Editor—Concern is growing that health service research will be impeded by “research governance” procedures, in addition to the difficulties of gaining ethical approval.1-3 We describe the problems experienced by an evaluation study team (funded by the Service Delivery and Organisation Research Programme) that wanted to assess the impact of modernising endoscopy services in 20 NHS Trusts in England. Ethical approval was given by a multicentre research ethics committee in 47 working days. Achieving research governance approval was more difficult.
The study used postal surveys of patients to assess the impact of endoscopy service innovations on waiting times and other outcomes, using validated quality of life, patient satisfaction instruments, and health economic data. A qualitative component used interviews with clinicians, change agents, and patients. No experimental intervention was undertaken.
The range of familiarity with research governance “approval” procedures in NHS trusts was wide, from full awareness to total ignorance, illustrated by two trusts providing immediate verbal approval. Substantial variation occurred in the application procedures. Many trusts required more information than the ethics committee, such as confirmation of sponsor and copies of peer review reports (often not made available to researchers). Documentation was “lost” in two trusts.
Some trusts gave approval authority to one person while others relied on research and development committees, which typically sat monthly. These committees often had long lead times and full agendas, resulting in delays. One such trust committee imposed its own “research” conditions, altering the protocol and leading to the site being abandoned, to the dismay of the trust clinician who wanted to participate.
In summary, the research governance framework has been interpreted in many different ways.4,5
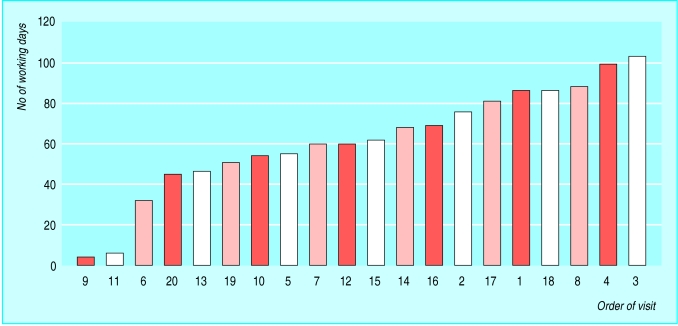
The figure shows the number of working days from application to final approval. The median time to approval was 61 days (95% confidence interval 51 to 81 days); the most time taken was 103 days (equivalent to 5 months). Applications took place from November 2003 to March 2004, and to exclude the possibility of improvements taking place over time we estimated a possible correlation (Spearman's r) between the order of the application and the time taken. We found a non-significant (P = 0.22) negative correlation (r = -0.28), indicating that order had no effect.
Figure 1.
Time taken to gain approval for research and development from trusts
Obtaining research governance approval for all 20 NHS trusts required 103 days. This is a rate limiting step, as a simultaneous start was needed across all 20 sites. With the addition of the 47 days taken to obtain ethical approval, this resulted in a total delay of 150 days.
Research studies requiring multiple NHS sites should build in substantial lag times before research processes can be initiated. We anticipate that failure to address this new obstacle to health service research will block evaluation work and accelerate the migration of clinical studies to other parts of the world.
Supplementary Material
 Details of the other four authors are on bmj.com
Details of the other four authors are on bmj.com
References
- 1.Dumville JC, Watson J, Raynor P, Torgerson DJ. Research governance: a barrier to ethical research. Q Rev Med 2004;97: 113-4. [DOI] [PubMed] [Google Scholar]
- 2.Wald DS. Bureaucracy of ethics applications. BMJ 2004;329: 282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamrozik K. Research ethics paperwork: what is the plot we seem to have lost? BMJ 2004;329: 286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health. Research governance framework for England. London: DoH, 2001.
- 5.Salisbury C, Leese B, McManus RJ. Ensuring that research governance supports rather than stifles research. Br J Gen Pract 2005;55: 4-5. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.