–1.5 cm2 as moderate and between 1.5–2.0 cm2 as mild MS.
2.2. Measuring LA size
Left atrium (LA) end-systolic diameter was measured in the parasternal long axis and apical 4-chamber view in M-mode at end systole. Antero-posterior, medio-lateral and apico-basal diameters of the left atrium were used to calculate its volume in milliliters (ml) with the ellipse method (the product of these three dimensions is further multiplied with the constant value 0.523). The LA volume was then indexed to body surface area and represented as ml/m2.
2.3. Spontanoeus echo contrast (SEC) and thrombi
The presence of thrombus was diagnosed when an intracavitary echo-dense mass with an echocardiographic appearance different from the atrial endocardium and the pectinate muscles was detected in atleast two orthogonal views. The presence of SEC was diagnosed when dynamic and swirling intracavitary smoke-like echoes were detected, which were differentiated from white noise artifact by their characteristic swirling pattern and by careful attention to the gain settings. The severity of SEC was scored as, 0 = no SEC, grade 1 + = mild SEC at some part of LA, grade 2 + = severe swirling SEC that appeared throughout LA. The same grading was used for SEC in the right atrial cavity.12
2.4. Holter analysis
Patients in normal sinus rhythm were put on 24 h holter monitoring for paroxysmal atrial tachycardia, paroxysmal atrial fibrillation, flutter and multifocal atrial tachycardia. The duration, number of episodes and ventricular rate of each tachycardia episode were carefully measured.
3. Blood sample collection
10 ml venous blood sample was obtained for quantitative measurement of hs-CRP and IL-6 levels from all patients. The samples were allowed to clot, serum separated and stored at less than −80 ° Celsius. The relevant markers were measured by standard ELISA kits, hs-CRP (BioCheck, Inc. Foster City) and IL-6 (Quantikine R&D systems, Minneapolis) according to the manufacturer’s instructions. ELISA was performed and absorbance taken at 450 nm wavelength The plate had starting wells with 6–8 standards with high to low concentrations decreasing in duplicates, wells for quality control both for samples and duplicates. Standard curve was made with absorbance on Y axis vs concentration on X axis. A Quality control was run to check the validity of the measurements.
4. Statistical analysis
SPSS statistics version 17(SPSS Inc, ILL) was used for analysis, differences between mean values were analyzed by Student’s unpaired t-test for those with a normal distribution and by Mann–Whitney U test for those without a normal distribution. To compare proportions chi-square test/fisher exact test were used. A two tailed p value of ≤0.05 was considered statistically significant with 95% confidence interval (CI). Mean and standard deviation (SD) was calculated for all values.
5. Results
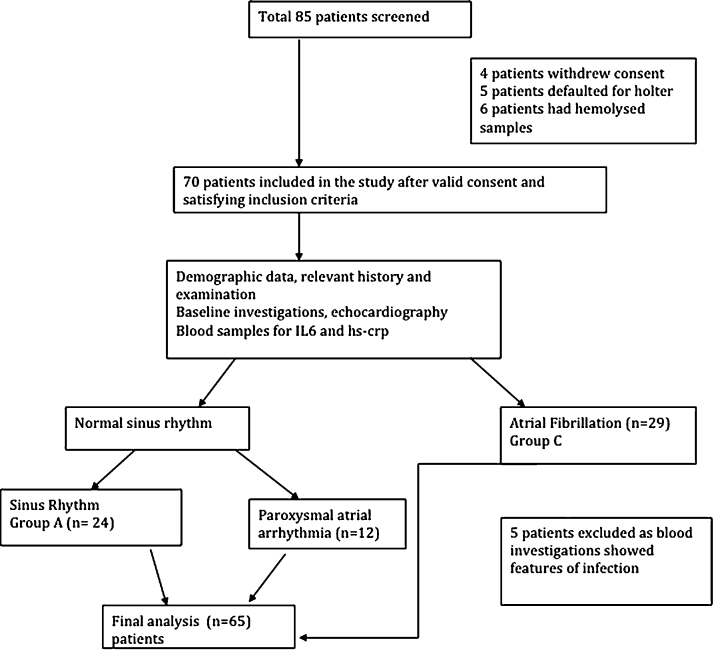
All prospective eligible patients were screened, out of which eighty five (n = 85) patients and ten (n = 10) age matched healthy controls were recruited, whereas sixty five patients (n = 65) were included in the final analysis (Fig. 1). Mean age ± SD for all patients was 33.8 ± 8.1 years with M:F = 1:1.3. Out of these patients, thirty six (n = 36) subjects had NSR on ecg strip and twenty nine (n = 29) patients were diagnosed with AF without P waves. The mean mitral valve area in all patients was 0.765 ± 0.19 cm2 with a mean gradient of 13.15 ± 4.4 mmHg indicating that moderate to severe mitral stenosis patients were studied (Table 1). The mean Wilkin score in all patients was 8.92 indicating significant valvular calcification with reduced mobility of the leaflets. The thickening of leaflet tissue was more than 8–10 mm (normal thickness is between 4 and 5 mm)
Fig. 1.
Flow chart depicting the study design.
Table 1.
Baseline characteristics of the study population.
| Baseline characteristics | N = 65 (%) | |
|---|---|---|
| Mean Age (years) ± SD | 33.83 ± 8.026 | |
| Sex | Female | 40 (61.55) |
| Male | 25 (38.5%) | |
| Dyspnea (class) | NYHA II | 26 (40.0%) |
| NYHA III | 38 (58.5%) | |
| NYHA IV | 11 (5%) | |
| Palpitations | 43 (66.2%) | |
| TIA/Stroke | 18 (27)% | |
| Mitral valve area (cm2) | 0.765 ± 0.19 (0.4−1.3) | |
| Mean mitral gradient (mm Hg) | 13.15 ± 4.448 (5–26) | |
| Severity of mitral stenosis | Severe | 59 (90.8%) |
| moderate | 7 (9.2%) | |
| LA volume (ml/m2 BSA) | 107.99 ± 45.43 (47.9–258.1) | |
| LA/LAA clot | 15 (23.1%) | |
| Spontaneous echo contrast (SEC) | Present | 37 (56.9%) |
| Grade 2 | 19 (29.2%) | |
| Grade 3 | 18 (27.7%) | |
| LA/LAA clot or SEC | 38 (58.5%) | |
| Rhythm | Sinus | 36 (55.4%) |
| Atrial fibrillation | 29 (44.6%) | |
| RVSP (mm Hg) | 46.94 ± 19.952 (20–130) | |
| On Penicillin prophylaxis | 65 (100%) | |
| Oral anticoagulants | Sinus (n = 36) | 0 |
| AF (n = 29) | 23 (79.3%) | |
AF: atrial fibrillation; BSA: Body surface area; ESR: erythrocyte sedimentation rate, LA: Left atrium; LAA: left atrial appendage; LVEF: left ventricular ejection fraction, NYHA: New York heart association, RVSP: right ventricular systolic pressure, SEC: spontaneous echo contrast; TIA: transient ischemic attack; TLC: total leucocyte count.
5.1. Holter analysis for rhythm
Thirty six (n = 36) patients in normal sinus rhythm were subjected to 24 h holter recording, out of which twelve patients (n = 12) had paroxysmal atrial tachyarrhythmias. 9 patients of these had cumulative 59 episodes of AF with mean duration of 5.27 ± 4.1 s, while others had cumulative 15 episodes of ectopic atrial tachycardia with mean duration of 3.2 ± 2.36 s. Isolated supraventricular ectopics (SVE) were encountered in 31 patients (86.1%) with six patients having frequent SVE >10/hour, while supraventricular couplets were seen in 3 patients (8.3%) (Table 2) Two patients had both episodes of atrial fibrillation and ectopic atrial tachycardia, so total of 12 patients had atrial tachyarrhythmias (9 + 5 − 2).
Table 2.
Holter analysis in 36 NSR patients.
| N = 36 | N% | Number of episodes | Duration (seconds) | |
|---|---|---|---|---|
| Isolated SVE | 31 | 86.1% | NA | NA |
| SV couplets | 3 | 8.3% | NA | NA |
| SVE (>10/h) | 6 | 16.7% | NA | NA |
| Atrial fibrillation | 9 | 25% | 59 | 5.27 ± 4.10 (1.8–15) |
| Ectopic atrial tachycardia | 5 | 13.89% | 15 | 3.2 ± 2.36 (1.3–7) |
| Atrial flutter | 0 | 0 | NA | NA |
| Any atrial arrhythmia | 12 | 33.33% | 74 | 4.7 ± 3.8(1.3–15) |
NA: Not applicable; NSR: Normal sinus rhythm; SV: supraventricular; SVE: supra-ventricular ectopic.
5.2. Inter group comparisons
After the holter analysis three groups were created. Patients not having any tachyarrhythmia on holter recording with NSR was designated as group A (n = 24). Those patients which showed paroxysmal arrhythmia (paroxysmal AF, atrial tachycardia) on holter analysis were designated as group B (n = 12), while patients with permanent/baseline atrial fibrillation at the time of recruitment formed group C (n = 29). The baseline characteristics along with biomarker levels in all three groups are shown in Table 3.
Table 3.
Intergroup analysis (with p values) of demographics, echocardiographic and laboratory measurements (hs-CRP and IL6) .
| Characteristics | Group A (n = 24) |
Group B (n = 12) |
Group C (n = 29) |
p (group A vs group B) |
p (group A vs group C) |
|
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||||
| Age (years) | 30.17 ± 7.57 | 31.50 ± 7.77 | 37.83 ± 6.79 | p = 0.62 | p < 0.0001 | |
| Dyspnea (class) | NYHA II | 14 (58.3%) | 4 (33.3%) | 8 (27.6%) | p = 0.29 | p = 0.06 |
| NYHA III | 10 (41.7%) | 8 (66.7%) | 20 (69%) | |||
| NYHA IV | 0 | 0 | 1 (3.4%) | |||
| TIA/Stroke | 2 (8.3%) | 2 (16.7%) | 14 (48.3%) | p = 0.59 | p = 0.002 | |
| Echocardiographic measurements | ||||||
| Mitral valve area (cm2) | 0.815 ± 0.17 | 0.753 ± 0.21 | 0.730 ± 0.19 | p = 0.25 | p = 0.09 | |
| Mean mitral gradient (mm Hg) | 14.75 ± 4.60 | 14.08 ± 5.31 | 13.07 ± 2.40 | p = 0.59 | p = 0.15 | |
| Severity of mitral stenosis | Severe | 22 (91.7%) | 10 (83.3%) | 27 (93.1%) | p = 0.59 | p = 1.0 |
| moderate | 2 (8.3%) | 2 (16.7%) | 2 (6.9%) | |||
| LAVi (ml/m2) | 85.13 ± 23.5 | 97.59 ± 15.3 | 131.21 ± 51.4 | p = 0.06 | p < 0.0001 | |
| Spontaneous echo contrast (SEC) | Present | 10 (41.7%) | 7 (58.4%) | 20 (68.9%) | p = 0.64 | p = 0.03 |
| Grade 2 | 7 (29.2%) | 5 (41.7%) | 7 (24.1%) | |||
| Grade 3 | 3 (12.5%) | 2 (16.7%) | 13 (44.8%) | |||
| LA/LAA clot or SEC | 10 (41.7%) | 7 (58.4%) | 21 (72.4%) | p = 0.49 | p = 0.02 | |
| LVEF (%) | 61.04 ± 2.69 | 60.17 ± 2.48 | 58.03 ± 9.29 | p = 0.41 | p = 0.35 | |
| Laboratory measurements | ||||||
| Mean IL 6 (pg/ml) | 1.403 ± 1.61 | 2.98 ± 1.75 | 5.413 ± 1.45 | p = 0.56 | p = 0.05 | |
| Mean hs-CRP (mg/L) | 2.67 ± 1.32 | 2.09 ± 1.25 | 5.08 ± 1.31 | p = 0.88 | p = 0.007 | |
| TLC (/mm3) | 7758.3 ± 1689.6 | 7108 ± 1728.1 | 7327.6 ± 2037.5 | p = 0.29 | p = 0.32 | |
| ESR (mm at 1 h) | 13.13 ± 5.11 | 12.17 ± 2.52 | 13.24 ± 5.761 | p = 0.79 | p = 0.44 | |
AF: atrial fibrillation; BSA: Body surface area; ESR: erythrocyte sedimentation rate, hs-CRP: high sensitive C reactive protein; IL-6: interleukin 6; LA: Left atrium; LAA: left atrial appendage; LAVi: left atrial ventricular index; LVEF: left ventricular ejection fraction, MVA: mitral valve area; NYHA: New York heart association, RVSP: right ventricular systolic pressure, SEC: spontaneous echo contrast; TIA: transient ischemic attack; TLC: total leucocyte count.
5.3. Biomarker levels across various groups
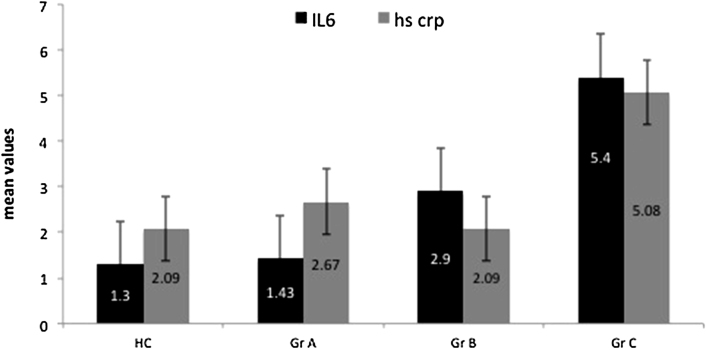
The lowest limit of detection for hs-CRP was 0.01 mg/L, >3 mg/L was designated as high with upper cut off detection limit as 10 mg/L. One way analysis of variance across the three groups was statistically significant (F = 4.9, p = 0.009) for hs-CRP. Post hoc analysis was later performed with hs-CRP of 2.093 ± 1.2 mg/L (mean ± SD) in healthy population while in group A, group B and group C as 2.67 ± 1.3 mg/L, 2.09 ± 1.2 and 5.08 ± 1.3 mg/L respectively. Group C showed significantly high hs-CRP when compared with group A (95% CI; 4.2 to 0.69, p = 0.007) and group B (95% CI: 5.4 to 0.54, p = 0.017) (Fig. 2). It was significantly high in permanent AF (group C) compared to healthy controls (95% CI: −3.94 to −2.04, p = 0.001), whereas group A and group B did not show any significant difference (p < 0.05).
Fig. 2.
hs-CRP (mg/L) and IL6 (pg/ml) levels in healthy controls and arrhythmia. Hs-CRP was found to be significantly raised in baseline AF (group C) compared with NSR group A (p = 0.007) and healthy controls (p < 0.05). IL6 showed graded increase in all patient groups (p = 0.05 between group A and C).
IL-6 had minimum sensitivity of 0.7 pg/ml with group C with significantly high levels (mean 5.41 ± 1.4 pg/ml) as compared to healthy controls (p = 0.005). It was also observed that the marker was statistically significant between group C and group A (95% CI; 1.2 to 0.89; p = 0.05) & group B and group C (95% CI: 1.13 to 3.76, p = 0.005) but non significant (p > 0.05) between group A (mean 1.403 ± 1.6 pg/ml) and B (mean 2.98 ± 1.7 pg/ml) (Fig. 2).
5.4. Correlation of hs-CRP with left atrial volume index (LAVi)
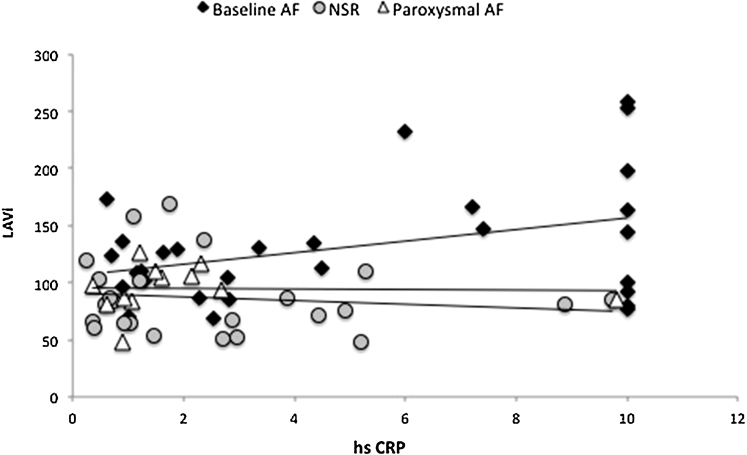
LAVi was statistically high in group C with a mean volume of 131.2 ± 51.4 ml/m2 to that of 85.1 ± 23.5 ml/m2 in group A (95% CI: 57.5 to 32.2, p = 0.002) and 97.5 ± 15.3 ml/m2 in group B (95% CI: 64.4 to 2.9; p = 0.03). A weak positive correlation was observed with hs-CRP and LAVi (r = 0.45, p = 0.06) in group C as compared to group A (Fig. 3).
Fig. 3.
Correlation of hs-CRP to LAVi in NSR and arrhythmia group. A weak positive correlation was observed with hs-CRP and LAVi (r = 0.55, p = 0.06) in AF patients.
5.5. Correlation between IL6 and hs-CRP values
hs-CRP values mirrored with IL 6 values in all patients in group B & C with consistent positive correlation between the two (r = 0.89, p = 0.01). All patients had a graded increase in hs-CRP values with a high hs-CRP of 10 mg/L corresponding to a high IL6 of 30 pg/ml.
5.6. Correlation of inflammatory markers to SEC
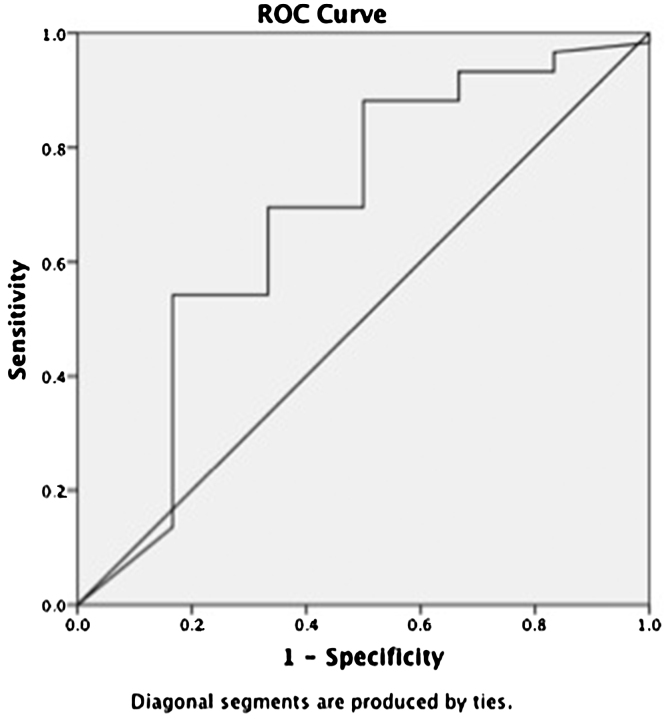
We observed that 68.2% of patients in group C (27/41) had moderate to severe SEC contrast through out LA as compared to 37.5% (10/24) in group A. Patients in group C (baseline AF) with SEC grade 3 had mean hs-CRP and IL6 of 4.47 ± 1.3 mg/L and 4.09 ± 1.2 pg/ml (r = 0.55, 95% CI; 3.6–2.3, p = 0.06), as compared to 1.34 ± 0.4 mg/L and 1.72 ± 0.07 pg/ml in group A. Receiver operating curve (ROC) curve was drawn to evaluate the usefulness of hs-CRP in predicting the occurrence of thrombogenicity. It had a sensitivity of 58%with a cut off of 2.3 mg/L, area under curve C-0.68, (95% CI ∼ 0.95–0.42) (Fig. 4).
Fig. 4.
ROC curve showing hs-CRP predicting thrombogenicity (cut off of 2.3 mg/L) with a sensitivity of 58%, area under curve C-0.68, (95% CI ∼ 0.95–0.42).
6. Discussion
The study reflects the importance of inflammation in rheumatic AF with hs-CRP and IL-6 being significantly high suggesting that chronic inflammation is linked to AF and may contribute to atrial fibrosis and dilatation of the left atrium.13, 8
A recent metanalysis revealed that hs-CRP is an independent predictor of AF recurrence after cardioversion (OR: 3.33; 95% CI 2.10–5.28) and another study showed significantly high CRP in paroxysmal arrhythmia group than normal sinus rhythm (95% CI; 1.1–17.5, p = 0.043) in rheumatic mitral stenosis.14 Our study also showed similar results with a significantly high hs-CRP in baseline AF as compared to paroxysmal AF subgroups ingeminating the hypothesis that this protein plays a cardinal role in promoting and maintenance of AF via atrial structural remodeling.15
High plasma IL-6 levels has been correlated with the presence and duration of AF and increased left atrial diameter. In a cross sectional analysis of 971 participants in the Heart Soul Study,16 IL-6 was the only biomarker significantly associated with AF (median IL-6 3.76 and 2.52 pg/ml in those with and without AF, respectively, p = 0.0005; OR = 1.77, p = 0.032). The IL-6/174CC genotype was significantly associated with the presence of AF in the adjusted analysis (odds ratio 2.34, p = 0.04) without any elevation in hs-CRP.17 In the present study, there was a graded rise in the biomarker levels suggesting that chronic inflammation is associated with development of atrial fibrillation.
Blood rheology, endothelial dysfunction, procoagulation, platelet activation and increased fibrinolytic activity may confer a prothrombotic environment in AF. The increased risk of stroke and thromboembolism after AF has been postulated due to prothrombotic or hypercoagulable states.18 Fourteen patients in permanent AF group, 2 each in NSR and paroxysmal AF group had stroke suggesting that valvular AF may carry a higher risk for thromboembolic events than does non-valvular AF. Previous studies reported an apparent link between thrombogenesis associated with fibrinogen and plasma viscosity in AF in which increased CRP is associated with an increased risk of SEC and vascular events.19 Contrarily, some recent studies20 did not show a significant correlation of SEC and LA clot with MMP-9, galactin 3 & hs-CRP. We observed a mild positive correlation with hs-CRP and LAVi (p = 0.06, r = 0.55) in atrial fibrillation which suggests that there is an association of hs-CRP with atrial structural remodeling.
Our study was non-conclusive of any association of IL6 and hs-CRP with thrombogenicity (r = 0.55, 95%CI; 3.6 to 2.3, p = 0.06) which is also evidenced by a recent study.21 hs-CRP was found to predict the occurrence of thrombi with a sensitivity of 58% & a cut off of 2.3 mg/L, area under curve C-0.68, (95% CI ∼ 0.95 to 0.42). Out of the two biomarkers, IL6 did not show any correlation (Fig. 4). It is also proven that the occurrence of spontaneous contrast not only depends on slow flow, but is also related to the hematocrit levels but we did not observe any discrepancy in these levels in our patients. A study by Li et al. investigated the role of inflammatory and oxidative stress biomarkers in lone and typical AF patients. They found significant increase of hs-CRP & IL6 without any increase in isoprostranes explaining strong correlation between inflammation and AF.22
We chose isolated rheumatic mitral stenosis without significant involvement of other valves, to avoid confounding factors as significant MR patients tend to have large LA size and patients with significant aortic valve lesions are more likely to have ventricular hypertrophy and LV dysfunction, all being independent risk factors for atrial fibrillation. Our study group comprised of relatively younger patients (mean 33.8 years) without risk factors like hypertension, diabetes, coronary artery disease, left ventricular hypertrophy and renal dysfunction, which are associated with generalized vascular inflammation and endothelial dysfunction. Non-valvular atrial fibrillation patients are associated with more widespread vascular inflammatory processes and platelet activation unlike patients of our cohort, in which the culprit source of inflammation seems left atrium without any significant vascular endothelial source. Liuba et al10 showed that patients with permanent AF had higher plasma levels of IL-8 in the samples from the femoral vein, right atrium, and coronary sinus than in the samples from the pulmonary veins, suggesting that a possible source of inflammation exists in the systemic circulation.
Continuing the debate whether chronic inflammation is the cause of atrial fibrillation or the arrhythmia itself leads to an increase in biomarkers-“the hen and egg” effect,23 we observed a graded rise in levels of hs-CRP and IL-6 in baseline AF group as compared to the paroxysmal arrhythmia and normal sinus group suggesting that inflammation may be the cause of atrial arrhythmia, as short duration arrhythmic events are unlikely to cause rise in level of inflammatory biomarkers.20
6.1. Study limitation
A relatively small sample size, single time biomarker estimation and a non-randomized group with a very small subset of patients with paroxysmal arrhythmia. The study suggests the possibility of anti inflammatory therapy reducing the recurrence of AF and thus opening the doors for further investigation, preferentially drugs less burdened with potential serious side effects such as with statins and glucocorticoids.24 The observations of this paper are restricted to hs-CRP and IL6 although other biomarkers like hs-troponin I and CD40 are being planned in future. Future studies would reckon prognostic information along with clinical risk scoring for long term cardiovascular events and death.
7. Conclusion
Serum proteonomic biomarkers may act as complementary tool for diagnosis, in prognostication and therapeutic monitoring of novel treatments in rheumatic atrial fibrillation. This study showed a link between chronic inflammatory processes and the development of AF in patients with rheumatic heart disease and mitral Stenosis, through upregulation of inflammatory markers like hs-CRP and IL6.25, 26 Increased hs-CRP and IL-6 levels in the paroxysmal and permanent AF group may favour the hypothesis that low grade chronic inflammation could be the cause of atrial fibrillation rather than the consequence. Despite similar severity of mitral stenosis, atrial arrhythmias were not present in all and when present, was associated with raised inflammatory markers i.e., hs-CRP and IL6. The elevation of inflammatory markers is proportional to the burden of atrial tachycardia.
Conflicts of interest
There are no conflicts of interest.
Funding agency
Indian Council of Medical Research, New Delhi, India. ICMR letter no-5/4/I-3/12/NCD-II.
Acknowledgement
All authors have contributed equally.
Contributor Information
Gautam Sharma, Email: drgautamsharma12@gmail.com.
Sudhir Shetkar, Email: drsudhirss@yahoo.com.
Ashu Bhasin, Email: ashu.bhasin@gmail.com.
Lakshmy Ramakrishnan, Email: lakshmy_ram@yahoo.com.
Rajnish Juneja, Email: rjuneja2@gmail.com.
Nitish Naik, Email: nitishnaik@yahoo.co.in.
Ambuj Roy, Email: drambujroy@gmail.com.
Sivasubramanian Ramakrishnan, Email: ramaaiims@gmail.com.
Balram Bhargava, Email: balrambhargava@yahoo.com.
Vinay Kumar Bahl, Email: vkbahl2002@yahoo.com.
References
- 1.Chopra P., Gulwani H. Pathology and pathogenesis of rheumatic heart disease. Indian J Pathol Microbiol. 2007;50(4):685–697. [PubMed] [Google Scholar]
- 2.Benjamin E.J., Wolf P.A., Agostino R.B. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Kabukcu M., Arslantas E., Ates I. Clinical echocardiographic and homodynamic characteristics of rheumatic mitral valve stenosis and atrial fibrillation. Angiology. 2005;56(2):159–163. doi: 10.1177/000331970505600206. [DOI] [PubMed] [Google Scholar]
- 4.Mihm M.J., Yu F., Carnes C.A. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 5.Engelmann M.D., Svendsen J.H. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083–2092. doi: 10.1093/eurheartj/ehi350. [DOI] [PubMed] [Google Scholar]
- 6.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y., Nakamura K., Fukushima-Kusano K. Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: possible involvement in intracardiac thrombogenesis. Thromb Res. 2003;111:137–142. doi: 10.1016/s0049-3848(03)00405-5. [DOI] [PubMed] [Google Scholar]
- 8.Liuba I., Ahlmroth H., Jonasson L. Source of inflammatory markers in patients with atrial fibrillation. Europace. 2008;10:848–855. doi: 10.1093/europace/eun111. [DOI] [PubMed] [Google Scholar]
- 9.Vılchez J.A., Roldan V., Hernandez D. Biomarkers in atrial fibrillation: an overview. Int J Clin Pract. 2014;68:408–409. doi: 10.1111/ijcp.12304. [DOI] [PubMed] [Google Scholar]
- 10.Issac T.T., Dokainish H., Lakkis N.M. Role of Inflammation in Initiation and Perpetuation of Atrial Fibrillation A Systematic Review of the Published Data. J Am Coll Cardiol. 2007;21:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 11.Aviles R., Martin D., Hansen C. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 12.Saddanand S., Sherrid S. Clinical and echocardiographic characteristics of left atrial spontaneous echo contrast in sinus rhythm. J Am Assoc Cardiol. 2000;35(7):1932–1939. doi: 10.1016/s0735-1097(00)00643-4. [DOI] [PubMed] [Google Scholar]
- 13.Korantzopoulos P., Kolettis T., Siogas K. Atrial fibrillation and electrical remodeling: the potential role of inflammation and oxidative stress. Med Sci Monit. 2003;9:225–229. [PubMed] [Google Scholar]
- 14.Dernellis J., Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375–380. doi: 10.2143/AC.56.6.2005701. [DOI] [PubMed] [Google Scholar]
- 15.Sata N., Hamada N., Horinouvhi T. C-reactive Protein and Atrial Fibrillation. Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J. 2004;45:441–445. doi: 10.1536/jhj.45.441. [DOI] [PubMed] [Google Scholar]
- 16.Marcus G.M., Whooley M.A., Glidden D. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155(2):303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudino M., Andreotti F., Zamparelli R. The 174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108:195–199. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 18.Conway D.S.G., Buggins P., Hughes E. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43:2075–2082. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 19.Roldan V., Marin F., Blann A.D. Interleukin-6, endothelial activation and thrombogenesis in chronic atrial fibrillation. Eur Heart J. 2003;24:1373–1380. doi: 10.1016/s0195-668x(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 20.Sahin T., Acar E., Cleikyurt U. Relation of hs-CRP and BNP levels with the atrial spontaneous echo contrast and thrombi in permanent atrial fibrillation patients with different etiologies. Med Sci Monit. 2012;18(2):CR78–92. doi: 10.12659/MSM.882461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black I.W., Chesterman C.N., Hopkins A.F. Hematologic correlates of left atrial spontaneous echo contrast and thromboembolism in non valvular atrial fibrillation. J Am Coll Cardiol. 1993;21:451–457. doi: 10.1016/0735-1097(93)90688-w. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Solus J., Chen Q. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lappegard K.T., Hovland A., Pop G.A. Atrial fibrillation: inflammation in disguise? Scan J Immun. 2013;78(2):112–119. doi: 10.1111/sji.12061. [DOI] [PubMed] [Google Scholar]
- 24.Young-Xu Y., Jabbour S., Goldberg R. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003;92:1379–1383. doi: 10.1016/j.amjcard.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Pan M., Zhu J.H., Jiang W.P. Inflammation: a possible pathogenic link to atrial fibrillation. Med Hypotheses. 2006;67:1305–1307. doi: 10.1016/j.mehy.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 26.Friedrichs K., Klinke A., Baldus S. Inflammatory pathways underlying atrial fibrillation. Trend Molec Med. 2011;17:556–563. doi: 10.1016/j.molmed.2011.05.007. [DOI] [PubMed] [Google Scholar]