Abstract
The prevalence of obesity in India is increasing and ranges from 8% to 38% in rural and 13% to 50% in urban areas. Obesity is a risk factor for development of type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, coronary heart disease and many cancers. In Asian Indians excess abdominal and hepatic fat is associated with increased risk for T2DM and cardiovascular disease. There is higher risk for development of obesity related non-communicable diseases at lower body mass index levels, compared to white Caucasians. Despite being a commonly encountered medical problem, obesity poses challenges in treatment. Many Indian physicians find themselves to be lacking time and expertise to prepare an appropriate obesity management plan and patients experience continuous weight gain over time despite being under regular medical supervision. In this article, we outline approaches to obesity management in ‘real life mode’ and in context to Asian Indian patients.
Keywords: Obesity, Management, Asian Indians, Lifestyle intervention, Pharmacotherapy
1. Introduction
The prevalence of obesity in India is increasing continuously and recent data shows that between 13% to 50% of the urban population and 8%–38.2% of the rural population suffers from obesity.1 Obesity is more commonly seen in women compared to men and is increasing in children and adolescents.1 The state of Punjab (North India) has the highest prevalence of 30% in women and 22% in men.2 The main contributors to this rise are adoption of sedentary lifestyle and consumption of energy dense foods.3, 4
The increase in obesity has led to increase in associated co-morbidities like T2DM, hypertension, dyslipidemia, coronary heart disease (CHD), non-alcoholic fatty liver disease (NAFLD), obstructive sleep apnea and certain cancers. The occurrence of multiple morbidities causes financial burden on the individual and the health care resources. Hence, it is important for physicians in India to diagnose and initiate early treatment to halt the progressive increase in body weight and development of co-morbidities.
In this article, we attempt to outline ‘real life’ approaches in dealing with patients with obesity in Asian Indians. We conducted a literature search on medical search engines,
PubMed (National Library of Medicine, Bethesda, MD, USA) and Google Scholar using the keywords, “obesity; Asian Indians; characteristics; diagnosis; co-morbidities; weight loss benefits; expectations; goals; counselling; management; diet; physical activity; lifestyle intervention; pharmacotherapy; bariatric surgery” for this purpose. Specifically; the following discussion takes into consideration published guidelines for Asian Indians; international guidelines and review articles from India and developed countries.
1.1. Obesity in asian indians: characteristics, diagnostic criteria and evaluation
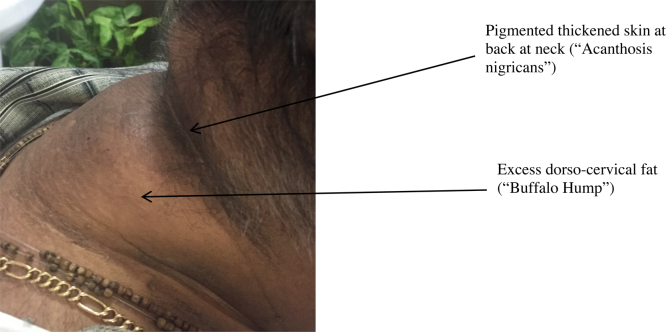
The distinctive features of obesity in Asian Indians include greater total, truncal, intra-abdominal and subcutaneous adipose tissue compared to white Caucasians.1, 5, 6 Fat deposition is seen in ectopic tissues like liver, pancreas, dorso-cervical region (‘buffalo hump’) and under the chin (‘double chin’) and this is closely associated with the metabolic syndrome.7, 8
Diagnosis of overweight and obesity is done using Body Mass Index (BMI) cut-offs of ≥23 kg/m2 and ≥25 kg/m2, respectively, as per Consensus Guidelines for Asian Indians. BMI is calculated as weight in kilogram divided by height in metre squared.9 Waist circumference (WC) should be measured as described in legend of Fig. 1. Waist hip ratio (WHR) is calculated by dividing WC by the maximum hip circumference. Measurement of body fat percentage through bioelectrical impedance analysis (BIA) and dual energy X-ray absorptiometry (DXA) can be done at advanced centres for obesity management; but are not required for routine management of obesity. The WC cut-offs WC for diagnosis of abdominal obesity in Asian Indian males and females are ≥90 cm and ≥80 cm, respectively, and WHR cut-offs in males and females are 0.88 and 0.80, respectively.9
Fig. 1.
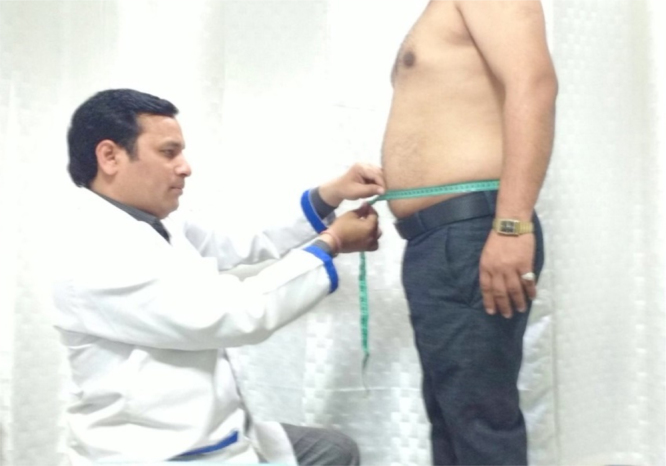
Waist circumference measurement in a 45 year old male patient with weight 72 kg and BMI 32 kg/m. The subject should be in the fasting state and standing erect and the observer should be sitting in front of the subject. Waist circumference is measured with a non-stretchable flexible tape in the horizontal position, just above the iliac crest at the end of normal expiration.9 In this case waist circumference was 105 cm, much above cut-offs for men (see text for details).
Evaluation of patients during the first clinic visit should be protocol-based and comprehensive. A detailed history should include onset and rate of weight gain, dietary history, physical activity, previous attempts at weight loss, current medications and
co-morbidities. An attempt must be made to identify factors associated with weight gain like smoking cessation and confinement due to illness or injury.
Patients should be examined for physical characteristics associated with obesity (Table 1). Acanthosis nigricans (Fig. 1), excess dorso-cervical fat deposition (Fig. 2), hepatomegaly, xanthalesma and arcus are some of the commonly observed phenotypic characteristics in patients with obesity. Investigations including 75 g oral glucose tolerance test, glycated hemoglobin (HbA1c), fasting lipid panel, thyroid function test, blood urea and serum creatinine should be checked. Secondary causes of weight gain (e.g. hypothyroidism, polycystic ovarian disease, Cushing’s disease, acromegaly etc.) should be investigated if suspected. Presence of obesity related co-morbidities (detailed below) should be documented.
Table 1.
Recommendations for medical management of Asian Indians with obesity.
| Recommendations | ||
|---|---|---|
| 1 | Clinical work up (excluding history) | Physical exam: Height, weight, waist circumference, waist-hip ratio, body mass index, estimation of percentage body fat,* Acanthosis nigricans (Fig. 2), skin tags, xanthalesma, arcus, double chin, ‘buffalo hump’ (Fig. 2), tendon xanthoma, gynecomazia, excess supra-clavicular fat pads, striae, thyromegaly, hepatomegaly |
| Laboratory tests: 75 g oral glucose tolerance test, glycated hemoglobin (HbA1c), fasting lipid panel, thyroid function test, blood urea and serum creatinine, serum cortisol# | ||
| 2 | Diet | Advise a hypo-caloric individualized diet. |
| 3 | Physical activity | Advise at least 60 min of physical activity (aerobic and resistance exercises) daily or 300 min of activity per week. |
| 4 | Pharmacotherapy | Orlistat (BMI ≥ 27 kg/m2 or a BMI of greater than 25 kg/m2 with co-morbidities) Liraglutide for carefully selected individuals Judicious use of metformin, SGLT2 inhibitors and other GLP-1 agonists in obese diabetic individuals. |
| 5 | Bariatric surgery | For patients with BMI of 32.5 kg/m2 in the presence of co-morbidities and 37.5 kg/m2 in the absence of co-morbidities |
*Optional investigations, # if clinically suspected; SGLT-2: sodium glucose transporter-2; GLP-1: glucagon like peptide-1.
Fig. 2.
Acanthosis nigricans and excess dorsocervical fat (‘buffalo hump’) in a 50 year old obese patient (BMI 35 kg/m2) with hypertension.
1.2. Educating the patients regarding obesity related morbidities along with the benefits of weight loss
Patients diagnosed with obesity may have associated co-morbidities including T2DM, hypertension, dyslipidemia, obstructive sleep apnea, NAFLD, proteinuria, osteoarthritis of weight bearing joints, varicose veins and lower limb edema.10, 11, 12, 13 Obesity is associated with cancers of endometrium, post-menopausal breast cancer, colon, oesophagus and kidney.14 Mood disorders, low self-esteem and poor quality of life can be found in many patients. These conditions should be identified and their management should be planned. Patients should be educated about the long term financial implications of these co-morbidities.
Patients should be encouraged to lose weight by educating them on the benefits of weight loss. Patients enrolled in lifestyle intervention programs like the Diabetes Prevention Program (DPP) which had the goal of a minimum weight loss of 7% body weight through dietary modification (hypo caloric diet) and 150 min of moderate intensity physical activity per week were found to have a 58% reduction in the incidence rate of diabetes.15 In the Look AHEAD (Action For Health in Diabetes) study, a 5–10% weight loss over one year led to improvement in glycemic control along with improvement in cardiovascular disease (CVD) risk factors (Table 2).16 Other benefits of weight loss include improvement in NAFLD, osteoarthritis, urinary incontinence, depression, obstructive sleep apnea, mobility and quality of life.17
Table 2.
Benefits Associated with 5–10% Weight Loss in Patients with Diabetes.
| S.No. | Benefit | Odds ratio (95% confidence intervals) |
|---|---|---|
| 1 | 0.5% reduction in HbA1c* | 3.52 (2.81–4.40) |
| 2 | 5 mm reduction in systolic blood pressure | 1.56 (1.27–1.91) |
| 3 | 5 mm reduction in diastolic blood pressure | 1.48(1.20–1.82) |
| 4 | 5 mg/dl increase in HDL cholesterol | 1.69 (1.37–2.07) |
| 5 | 40 mg/dl reduction in triglycerides | 2.20 (1.71–2.83) |
Based on data from Look Ahead Study16.
Optimum physical activity and diet to maintain weight in ideal range is a preferred strategy for primary prevention of obesity as well as for weight loss. A discussion on these (as elaborated below) will be particularly helpful for those with a familial tendency of weight gain.
1.3. Educating patient(s) regarding weight loss expectations and goal setting
It is important to discuss realistic weight loss goals and long term expectations as patients might perceive the loss to be slower and lesser than expected.18 In a randomized trial, dietary changes lead to an average weight loss of 2–3 kg after one year.19 Patients who are taking weight loss medications have greater probability of achieving weight loss. Comparison of effect of weight loss medication (orlistat) to dietary changes alone showed that the number of patients losing more than 5% initial body weight after one year was >35% with orlistat use compared to 25% with diet alone. There were more patients who lost >10% initial weight in the orlistat group (14%) compared to diet alone (10%).19, 20
It should be stressed that good adherence to lifestyle practices and sustained efforts will lead to greater weight loss. There are inter-individual variations in weight loss and a goal of a minimum weight loss of one kg per month may be suggested. A weight loss of >2% of baseline weight in the first month and >3% in the second month is associated with greater likelihood of maintaining >5% weight loss in the long term.21
Patients may regain weight with passage of time due to decreased compliance with lifestyle changes. About 30% of the weight may be regained one year after treatment and up to 50% patients may go back to base-line weight after 4–5 years.22, 23 Factors associated with maintenance of weight loss include weight monitoring, regular physician visits, sustained adherence to hypocaloric diets and high levels of physical activity.24, 25, 26, 27 Hence, importance of lifestyle changes should be stressed at every visit and particularly when weight loss medications are being tapered off.
1.4. Advising appropriate dietary modifications
Patients should be educated that Asian Indian diets are high in carbohydrate, total fat, saturated fat and trans fat and low in fibre, monounsaturated fatty acids (MUFA) and ω −3 polyunsaturated fatty acids (PUFA).28 The high carbohydrate content of Asian Indian diet is associated with hyperinsulinemia and insulin resistance.28, 29
Although, several popular weight loss diets are available, a randomized controlled trial showed that these diets result in a modest weight loss (2–3 kg) at one year.19 These results were limited to those patients who were able to achieve clinically meaningful adherence to the diet plan (25% of total patients). Long term adherence is generally low independent of the type of the diet and usually decreases after 6 months of dietary adherence.19, 30 Overall, low carbohydrate calorie-restricted diets have been shown to be effective in reducing weight, insulin resistance, serum triglycerides and increase HDL cholesterol levels.31, 32
Patients should be advised to reduce calorie intake and increase consumption of complex carbohydrates, proteins, fruits, vegetable, berries and nuts. Reduction in the consumption of saturated fats and partially hydrogenated vegetable oils (vanaspati) should be recommended.4, 17, 33 Increase in consumption of fibre through intake of fruits, vegetables, whole grain cereals, pulses and nuts should be encouraged.33 Meal replacement products can be used in those patients who have difficulty in controlling and keeping track of food intake or have hectic lifestyles. A six month Asian Indian lifestyle interventional trial using pistachio nuts (20% energy as compared to equicaloric diet) lead to reduction of waist circumference and improvement of cardio metabolic profile.34 We have observed (at FORTIS C-DOC Hospital) that the use of high protein diets in patients with normal renal function was associated with adequate satiety which may improve dietary adherence (Misra, A unpublished data). Counselling on reducing portion sizes and reducing frequency of dining out is important. Comprehensive dietary guidelines for healthy eating and weight loss for Asian Indians are available for reference.33
Many physicians can provide only rudimentary dietary advice due to lack of time. Challenges to successful diet adherence in Asian Indians include lack of knowledge about healthy diet, frequent festivals and socializing and lack of access to scientific nutrition advice. Patients should be referred to trained nutritionists who can devote adequate time in advising appropriate dietary management. Patients should be monitored for dietary adherence in follow up visits and importance of healthy eating should be reinforced frequently.
1.5. Advising changes in physical activity
Physical activity levels are frequently low in most Asian Indians as compared to white Caucasians.33 Sedentary behavior increases in Asian Indians at around 35 to 45 years in women and above 45 years in men.35 Evaluation of the physical activity levels and appropriate recommending regarding exercise is essential. Increase in physical activity improves weight loss and helps in maintaining it.
The patient’s age, physical conditioning and health status must be kept in mind while suggesting an exercise regimen. Patients with cardiac or respiratory symptoms need evaluation and optimization of medications. Patients with chronic health problems like T2DM who have been sedentary for long time intervals should also have a basic cardiac screening (electrocardiogram and echocardiography).
A combination of aerobic and muscle strengthening (resistance) exercises is beneficial. Patients who have physical deconditioning should be advised to start walking for about 15–20 min daily and increase time and intensity at weekly intervals. A goal of 60 min of physical activity daily or ≥300 min of weekly moderate intensity physical activity is an effective strategy for weight loss. Guidelines for physical activity for Asian Indians are available for reference.36 Lack of time, absence of facilities for exercise, extremes of climate, social and security reasons (for women) are frequently cited as reasons for inability to do adequate exercise. Patients should be motivated to make time for exercise, join gymnasiums or exercise at time of the day when weather is suitable. Establishment of an exercise apparatus (stationary exercise cycle, treadmill) is encouraged for those who can afford them. A discussion of health benefits of exercise including prevention of development of T2DM and cardiovascular disease and improvement in hypertension, dyslipidemia, bone density, muscle mass and sense of well-being can encourage patients to exercise.37, 38, 39, 40 Benefits of Yogic Asanas on weight loss, if any, remain poorly researched. Physical activity levels should be assessed in subsequent visits and patients should be encouraged to increase time and intensity to improve weight loss. Patients who reduce or stop physical activity should be counseled on resuming physical activity.
1.6. Advising appropriate choice of medications to avoid weight gain
Many commonly used medicines including certain anti-hyperglycemic drugs, glucocorticoids, atypical antipsychotics, anticonvulsants, mood-stabilizers and antidepressants are associated with weight gain.41 These drugs must be identified and substituted wherever possible with alternative drugs that are weight neutral or associated with weight loss (Table 3). In obese patients with T2DM, anti-hyperglycemic medications which are weight neutral or are associated with weight loss are preferred [metformin, sodium glucose transporter-2 (SGLT2) inhibitors, glucagon like peptide 1 (GLP-1) agonists].
Table 3.
Weight Changes with Common Medications used in India.a
| Weight gain | Weight neutral | Weight loss | |
|---|---|---|---|
| 1. Anti −hyperglycemic agents | pioglitazone, sulphonylureas | metformin, GLP-1 agonists, SGLT2 inhibitors | |
| 2. Hormones | glucocorticoids | testosteroneb, leuprolideb medroxyprogesteroneb | |
| 3. Atypical antipsychotics | olanzapine, quietiapine risperidone, aripiprazoleb | ziprasidoneb | |
| 4. Anticonvulsants and mood-stabilizers | gabapentin, carbamazepineb, divalproexb |
lithiumb, lamotrigineb | zonisamide, topiramate |
| 5. Antidepressants | amitriptyline, mirtazapine | bupropion, fluoxetine, sertralineb, venlafaxineb, duloxetineb |
Based on data from a systematic review and meta-analysis41.
Low quality evidence, GLP-1: glucagon like peptide-1, SGLT-2: sodium glucose transporter-2.
1.7. Adding pharmacotherapy for weight loss
Pharmacotherapy can be added to lifestyle changes for increasing weight loss. The recommended cut offs for use of pharmacotherapy in Asian Indians are BMI ≥27 kg/m2 without co-morbidities and ≥25 kg/m2 with co-morbidities.9 Additionally, it may be considered if WC is 10 cm above the upper limit of gender specific normal values for Asian Indians.9 Patients with medical conditions associated with obesity e.g. hypothyroidism, PCOD, Cushing’s disease should be treated for the underlying medical condition. Weight loss medications may be considered on a case-to-case basis after the primary condition has been adequately treated.
Medications for obesity need to be taken on a regular basis and over a long time interval to achieve meaningful weight loss. Patients who do not achieve a loss of 5% of initial weight in 3 months should be taken off treatment. Medications approved by US Federal Drug Administration (US FDA) for weight loss include orlistat, liraglutide, lorcaserin, phentermine-topiramate combination and bupropion-naltrexone combination. Of these, medications, orlistat and liraglutide are available in India.
Orlistat is a pancreatic lipase inhibitor, taken before meals and associated with weight loss of up to 10% of initial weight over one year in study settings.42 Use of orlistat has been shown to be cost-effective in the treatment of obesity.43 Adverse drug reactions are predominantly gastrointestinal and include cramps, oily stools, faecal urgency and bloating. Cetilistat is another pancreatic lipase inhibitor which is approved in Japan for treatment of obesity and is available in India. It is reported to have lower incidence of gastrointestinal adverse events (steatorrhea) compared to orlistat which may lead to lesser rate of discontinuation of treatment.46
Liraglutide is a GLP-1 agonist used in the treatment of T2DM (brand name Victoza) and its use is associated with weight loss in these patients. It has to be injected daily at doses up to 1.8 mg daily. It is approved as a weight loss medication for adults with BMI ≥30 kg/m2 without co-morbidities, and BMI ≥27 kg/m2 with co-morbidities. The dose for weight loss is 3.0 mg daily and it is available under brand name Saxenda. Patients on Liraglutide 3 mg subcutaneous injections daily for 56 weeks had an average weight loss of 8%, and >10% weight loss was seen in 33% of the patients.44 There may be improvement in non-alcoholic steatohepatitis (NASH) as histological resolution was noted in 39% of overweight patients,45 although long term studies are still lacking. The most commonly reported adverse reactions include nausea, diarrhoea, constipation, vomiting, fatigue, dizziness and dyspepsia. It is contraindicated in pregnancy, family history of medullary thyroid carcinoma and Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). It should not be used in patients with history of pancreatitis and used with caution in patients with renal impairment. In India, Liraglutide (brand name Victoza) is available for treatment of T2DM.
Barriers to successful pharmacotherapy include lack of acceptance of need for treatment, failure to take medications on a regular basis for long time intervals, and reluctance to take injectable treatment (Liraglutide). Some patients fear taking medications as serious adverse events like pulmonary hypertension, valvular heart disease, suicidal tendencies and adverse cardiovascular events were reported with older weight loss medications. Patients should be counselled that these medications (e.g. fenfluramine, dexfenfluramine, rimonabant and sibutramine) have been withdrawn and currently available medications undergo rigorous safety monitoring before approval. Alternative unproven therapies for weight loss should be strongly discouraged.
1.8. Advising bariatric surgery
Patients with severe obesity and with or at risk of co-morbidities should be offered bariatric surgery. Guidelines for Asian Indians recommend bariatric surgery in those with a BMI of ≥32.5 kg/m2 in the presence of co-morbidities and ≥37.5 kg/m2 in the absence of co-morbidities.9 Commonly performed surgeries include laparoscopic adjustable gastric banding (LAGB), laparoscopic sleeve gastrectomy (LSG), laparoscopic Roux-en-Y gastric bypass (LRYGB) and biliopancreatic diversion with duodenal switch (BPDS).
Bariatric surgery leads to higher levels of percentage excess weight loss and remission of T2DM compared to medical therapy (including very low calorie diet and intensive lifestyle program) after a mean follow up of 17 months.47 A study on Asian Indians patients with diabetes of less than five years duration showed 100% diabetes remission after LRYGB.48 Other benefits include improvement in hypertension, dyslipidemia, osteoarthritis, NAFLD and obstructive sleep apnea.49, 50
Patients who are candidates for bariatric surgery should be referred to the bariatric team to discuss the benefits, possibility of complications, need for long term micro-nutrient supplementation, long-term medical follow up and cost-implications of bariatric surgery. Barriers to bariatric surgery include fear of ‘surgery’, lack of acceptance of need for surgery, apprehension about development of complications and financial constraints.
2. Conclusions
Rising obesity prevalence in India needs appropriate measures for prevention and management. Obesity characteristics (including ectopic fat) are more adverse in Asian Indians and lead to morbidities at lower BMI levels compared to white Caucasians. Lifestyle management should be advised at lower limits of BMI and waist circumference according to Indian guidelines. Diet and exercise recommendations should be more intensive to prevent and treat obesity and its co-morbidities. Pharmacotherapy for weight loss should be prescribed after discussing the adverse effects and benefits of treatment. Medications which are weight neutral or are associated with weight loss benefits should be preferred for use wherever possible. Bariatric surgery leads to improvement of multiple co-morbidities and should be judiciously offered to selected patients.
References
- 1.Misra A., Shrivastava U. Obesity and dyslipidemia in South Asians. Nutrients. 2013;5(July (7)):2708–2733. doi: 10.3390/nu5072708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Family Health Survey (NFHS-3). Available online: https://dhsprogramcom/pubs/pdf/FRIND3/FRIND3-Vol1[Oct-17-2008]pdf (Accessed February 15, 2016). 2005-06.
- 3.Misra A., Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008 Nov;93(11 Suppl 1):S9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 4.Misra A., Khurana L. Obesity-related non-communicable diseases: south Asians vs white caucasians. Int J Obes (Lond) 2011;35(February (2)):167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- 5.Hughes K., Aw T.C., Kuperan P. Central obesity, insulin resistance, syndrome X, lipoprotein(a), and cardiovascular risk in Indians, Malays, and Chinese in Singapore. J Epidemiol Commun Health. 1997;51(August (4)):394–399. doi: 10.1136/jech.51.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raji A., Seely E.W., Arky R.A. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86(November (11)):5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 7.Misra A., Jaiswal A., Shakti D. Novel phenotypic markers and screening score for the metabolic syndrome in adult Asian Indians. Diabetes Res Clin Pract. 2008;79(February (2)):e1–e5. doi: 10.1016/j.diabres.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Misra A., Anoop S., Gulati S. Body fat patterning, hepatic fat and pancreatic volume of non-obese Asian Indians with type 2 diabetes in north India: a case-control study. PLoS One. 2015;10(10):e0140447. doi: 10.1371/journal.pone.0140447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misra A., Chowbey P., Makkar B.M. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Phys India. 2009;57(February):163–170. [PubMed] [Google Scholar]
- 10.Neeland I.J., Turer A.T., Ayers C.R. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(September (11)):1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson P.W., D'Agostino R.B., Sullivan L. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(September (16)):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 12.Hsu C.Y., McCulloch C.E., Iribarren C. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(January (1)):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 13.Adams L.A., Lymp J.F., St Sauver J. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(July (1)):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Wolin K.Y., Carson K., Colditz G.A. Obesity and cancer. Oncologist. 2010;15(6):556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The diabetes prevention program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(December (12)):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing R.R., Lang W., Wadden T.A. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(July (7)):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behl S., Gulati S., Bhardwaj S. Medical management of obesity. J Clin Prevent Cardiol. 2012;(April (1)):9. [Google Scholar]
- 18.Grave R.D., Calugi S., Molinari E. Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obes Res. 2005;13(November (11)):1961–1969. doi: 10.1038/oby.2005.241. [DOI] [PubMed] [Google Scholar]
- 19.Dansinger M.L., Gleason J.A., Griffith J.L. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(January (1)):43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 20.Yanovski S.Z., Yanovski J.A. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311(January (1)):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unick J.L., Neiberg R.H., Hogan P.E. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring). 2015;23(July (7)):1353–1356. doi: 10.1002/oby.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadden T.A., Neiberg R.H., Wing R.R. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19(October (10)):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarwer D.B., von Sydow Green A., Vetter M.L. Behavior therapy for obesity: where are we now? Curr Opin Endocrinol Diabetes Obes. 2009;16(October (5)):347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 24.Svetkey L.P., Stevens V.J., Brantley P.J. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(March (10)):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery R.W., Wing R.R., Sherwood N.E. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78(October (4)):684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 26.Wing R.R., Papandonatos G., Fava J.L. Maintaining large weight losses: the role of behavioral and psychological factors. J Consult Clin Psychol. 2008;76(December (6)):1015–1021. doi: 10.1037/a0014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelan S., Wyatt H.R., Hill J.O. Are the eating and exercise habits of successful weight losers changing? Obesity (Silver Spring) 2006;14(April (4)):710–716. doi: 10.1038/oby.2006.81. [DOI] [PubMed] [Google Scholar]
- 28.Misra A., Khurana L., Isharwal S. South Asian diets and insulin resistance. Br J Nutr. 2009;101(February (4)):465–473. doi: 10.1017/S0007114508073649. [DOI] [PubMed] [Google Scholar]
- 29.Sevak L., McKeigue P.M., Marmot M.G. Relationship of hyperinsulinemia to dietary intake in south Asian and European men. Am J Clin Nutr. 1994;59(May (5)):1069–1074. doi: 10.1093/ajcn/59.5.1069. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg I., Stampfer M.J., Schwarzfuchs D. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT) J Am Coll Nutr. 2009;28(April (2)):159–168. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- 31.Backes A.C., Abbasi F., Lamendola C. Clinical experience with a relatively low carbohydrate, calorie-restricted diet improves insulin sensitivity and associated metabolic abnormalities in overweight, insulin resistant South Asian Indian women. Asia Pac J Clin Nutr. 2008;17(4):669–671. [PubMed] [Google Scholar]
- 32.Foster G.D., Wyatt H.R., Hill J.O. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(May (21)):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 33.Misra A., Sharma R., Gulati S. Consensus dietary guidelines for healthy living and prevention of obesity, the metabolic syndrome, diabetes, and related disorders in Asian Indians. Diabetes Technol Ther. 2011;13(June (6)):683–694. doi: 10.1089/dia.2010.0198. [DOI] [PubMed] [Google Scholar]
- 34.Gulati S., Misra A., Pandey R.M. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. 2014;30(February (2)):192–197. doi: 10.1016/j.nut.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Singh R.B., Pella D., Mechirova V. Prevalence of obesity, physical inactivity and undernutrition, a triple burden of diseases during transition in a developing economy. The Five City Study Group. Acta Cardiol. 2007;62(April (2)):119–127. doi: 10.2143/AC.62.2.2020231. [DOI] [PubMed] [Google Scholar]
- 36.Misra A., Nigam P., Hills A.P. Consensus physical activity guidelines for Asian Indians. Diabetes Technol Ther. 2012;14(January (1)):83–98. doi: 10.1089/dia.2011.0111. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson H.O., Mason J.M., Nicolson D.J. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24(February (2)):215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 38.Jeon C.Y., Lokken R.P., Hu F.B. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(March (3)):744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 39.Howe T.E., Shea B., Dawson L.J. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7) doi: 10.1002/14651858.CD000333.pub2. [CD000333] [DOI] [PubMed] [Google Scholar]
- 40.Kraus W.E., Houmard J.A., Duscha B.D. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(November (19)):1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 41.Domecq J.P., Prutsky G., Wang Z. Drugs commonly associated with weight change: umbrella systematic review and meta-analysis (protocol) Syst Rev. 2012;1:44. doi: 10.1186/2046-4053-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjostrom L., Rissanen A., Andersen T. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(July (9123)):167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 43.Ara R., Blake L., Gray L. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technol Assess. 2012;16(5):1–195. doi: 10.3310/hta16050. [iii–xiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pi-Sunyer X., Astrup A., Fujioka K. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(July (1)):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong M.J., Gaunt P., Aithal G.P. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2015;(November):19. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 46.Kopelman P., Groot Gde H., Rissanen A. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical) Obesity (Silver Spring) 2010;18(January (1)):108–115. doi: 10.1038/oby.2009.155. [DOI] [PubMed] [Google Scholar]
- 47.Ribaric G., Buchwald J.N., McGlennon T.W. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24(March (3)):437–455. doi: 10.1007/s11695-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhasker A.G., Remedios C., Batra P. Predictors of remission of T2DM and metabolic effects after laparoscopic roux-en-y gastric bypass in obese indian diabetics-a 5-year study. Obes Surg. 2015;25(July (7)):1191–1197. doi: 10.1007/s11695-014-1501-x. [DOI] [PubMed] [Google Scholar]
- 49.Sjostrom L., Peltonen M., Jacobson P. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(January (1)):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 50.Vander Naalt S.J., Gurria J.P., Holterman A.L. Surgical treatment of nonalcoholic fatty liver disease in severely obese patients. Hepat Med. 2014;6:103–112. doi: 10.2147/HMER.S64819. [DOI] [PMC free article] [PubMed] [Google Scholar]