Abstract
Background
Resistant hypertension is a well-recognized clinical challenge yet there are no reported data on its prevalence in Pakistan. These patients are subjected to a higher risk of developing hypertensive complications. The objective of our study was to evaluate the prevalence and determinants of resistant hypertension in an Asian cohort of hypertensive patients.
Methods
This cross-sectional study was carried out among hypertensive patients visiting a tertiary care hospital in Karachi from September-December 2015. Patient data and characteristics were recorded using a pre-coded questionnaire. Morisky and Berlin questionnaires were used to assess compliance to medications and determine the risk of developing obstructive sleep apnea, respectively. Pearson's chi-square test was used to analyze statistical differences between hypertensive patients and related factors.
Results
A total of 515 patients were included in the study. Overall, 12% of the total patients (n = 62) were resistant hypertensives and 25% (n = 129) had pseudo-resistant hypertension. Resistant patients were more often females, older and had a higher body mass index (all P < 0.001). Use of painkillers and noncompliance to dietary recommendations were found to be significant determinants of resistant hypertension. Prevalence of comorbid conditions, including diabetes (p = 0.33), hyperlipidemia (p = 0.46), and chronic kidney disease (p = 0.23), was not significantly higher in patients with resistant hypertension.
Conclusion
Nearly one in ten hypertensive patients had true resistant hypertension, and twenty-five percent of patients had pseudo-resistance. Resistance hypertensions is significantly associated with female gender, older age, obesity, dietary noncompliance and increased use of NSAIDs.
Keywords: Resistant hypertension, Prevalence, Pakistan
1. Introduction
Hypertension is one of the most important risk factors for cardiovascular and renal diseases. Approximately 1 billion adults have hypertension and the prevalence is increasing.1 Despite best efforts to control blood pressure, resistant hypertension (RH) may occur.2 In spite of increased recognition and therapeutic interventions, studies show that the efficiency of blood pressure control in hypertensive patients continues to decline,1, 3, 4 with an increase in the incidence of RH worldwide.5, 6, 7, 8 The failure to achieve target values of blood pressure has several causes such as poor treatment adherence, scarcity of resources and failure to intensify treatment in a timely manner.9
Nearly 1/5th of the population of Pakistan suffers from hypertension with prevalence expected to rise in the coming years.10 However, RH prevalence values have yet to be reported. Despite an increase in the number of clinical studies on the prevalence of RH, the growing incidence of this condition among treated hypertensive patients is established to be between 5 and 30% in the developed part of the world.2, 4, 10, 11, 12 This disparity is primarily because of the difference in definition employed in the study or failure to rule out pseudo-hypertension. There is a dearth of published literature providing up-to-date estimates of the prevalence of RH across developing regions of the world.
Existing researches have, hitherto, mainly focused on Western population while the prevalence of RH in South Asia has not been well explored. Therefore, these RH prevalence estimates may differ from what would be found in a middle-income metropolis like that of Karachi.
An interesting development is the proof offered by prevalence surveys and genome-wide association studies that the incidence of hypertension and its resistant category has a genetic component and predisposes population of South Asians and Central and Eastern.13, 14, 15 However, the disease remains idiopathic in its essence, with its proximal causes still not well defined.16
Defining optimal treatment for patients with RH is a clinical challenge. Hence, a greater understanding of this subgroup of hypertensive patients is crucial for establishing known determinants and facilitate the development of targeted strategies for prevention, treatment, and control. Thus, the objective of our study was to assess the prevalence and predictors of resistant hypertension in an Asian cohort of hypertensive patients.
2. Method
This cross-sectional study was conducted at a tertiary care hospital in Karachi, Pakistan for a duration of four months from September 1, 2015 to December 31, 2015. It consisted of a survey of all patients older than 18 years, previously diagnosed with essential hypertension, who had records of 3 or more blood pressure measurements within the past one year, presenting to the clinics. Essential hypertension was defined as a systolic blood pressure that is persistently ≥ 140 mmHg on 2 or more occasions, or a diastolic blood pressure persistently ≥ 90 mmHg on 2 or more occasions, when an average of each is measured at every visit using at least 2 values.17 Patients who were known cases of hypertension secondary to tumors or other diseases of the endocrine system, obesity, pregnancy, coarctation of aorta, and use of hormonal contraceptives were excluded from the study. The study was approved by the Institutional Review Board of Dow University of Health Sciences (DUHS)
Participants were selected via convenience sampling; all patients visiting the clinic on a given day were interviewed after written consent. Participant cooperation rate was 96%, which yielded a total of 515 complete cases for analyses. For the purpose of this study, we used a pilot-tested questionnaire that was administered to each patient with the help of an interviewer. Moreover, interviewer bias was eliminated by training interviewers who were kept unaware of the outcome of interest for the study. From each patient, we obtained information on socio-demographic details, duration of hypertension, current medications, co-morbid conditions, lifestyle and family history of hypertension. In addition to these, Berlin questionnaire was used to determine the risk of developing obstructive sleep apnea (OSA), dividing the patients into high and low-risk categories. Morisky questionnaire was utilized to assess adherence to antihypertensive medications, the results of which were classified into high, average and poor adherence, following which all cases of RH were further classified into those with pseudo-resistance and those with true resistant hypertension. True resistant hypertension (tRH) was defined as blood pressure that exceeds target values in spite of best possible regimen consisting of 3 antihypertensive drugs of different classes, where one should ideally be a diuretic.18 Pseudo-resistance was defined as uncontrolled hypertension as a result of inaccurate measurement, poor adherence to prescribed medications, incorrect drug choices/doses, or white-coat effect.19 Moreover, to measure blood pressure (BP) of each patient, the physician used a mercury sphygmomanometer. After an initial resting period of 5 minutes, two measurements were taken with an interval of 10 min in between, and mean values of both systolic and diastolic blood pressure were recorded. In addition to these, 3 previous blood pressure values were extracted from the patients’ files. WHO defines a BMI ≥ 25 kg/m2 as overweight, whereas obesity is categorized as BMI ≥ 30 kg/m2.20
For the purpose of this study, all data entry and analyses were done using the IBM Statistical Package for Social Sciences (SPSS), version 23 for Windows. Frequencies with percentages were calculated for all categorical variables and the Chi-square test was used to check the significance of relationships between characteristics of patients with essential hypertension and those with true resistant hypertension. No imputation methods were used to replace missing values, and only completely filled questionnaires were considered for our use. A p-value of less than 0.05 was considered to be significant.
3. Results
The mean age of patients was 64.3 ± 12.3 years, with a mean BMI of 29.1 ± 4.5 kg/m2. The mean systolic and diastolic blood pressures were 142 ± 15.2 mmHg and 88 ± 11.7 mmHg, respectively. More than half the patients had diabetes mellitus (n = 287, 55.7%), while 78 patients (15.1%) had a positive family history for hypertension. Approximately a third of the total patients (n = 174, 33.8%) were non-compliant to dietary recommendations. It was found that patients were (n = 317, 61.6%) moderately adherent to prescribed antihypertensive medications. Among the sample cohort, 112 patients (21.7%) were at a high risk of developing OSA. Table 1 summarizes the baseline characteristics of the sample population.
Table 1.
Baseline characteristics of patients.
| Characteristics | |
|---|---|
| Male, n (%) | 309 (60.0) |
| Married, n (%) | 463 (89.9) |
| Average systolic blood pressure, mmHg | 142 ± 15.2 |
| Average diastolic blood pressure, mmHg | 88 ± 11.7 |
| Mean BMI, (kg/m2) | 29.1 ± 4.5 |
| Drugs, n (%) | |
| Steroids | 33 (6.4) |
| Painkillers | 171 (33.2) |
| Lifestyle risk factors, n (%) | |
| Dietary non-compliance | 174 (33.8) |
| Smoking | 194 (37.7) |
| Alcohol | 26 (5.0) |
| Co-morbidities, n (%) | |
| Diabetes Mellitus | 287 (55.7) |
| Hyperlipidemia | 114 (22.1) |
| Chronic kidney disease | 39 (7.6) |
| Family history, n (%)a | 78 (15.1) |
| Adherence, n (%)b | |
| High | 151 (29.3) |
| Average | 317 (61.6) |
| Poor | 47 (9.1) |
| Risk of OSA, n (%)c | |
| High | 112 (21.7) |
| Low | 403 (78.3) |
Patients with positive family history for hypertension.
Adherence to prescribed antihypertensive agents.
Obstructive sleep apnea.
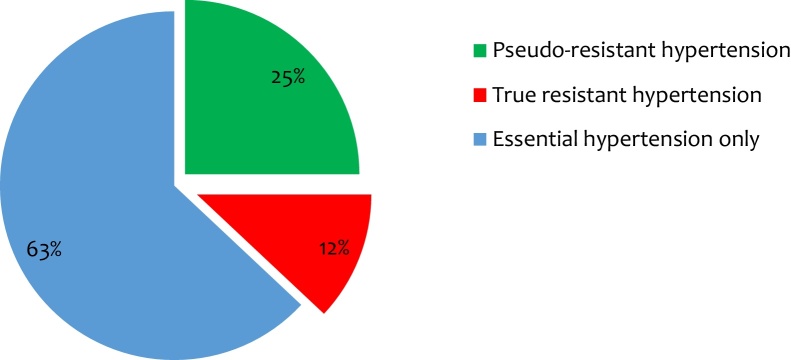
Prevalence of true resistant hypertension was low (n = 62, 12%), a quarter (n = 129, 25%) of the population were pseudo-resistant hypertensive patients while most (n = 320; 63%) had essential hypertension with no form of RH (Fig. 1). The study population was divided into two groups according to patients exhibiting essential hypertension (n = 453) and true resistant hypertension (n = 62). The comparison of different characteristics is seen in Table 2. The resistant population was significantly older (66.1% vs 21.2; p < 0.001), a greater proportion was female (80.6% vs 34.4%; p < 0.001) and was associated (58.1% vs 16.8; p < 0.001) with obesity (BMI ≥30 kg/m2) when compared to the non-RH population. Similarly, in comparison to those with non-RH, patients with RH were associated with OSA (53.2% vs 17.4%; p < 0.001) and the use of painkillers (45.2% vs 31.6%; p = 0.033) was significantly higher. A significant proportion of patients (n = 37, 59.7%; p = 0.004) with RH were non-compliant to the dietary recommendations. The prevalence of diabetes mellitus (p = 0.33), chronic kidney disease (p = 0.23) and hyperlipidemia (p = 0.46) was not statistically significant between the two groups.
Fig. 1.
Distribution of patients with hypertension.
Table 2.
Comparison of characteristics between patients with true resistant hypertension and essential hypertension.
| Essential Hypertension (n = 453, %)a | True Resistant Hypertension (n = 62, %) | p-valueb | |
|---|---|---|---|
| Males | 297 (65.6) | 12 (19.3) | <0.001 |
| Age >65, years | 96 (21.2) | 41 (66.1) | <0.001 |
| BMI >30, kg/m2 | 76 (16.8) | 36 (58.1) | <0.001 |
| Dietary non-compliance | 137 (30.2) | 37 (59.7) | 0.004 |
| Alcohol | 21 (4.64) | 5 (8.06) | 0.25 |
| High risk of OSA c | 79 (17.4) | 33 (53.2) | <0.001 |
| Diabetes | 256 (56.5) | 31 (50.0) | 0.33 |
| Hyperlipidemia | 98 (21.6) | 16 (25.8) | 0.46 |
| Chronic kidney disease | 32 (7.03) | 7 (11.3) | 0.23 |
| Painkillers | 143 (31.6) | 28 (45.2) | 0.033 |
Patients who do not have true resistant hyperension.
p-values <0.05 were considered statistically significant.
4. Discussion
The prevalence of RH among our study population was 12%. This finding is consistent with other observational studies,4, 21, 22 however it is still lower than several studies conducted in Europe and North America. A possible reason for the lower prevalence of RH could be the different conditions of our study. In the Controlled Onset Verapamil Investigation of Cardiovascular Endpoints trial, the prevalence of RH was 18%.23 In this study, data was collected from patients who were 55 years or older and had additional cardiovascular risk factors. However, our study includes all hypertensive patients older than 18 years of age with no strict inclusion criteria for cardiovascular risk factors. Prior studies have often also over-estimated tRH without ruling out pseudo-resistance.4, 18 In our study, a quarter of the total patients were pseudo-resistant hypertensives. A phenomenon that can be caused by various factors, including inaccurate measurement of BP, poor adherence or suboptimal treatment regimens, physician’s inertia or white-coat effect, produces a false impression of tRH if not taken into account.7 The incapacity of ruling out pseudo-resistance is a matter of great importance in order to avoid over treatment and avoid complications. Despite the increased recognition of the high-risk associated with the condition, cases with RH have been poorly characterized in literature especially in South Asian countries including Pakistan. The limited data that could be gathered from the studies conducted in our neighboring countries show that the prevalence of RH is on a steady rise.10, 14, 16 According to Daugherty et al., approximately 1 in 6 patients on 3 or more antihypertensive drugs will continue to meet the criteria for RH after 1 year.6 Physicians can expect to encounter RH one in every ten hypertensive patients on treatment. This situation is worrisome and highlights that treatment resistant BP will continue to be a clinically important hypertension phenotype.
Our results also show that patients above 65 were significantly more likely to develop RH, a trend which has been observed in most epidemiological studies.22, 23 A study carried on hypertensive patients reported that prevalence of RH increased with age, particularly in females as compared to males; implying that gender and age may be used to predict development of RH.22 Parallel to this observation, the Framingham study data identified old age as the strongest predictor of tRH.24 It may be attributed to age-related changes in blood vessels and an increase in frequency of all related conditions (e.g., obesity, diabetes mellitus, CKD). Moreover, we also observed a significant association with the female gender. Several studies have reported sexual dimorphism in BP such that incidence of hypertension is much higher in males during early adulthood, but this level alters after the sixth decade of life. The rise in the BP after the age of 55 is steeper in females as compared to males, which also shows features of treatment resistance.18, 25, 26 Women are more likely to have difficulty adhering to optimum BP, have a higher prevalence of co-morbidities and obesity influenced by estrogen withdrawal and, as a consequence, are more likely to develop RH.
A statistically significant relationship was associated between obesity and RH in our study; more than half of the patients with RH were obese while only one-fifth of those with essential hypertension had BMI > 30, kg/m2. Obesity, the sixth most important predisposing factor for RH,27 elevates the need for increased number of antihypertensive medications and also increases tendency of never achieving required BP control.28 Increased body weight predisposes to tRH by causing decreased sodium excretion, insulin resistance, increased activity of sympathetic nervous system and lastly renal injury by the activation of the renin-angiotensin system.29 The obesity pandemic poses a substantial threat to society and proper measures towards lifestyle modification, including low fat diet and routine exercises, can help reduce the incidence of RH.
In our study, dietary non-compliance had a significant impact on RH exemplifying the role of adequate diet in hypertension prevalence, which has been long recognized and established across numerous studies. Previous literature suggests high salt diet as the main culprit causing resistance to antihypertensive agents. This may occur by increment of BP or by blunting the BP-lowering effect of antihypertensive agents.27, 28, 29, 30, 31, 32 Resistant hypertensive patients tend to exhibit a higher salt sensitivity as compared to patients with essential hypertension.14 This may be due to the use of renin-angiotensin system blockers, as a part of the treatment regime, which heighten the hypertensive response to high salt intake.6, 25 Therefore, it is important to understand that dietary noncompliance can negate or nullify the effect of even the most ideal drug therapy.33 Other dietary factors such as high potassium intake, vegetarian diet and cessation of tobacco smoking have also been reported to be involved in BP control.34 However, these dietary conditions are less likely to be adopted by the younger age group (<65 years). Regular family meals at home are infrequent in this group instead they are more likely to consume fast-food on a regular basis with friends and family, ultimately leading to an increase in the intake of oily, salty food, which in general could adversely affect people of all age groups. Given the strong relationship, adequate BP control is paramount. Proper counseling may largely contribute to resolving noncompliance along with monitoring and moral support by family members. In addition, the use of written instructions and pictograms have also proved to play an elementary role in patients’ dietary compliance.35, 36
Interestingly, our study did not find a significant relationship between chronic kidney disease and RH. This finding is at odds with previous epidemiological studies,4, 6, 11, 13 identifying renal disease both as a cause and consequence of RH. It is reasonable to assume that the small sample size drawn from a single tertiary care center may have excluded patients with chronic kidney disease who may have been referred to the nephrology clinic. The number of patients with previously existing renal disease was also moderately low. Mechanisms involving sodium and fluid retention, activation of the renin–angiotensin–aldosterone hormonal axis could plausibly account for the reported rise in treatment resistance.
Another important finding of this study is the evaluation of OSA in patients with RH compared to those with essential hypertension. RH was associated with a significantly increased risk of developing OSA. The intermittent cessation in airflow may cause hypoxia leading to the release of endothelin from vascular endothelium ultimately causing vasoconstriction. These cyclical changes in the levels of endothelin and repeated oxygen desaturation leads to the development of RH.37 Logan AG et al., evaluated 41 consecutive patients with t-RH of which 83% patients suffered from unsuspected sleep apnea.38 However, a study conducted by Min HJ et al. concluded that the potential causal association between OSA and hypertension is in fact due to common risk factors, primarily obesity, between the two conditions.39 Patients with OSA may also be at increased risk for weight gain. Therefore, physicians must not disregard correction of risk factors in the treatment of RH patients.
Our results raise the prospect that an increased use of painkillers contributes to the poor control found in RH. Given the widespread use of non-narcotic analgesics including the NSAIDs, aspirin and acetaminophen they are most likely the offending agents concerning BP control40, 41; their role well established in literature.42, 43 We believe that the number of people who took excessive painkillers for controlling their respective pains were not aware of the side effects of these drugs and their role in decreasing the efficacy of the BP controlling medications. In addition, other painkillers, like acetaminophen, have been found to develop hypertension,41 but in comparison to NSAIDS (particularly ibuprofen), are less likely to worsen BP control in treated patients.42 Thus, such medications should be avoided or withdrawn in resistant hypertensive patients. As this may be clinically difficult, the lowest effective dose should be administered when needed.
We could not find any association between diabetes mellitus and resistant hypertension. This observation is discordant with other previous studies which have shown a positive relation.11, 22 A possible reason could be because of our small sample size of patients. Moreover, it could also be due to an overall high prevalence of diabetes mellitus in the existing sample; more than half of the patients were diabetic.
There are several limitations in our study which need to be considered. First, we collected data from a single tertiary care hospital yielding a small size of patients; however RH prevalence is in line with previous reports. Second, the cross-sectional design of our study limits the inference of reasons related for development of the identified risk factors and is designed to suggest association only and does not assess causality. Therefore, it is important to conduct prospective studies in order to determine antecedent factors associated with the predisposition of RH. Third, we employed convenience sampling in our study. Since data was collected in accordance to the convenience of the interviewers, this might have neglected some people with RH visiting the hospital on other days. We also relied on patient’s self-reporting for compliance to antihypertensive medications and dietary intake as it was feasible and cost effective. Lastly, patients in the study were spread over a wide age range accounting for significant differences and the findings should thus be generalized with caution. We believe consideration of these important points can help better understand the key role players in the development, progression and prognosis of RH.
5. Conclusion
The prevalence of RH is on a road to rise and has the potential of becoming a major problem in the upcoming years, imposing a heavy social and economic burden on the country. Attention to metabolic risk factors, reinforcing lifestyle modifications and avoiding agents that can elevate the BP, may be the key. Although the results need to be confirmed by prospective studies evaluating the relationship between the aforementioned risk factors and delineating pathogenesis and interventional studies investigating the role of diagnostic evaluation; they do establish that early identification is prudent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Cifkova R., Fodor G., Wohlfahrt P. Changes in hypertension prevalence, awareness, treatment, and control in high-, middle-, and low-income countries: an update. Curr Hypertens Rep. 2016;18:62. doi: 10.1007/s11906-016-0669-y. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar I., Kotchen J.M., Kotchen T.A. Hypertension: trends in prevalence, incidence, and control. Annu Rev Public Health. 2006;27:465–490. doi: 10.1146/annurev.publhealth.27.021405.102132. [DOI] [PubMed] [Google Scholar]
- 3.Hajjar I., Kotchen T.A. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Egan B.M., Zhao Y., Axon R.N. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandal P.K., Dey A.K., Pramanik S. The prevalence and associated cardiovascular risk factors in resistant hypertensive subjects in Eastern India. Asian J Med Sci. 2014;5:1–5. [Google Scholar]
- 6.Daugherty S.L., Powers J.D., Magid D.J. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barochiner J., Alfie J., Aparicio L.S. Prevalence and clinical profile of resistant hypertension among treated hypertensive subjects. ClinExpHypertens. 2013;35:412–417. doi: 10.3109/10641963.2012.739236. [DOI] [PubMed] [Google Scholar]
- 8.Pimenta E., Calhoun D.A., Oparil S. Mechanisms and treatment of resistant hypertension. Arq Bras Cardiol. 2007;88(June (6)):683–692. doi: 10.1590/s0066-782x2007000600009. [DOI] [PubMed] [Google Scholar]
- 9.Balazovjech I., Hnilica P., Jr. Compliance with antihypertensive treatment in consultation rooms for hypertensive patients. J Human Hypertension. 1993;7:581–583. [PubMed] [Google Scholar]
- 10.Saleem F., Hassali A.A., Shafie A.A. Hypertension in Pakistan: time to take some serious action. Br J Gen Pract. 2010;60:449–450. doi: 10.3399/bjgp10X502182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persell S.D. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076 10–80. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 12.Cushman W.C., Ford C.E., Cutler J.A. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla G., Bombelli M., Seravalle G. Prevalence and clinical characteristics of patients with true resistant hypertension in central and Eastern Europe: data from the BP-CARE study. J Hypertens. 2013;31:2018–2024. doi: 10.1097/HJH.0b013e328363823f. [DOI] [PubMed] [Google Scholar]
- 14.Leenen F.H., Dumais J., McInnis N.H. Results of the Ontario survey on the prevalence and control of hypertension. CMAJ. 2008;178:1441–1449. doi: 10.1503/cmaj.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato N. Ethnic differences in genetic predisposition to hypertension. Hypertens Res. 2012;35:574–581. doi: 10.1038/hr.2012.44. [DOI] [PubMed] [Google Scholar]
- 16.Kumara W.A., Perera T., Dissanayake M. Prevalence and risk factors for resistant hypertension among hypertensive patients from a developing country. BMC Res Notes. 2013;6:373. doi: 10.1186/1756-0500-6-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carretero O.A., Oparil S. Essential hypertension: part I: definition and etiology. Circulation. 2000;101:329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun D.A., Jones D., Textor S. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for high blood pressure research. Circulation. 2008;117:e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 19.Pimenta E., Gaddam K.K., Oparil S. Mechanisms and treatment of resistant hypertension. J ClinHypertens (Greenwich) 2008;10:239–244. doi: 10.1111/j.1751-7176.2008.08143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization, Obesity and overweight fact sheet. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 21.Gijón-Conde T., Graciani A., Banegas J.R. Resistant hypertension: demography and clinical characteristics in 6292 patients in a primary health care setting. Rev EspCardiol (Engl Ed) 2014;67:270–276. doi: 10.1016/j.rec.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Sim J.J., Bhandari S.K., Shi J. Characteristics of resistant hypertension in a large ethnically diverse hypertension population of an integrated health system. Mayo ClinProc. 2013;88:1099–1107. doi: 10.1016/j.mayocp.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black H.R., Elliott W.J., Grandits G. Principal results of the controlled onset verapamil investigation of cardiovascular end points (CONVINCE) trial. JAMA. 2003;289:2073–2082. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones D.M., Evans J.C., Larson M.G. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594–599. doi: 10.1161/01.hyp.36.4.594. [DOI] [PubMed] [Google Scholar]
- 25.Kannel W.B., Wolf P.A., McGee D.L. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA. 1981;245:1225–1229. [PubMed] [Google Scholar]
- 26.Hajjar I.M., Grim C.E., George V. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med. 2001;161:589–593. doi: 10.1001/archinte.161.4.589. [DOI] [PubMed] [Google Scholar]
- 27.Haslam D.W., James W.P. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 28.Bramlage P., Pittrow D., Wittchen H.U. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17:904–910. doi: 10.1016/j.amjhyper.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Hall J.E. The kidney, hypertension and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 30.He F.J., Li J., Macgregor G.A. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;4:CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luft F.C., Weinberger M.H. Review of salt restriction and the response to antihypertensive drugs: satellite symposium on calcium antagonists. Hypertension. 1988;11:I229–32. doi: 10.1161/01.hyp.11.2_pt_2.i229. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger M.H., Cohen S.J., Miller J.Z. Dietary sodium restriction as adjunctive treatment of hypertension. JAMA. 1988;259:2561–2565. [PubMed] [Google Scholar]
- 33.Khan M.S., Bawany F.I., Mirza A. Frequency and predictors of non-compliance to dietary recommendations among hypertensive patients. J Community Health. 2014;39:732–736. doi: 10.1007/s10900-014-9819-9. [DOI] [PubMed] [Google Scholar]
- 34.van der Wal M.H., Jaarsma T., van Veldhuisen D.J. Non-compliance in patients with heart failure; how can we manage it? Eur J Heart Fail. 2005;7:5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Segador J., Gil-Guillen V.F., Orozco D. The effect of written information on adherence to antibiotic treatment in acute sore throat. Int J Antimicrob Agents. 2005;26:56–61. doi: 10.1016/j.ijantimicag.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Dowse R., Ehlers M. Medicine labels incorporating pictograms: do they influence understanding and adherence? Patient EducCouns. 2005;58:63–70. doi: 10.1016/j.pec.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Phillips B.G., Narkiewicz K., Pesek C.A. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–66. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 38.Logan A.G., Perlikowski S.M., Mente A. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Min H.J., Cho Y.J., Kim C.H. Clinical features of obstructive sleep apnea that determine its high prevalence in resistant hypertension. Yonsei Med J. 2015;56:1258–1265. doi: 10.3349/ymj.2015.56.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dedier J., Stampfer M.J., Hankinson S.E. Non-narcotic analgesic use and the risk of hypertension in US women. Hypertension. 2002;40:604–608. doi: 10.1161/01.hyp.0000035856.77718.da. [DOI] [PubMed] [Google Scholar]
- 41.Forman J.P., Stampfer M.J., Curhan G.C. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension. 2005;46:500–507. doi: 10.1161/01.HYP.0000177437.07240.70. [DOI] [PubMed] [Google Scholar]
- 42.Radack K.L., Deck C.C., Bloomfield S.S. Ibuprofen interferes with the efficacy of antihypertensive drugs: a randomized, double-blind, placebo-controlled trial of ibuprofen compared with acetaminophen. Ann Intern Med. 1987;107:628–635. doi: 10.7326/0003-4819-107-5-628. [DOI] [PubMed] [Google Scholar]
- 43.Conlin P.R., Moore T.J., Swartz S.L. Effect of indomethacin on blood pressure lowering by captopril and losartan in hypertensive patients. Hypertension. 2000;36:461–465. doi: 10.1161/01.hyp.36.3.461. [DOI] [PubMed] [Google Scholar]