Abstract
OBJECTIVES
Only 40% of patients with hepatocellular carcinoma (HCC) are diagnosed at an early stage, suggesting breakdowns in the surveillance process. The aim of our study was to assess the reasons behind surveillance process failures among patients in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis Trial (HALT-C), which prospectively collected HCC surveillance data on a large cohort of patients.
METHODS
Binary regression analysis was used to identify predictors of consistent surveillance, which was defined as having an ultrasound and alpha-fetoprotein every 12 months. Surveillance failures among patients who developed HCC were classified into one of three categories: absence of screening, absence of follow-up, or absence of detection.
RESULTS
Over a mean follow-up of 6.1 years, 692 (68.9%) of 1,005 patients had consistent surveillance. Study site was the strongest predictor of consistent surveillance (P < 0.001). After adjusting for study site, patient-level predictors of consistent surveillance included platelet count >150,000/mm3 (hazard ratio (HR) 1.28; 95% confidence interval (CI): 1.05–1.56) and complete clinic visit adherence (HR 1.72, 95% CI: 1.11–2.63). Of 83 patients with HCC, 23 (27.7%) were detected beyond Milan criteria. Three (13%) had late-stage HCC due to the absence of screening, 4 (17%) due to the absence of follow-up, and 16 (70%) due to the absence of detection.
CONCLUSIONS
Surveillance process failures, including absence of screening or follow-up, are common and potentially contribute to late-stage tumors in one-third of cases. However, the most common reason for finding HCC at a late stage was an absence of detection, suggesting better surveillance strategies are needed.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and one of the leading causes of death among patients with cirrhosis. Its incidence in the United States is increasing due to the current epidemic of non-alcoholic fatty liver disease and hepatitis C virus (HCV) infection (1). Prognosis for patients with HCC depends on tumor stage, with curative options available for patients diagnosed at an early stage (2). Patients with early HCC achieve 5-year survival rates of 70% with resection or transplantation, whereas those with advanced HCC have a median survival of less than 1 year (3,4).
Surveillance is recommended in patients with cirrhosis to detect HCC at an early stage (5). Although surveillance can detect early HCC (6), over 60 % of tumors in clinical practice are diagnosed at late stages, suggesting failures in the surveillance process (7). The Quality in the Continuum of Cancer Care conceptual model categorizes surveillance process failures as an (a) absence of screening, (b) absence of follow-up for abnormal tests, or (c) absence of detection despite completing screening and follow-up (8). The model has been successfully used to examine factors associated with failures in breast and cervical cancer screening (9,10) but has not been systematically applied to evaluate HCC surveillance (11).
Prior studies have demonstrated low utilization rates for HCC surveillance; however, most used operational definitions for recent surveillance (e.g., ultrasound in the past 2 years) and few assessed the receipt of consistent surveillance (12 – 15). Furthermore, most studies have only assessed surveillance process failures (absence of screening or follow-up) without linking it to downstream outcomes (development of late-stage tumors). Although process measures are more sensitive to differences in quality of care, outcomes are often of greater interest (16).
The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial prospectively collected HCC surveillance data on a large cohort of HCV patients with advanced liver disease, who were followed by expert hepatologists in academic liver centers for up to 8.7 years. HCC surveillance in this cohort was conducted in a near-optimal setting, where patients had been selected for compliance and followed systematically in academic liver centers throughout the United States. The aim of our study was to explore HCC outcomes among patients in a formalized surveillance program and identify factors associated with surveillance process failures.
METHODS
Study population
The primary aim of the HALT-C Trial was to determine the impact of maintenance pegylated interferon therapy on long-term outcomes in patients with advanced hepatitis C, and its design has been previously described (17). In brief, patients were included if they had chronic HCV infection with advanced fibrosis or cirrhosis (Ishak score ≥3) without decompensation and had failed to achieve sustained virological response after previous interferon treatment. After 24 weeks of pegylated interferon and ribavirin, those who remained viremic at week 20 were randomized to weekly pegylated interferon maintenance therapy or no further therapy for the next 3.5 years. Study end-points/outcomes included progression of fibrosis by at least two points on Ishak score or the development of any complications of cirrhosis including ascites, encephalopathy, variceal hemorrhage, HCC, death, or liver transplantation. All patients had imaging and alpha-fetopro-tein (AFP) before enrollment; patients were excluded if they had an AFP >200 ng/ml or a suspicious mass on abdominal imaging —ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI). After the initial 3.5 years of follow-up, patients were invited to extend study participation without treatment, until study termination in October 2009.
We reviewed HCC surveillance data for all patients to assess consistent surveillance rates and to identify reasons for surveillance process failures. We excluded patients with < 1 year of follow-up after enrollment, so adequate time was available to accurately assess failure rates.
HALT-C HCC surveillance protocol
Patients had clinic visits scheduled every 3 months during the 3.5 years of the randomized trial and every 6 months thereafter, with AFP levels checked at each visit. Ultrasound examinations were performed at randomization, 6 months after randomization, and every 6–12 months thereafter. Ultrasound was recommended at 12-month intervals during the first 3.5 years of the trial and every 6 months during extended follow-up. Patients with an elevated or rising AFP and those with new lesions on ultrasound were to be evaluated with cross-sectional imaging (CT or MRI).
Definite HCC was defined by (a) imaging demonstrating a mass with AFP levels >1,000 ng/ml or (b) histological confirmation. Presumed HCC was defined as a new mass on ultrasound, in the absence of histology and AFP <1,000ng/ml, with one of the following characteristics: (a) two imaging studies with characteristic findings of HCC, (b) progressively enlarging lesion on ultrasound leading to patient death, (c) one imaging study demonstrating an enlarging mass with characteristics of HCC, or (d) one imaging study demonstrating a mass and increasing AFP levels. An Outcomes Review Panel, comprised of a rotating panel with three trial investigators, adjudicated all cases of presumed and definite HCC. Modified tumor, node, and metastases (TNM) criteria were used for tumor staging. Earlier reports included patients with HCC up to the final study visit on 20 October 2009 (18,19).
Statistical analysis
Prevalence and correlates of consistent surveillance
Consistent surveillance was defined as having at least one ultrasound and AFP testing within every 12-month period of follow-up. We chose a 1-year interval based on recommendations in the HALT-C surveillance protocol, which were created before AASLD and EASL guidelines (20). Patients without an ultrasound and AFP in any 12-month follow-up period were coded as inconsistent surveillance. Patients with one test performed in isolation (that is, ultrasound without AFP or vice-versa) were coded as inconsistent surveillance. We were unable to discriminate between cases in which HCC screening was not ordered and those in which patients did not comply with scheduled tests. Patients were censored at HCC diagnosis, liver transplantation, death, or last clinic visit. We used the Clopper–Pearson exact confidence interval method to obtain 95% confidence interval (CI) estimates for surveillance rates.
Demographics, clinical features, and tumor characteristics were compared between patients with and without consistent surveillance using Fisher exact and Mann–Whitney rank-sum tests for categorical and continuous variables, respectively. We assessed patient socio-demographic and clinical characteristics, including age, gender, race/ethnicity, body mass index (BMI), marital status, household size (number of people living with the subject), housing status (house/apartment vs. other), highest level of education (no high school vs. high school vs. college), employment status (full time vs. other), insurance status, alcohol and smoking history, clinic visit adherence, platelet count, fibrosis stage, and study site. Platelet count and AFP were evaluated as continuous and dichotomous variables and yielded similar results. Clinic visit adherence was dichotomized as complete and incomplete. Complete visit adherence was defined as patients coming to all clinic appointments within 6-week visit windows. Patients who missed appointments or arrived later than the 6-week window were classified as having incomplete visit adherence.
To identify factors associated with consistent surveillance, we constructed predictive models for screening failure with binary correlated regression models, using a generalized estimating equations approach to account for the correlation within study site. Initial models included all explanatory factors, variables of clinical importance (e.g., bilirubin, stage of fibrosis), variables with univariate P values < 0.25, and selected interactions (e.g., race and gender, visit adherence and study site). The final multivariate model includes all predictors with P value <0.05. Given that practice guidelines recommend HCC surveillance only among patients with cirrhosis, we performed this analysis on the entire HALT-C cohort as well as separately on those with cirrhosis at enrollment.
Surveillance process failures associated with late-stage HCC
We reviewed HCC surveillance data for patients who developed HCC to determine reasons for failure in the surveillance process. Patients who had surveillance by CT or MRI during the year before HCC diagnosis (n = 7) were excluded from this analysis. These patients were excluded for several reasons: (a) we were unable to determine if tests were performed for surveillance or diagnostic purposes, (b) there are insufficient data to support CT and MRI as routine surveillance tests, and (c) CT and MRI are diagnostic tests so absence of follow-up would not be possible. HCC was classified as early or late stage, with late stage being defined as tumors detected beyond Milan criteria (one tumor < 5 cm in maximum diameter or three tumors, each < 3 cm, with no vascular invasion or distant metastases). We also performed an analysis of HCC cases detected beyond stage T1 (one tumor < 2 cm in maximum diameter with no vascular invasion or distant metastases) given the goal of surveillance is to detect tumors as early as possible (21).
Surveillance process failures were classified into one of three mutually exclusive categories: absence of screening, absence of follow-up, and absence of detection. Absence of screening was defined as lack of ultrasound and AFP performed within the 12-month period before HCC diagnosis. Absence of follow-up was defined as lack of cross-sectional imaging within 6 months of a positive screening test. A positive screening test included: (a) suspicious mass on ultrasound, (b) new AFP elevation greater than 20 ng/ml, or (c) a doubling of AFP from prior visit if previously >20 ng/ml. We chose a 6-month interval based on the frequency of HALT-C clinic visits, although a more strict cutoff could have been considered given an approximate tumor doubling time of 3 months (22). Absence of detection was defined as cases of late-stage HCC despite completion of screening and follow-up. Absence of detection was a diagnosis of exclusion, so it was not coded in cases with an absence of screening or absence of follow-up. All data analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics
Table 1 summarizes the characteristics of the patients at the time of enrollment into the HALT-C Trial. Of the 1,050 enrolled patients, 45 were excluded because they were followed for < 1 year after enrollment. The mean follow-up for the remaining patients was 6.1 years. Two hundred seventy-eight patients (27.6%) completed all clinic visits in accordance with the study protocol, whereas 727 (72.4%) patients had incomplete visit adherence. Of those with incomplete visit adherence, 531 (73 %) patients missed or delayed the appointment on multiple occasions.
Table 1.
Baseline patient characteristics
| Variable | Consistent surveillance (n=692) | Inconsistent surveillance (n=313) | P value |
|---|---|---|---|
| Age | 50.2±6.9 | 50.2±7.7 | 0.80 |
| Gender (% male) | 489 (70.7%) | 203 (71.6%) | 0.82 |
| Race | |||
| Caucasian | 500 (72.3%) | 222 (70.9%) | 0.68 |
| Black | 127 (18.4%) | 55 (17.6%) | |
| Hispanic | 49 (7.1%) | 29 (9.3%) | |
| Other | 16 (2.3%) | 7 (2.2%) | |
| Insurance status | |||
| Medicaid | 32 (4.6%) | 16 (5.1%) | 0.56 |
| Medicare | 63 (9.1%) | 28 (8.9%) | |
| HMO | 275 (39.7%) | 134 (42.8%) | |
| Private | 276 (39.9%) | 108 (34.5%) | |
| BMI | 30.0±5.5 | 29.5±5.4 | 0.07 |
| Alcohol history (% ever drank) | 565 (81.8%) | 264 (84.6%) | 0.28 |
| Smoking history (pack years) | 15.0±17.1 | 13.9±15.9 | 0.56 |
| Smoking status (% active smoker) | 191 (27.6%) | 99 (31.6%) | 0.20 |
| Marital status (% married) | 471 (68.2%) | 203 (65.3%) | 0.38 |
| Number of people in household | 2.8±1.3 | 2.9±1.5 | 0.20 |
| Employment status (% full time) | 450 (65.1%) | 210 (67.5%) | 0.47 |
| Education | |||
| Less than high school | 66 (9.6%) | 28 (9.0%) | 0.77 |
| High school graduate/GED | 501 (72.6%) | 221 (71.3%) | |
| College graduate | 123 (17.8%) | 61 (19.7%) | |
| Duration of infection (years) | 28.1±8.1 | 28.3±8.0 | 0.99 |
| Complete clinic visit adherence | 211 (30.5%) | 67 (21.4%) | 0.003 |
| Platelet count (*1000/mm3) | 169.6±67.2 | 154.6±60.7 | 0.001 |
| Platelet count >150,000/mm3 | 403 (58.2%) | 154 (49.2%) | 0.01 |
| AST (U/l) | 86.5±61.5 | 91.1±54.1 | 0.02 |
| ALT (U/l) | 105.5±80.7 | 111.1±72.2 | 0.01 |
| Alkaline phosphatase (U/l) | 98.7±44.6 | 101.5±48.1 | 0.84 |
| Bilirubin (mg/dl) | 0.8±0.4 | 0.8±0.4 | 0.99 |
| Albumin (g/dl) | 3.9±0.4 | 3.9±0.4 | 0.31 |
| INR | 1.0±0.1 | 1.0±0.1 | 0.02 |
| AFP (ng/ml) | 15.9±26.7 | 20.9±34.4 | 0.04 |
| AFP > 20 ng/ml | 118 (17.1%) | 76 (24.3%) | 0.01 |
| Child-Pugh Score | 5.2±0.4 | 5.2±0.5 | 0.27 |
| Stage of disease | |||
| Minimal fibrosis (Ishak 0–2) | 60 (8.7%) | 16 (5.1%) | 0.13 |
| Significant fibrosis (Ishak 3–4) | 356 (51.5%) | 165 (52.7%) | |
| Cirrhosis (Ishak 5–6) | 276 (39.9%) | 132 (42.2%) | |
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate amino-transferase; BMI, body mass index; GED, general educational development; HMO, health maintenance organization; INR, international normalized ratio.
The mean age of the patients was 50 years and 71% were male. Over 70 % were non-Hispanic white, 18% were Black, and 8% were Hispanic. Cirrhosis was present at baseline in 41 % of patients, with all cirrhotic patients having Child-Pugh A disease. The mean baseline platelet count was 159 * 109/l, with 18% of patients having a platelet count below 100×109/l. The mean baseline AFP level was 17 ng/ml, with 19 % of patients having AFP levels >20 ng/ml and only 3% having AFP levels >100 ng/ml. HCC developed in 90 patients, of whom 19 (21.1 %) were found at TNM T1 stage and 66 (73.3%) within Milan criteria.
Prevalence and correlates of consistent surveillance
Of the 1,005 patients with at least 1 year of follow-up, 692 (68.9 %). had consistent surveillance whereas 313 (31.1 %) had inconsistent surveillance. Over the entire 6,120 patient-year follow-up period, patients did not receive HCC screening 7.5 % (95% CI: 6.8 –8.2%) of the time —related to a lack of ultrasound in 431 (94.1 %) cases, lack of AFP in 12 (2.6 %) cases, and lack of both tests in 15 (3.3%) cases. The percent of patients without HCC screening was 6.5 % (65/1,005) during year 1, peaked at 11.0% (84/763) in year 5, and decreased back to 4.2 % (13/312) by year 8. Of the 1,005 patients, 797 (79.3 %) had surveillance done at a 6-month interval at least once during follow-up, but no patient received consistent 6-monthly surveillance over the entire study period.
On univariate analysis, study site, baseline platelet count 150,000/mm3, complete clinic visit adherence, and baseline AFP level >20 ng/ml were predictors of consistent surveillance. Consistent surveillance rates did not differ by patient characteristics including age, gender, race, education level, or fibrosis stage. Markers of social support including marital status, number of people in household, and insurance status were also not predictors of consistent surveillance.
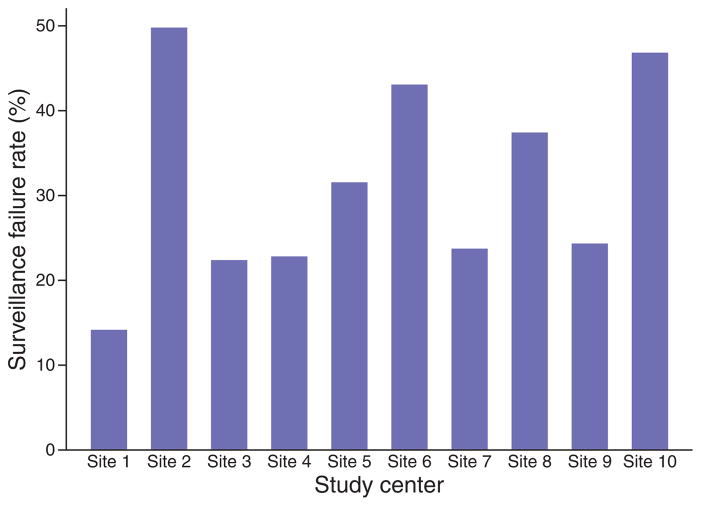
On multivariate analysis, study site remained the strongest independent predictor of consistent surveillance (P<0.001). There was substantial variation in consistent surveillance rates between study sites, ranging from 85.7 % in one center to 50.0 % in another (Figure 1). The study site with the highest patient enrollment (n=208) had consistent surveillance rates of 76.0% (95% CI: 69.7– 81.3). Although four study sites with lower patient enrollment had significantly lower consistent surveillance rates, there was no correlation between consistent surveillance rates and number of patients enrolled at each site (P=0.77). Complete visit adherence rates ranged from 6 to 68 % between study sites, but this did not correlate with inter-site differences in consistent surveillance rates (P=0.49). Rates of consistent surveillance also did not differ by the presence of general clinical research centers (n=8) (P=0.63) or number of site investigators (P=0.15).
Figure 1.
Hepatocellular carcinoma (HCC) surveillance rates by study site.
Accounting for study site, independent predictors of consistent surveillance included baseline platelet count >150,000/mm3 (hazard ratio (HR) 1.28; 95 % CI: 1.05 –1.56) and complete clinic visit adherence (HR 1.72, 95 % CI: 1.11 –2.63) (Table 2). Patients with baseline platelet count >150,000/mm3 had significantly higher rates of consistent surveillance than those with thrombocytopenia (72.4 vs. 64.5 %, respectively). This relationship may be mediated by poorer health status among patients with thrombocytopenia, although we were unable to determine reasons behind this association. Consistent surveillance was performed in 75.9 % of patients with complete clinic visit adherence, which was significantly higher than the 66.2 % consistent surveillance rates among patients with incomplete clinic visit adherence. This association with clinic visit adherence raises the possibility that patient compliance may be a potential issue in HCC surveillance.
Table 2.
Predictors of consistent surveillance
| Patient characteristic | Univariate analysisa | Multivariate analysisa | ||
|---|---|---|---|---|
|
|
|
|||
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Complete clinic visit adherence | 1.71 | 1.09–2.68 | 1.72 | 1.11–2.63 |
|
| ||||
| Baseline platelet count > 150,000/mm3 | 1.39 | 1.14–1.69 | 1.28 | 1.05–1.56 |
|
| ||||
| Baseline AFP level < 20 ng/ml | 1.57 | 1.08–2.28 | 1.48 | 0.99–2.20 |
AFP, alpha-fetoprotein; CI, confidence interval.
Analyses account for the correlations within study sites, using the generalized estimating equation approach.
Prevalence and correlates of consistent surveillance in patients with cirrhosis
Consistent surveillance rates did not differ by stage of fibrosis—67.6 vs. 69.7 % in those with vs. without cirrhosis (P=0.49). Among the 408 patients with cirrhosis at enrollment, 276 (67.6 %) had consistent surveillance. Over the entire 2,358 patient-year follow-up period, patients with cirrhosis did not receive HCC screening 8.0% (95% CI: 7.0–9.2%) of the time—related to a lack of ultrasound in 181 (95.8 %) cases, lack of AFP in 3 (1.6 %) cases, and lack of both tests in 5 (2.6%) cases. On univariate analysis, study site, Caucasian race, household size, and baseline AFP level >20 ng/ml were predictors of consistent surveillance. On multivariate analysis, study site was the strongest independent predictor of consistent surveillance (P=0.005). Accounting for study site, independent predictors of consistent surveillance included household size (OR 0.84, 95 % CI: 0.77 – 0.92) and baseline AFP level >20ng/ml (OR 0.69, 95% CI: 0.50–0.95).
Surveillance process failures associated with HCC beyond milan criteria
Of 83 HCC patients eligible for analysis, 23 patients (27.7%). had tumors detected beyond Milan criteria. Figure 2 describes reasons for surveillance process failure among those with HCC beyond Milan criteria. Three (13.0%) patients were categorized as an absence of screening, as they had not undergone HCC screening within 12 months of diagnosis— two patients did not have an ultrasound or AFP, and one patient only had an AFP without abdominal imaging. Two patients did not have abdominal imaging recorded for over 3 years, whereas the third patient had their most recent ultrasound 1.3 years before HCC diagnosis.
Figure 2.
Hepatocellular carcinoma (HCC) surveillance outcome failures for patients beyond Milan criteria.
Four (17.4 %) patients had absence of follow-up for positive surveillance tests. One patient had a suspicious mass on ultrasound, two had a positive AFP level (both with doubling of AFP while elevated over 20 ng/ml), and one patient had both a mass on ultrasound and positive AFP level (doubling of AFP and new elevation >20ng/ml). The suspicious masses on ultrasound were found 0.6 and 1.3 years before HCC diagnosis, while the positive AFP levels occurred a median of 1.0 year before HCC diagnosis.
The remaining 16 (69.6 %) patients had absence of detection, with tumors discovered beyond Milan criteria despite appropriate surveillance and follow-up. Of those with failure of detection, seven patients had negative surveillance testing (both ultrasound and AFP) within 6 months of HCC diagnosis, 5 patients within 6–9 months, and 4 patients had tumors missed by surveillance testing 9–12 months before diagnosis. None of the patients with tumors beyond Milan criteria and an absence of detection had consistent surveillance at 6-month intervals during the year before HCC diagnosis. Cirrhosis was present in one (33.3%) of the patients with absence of screening, two (50%) of the patients with absence of follow-up, and nine (56.3%) of the patients with absence of detection.
Surveillance process failures also occurred in the 60 patients with HCC detected within Milan criteria- 8 (13.3 %) of these patients had an absence of screening and 14 (23.3 %) had an absence of follow-up during the year before HCC diagnosis.
Surveillance process failures associated with HCC beyond TNM stage T1
Sixty-four (77.1%). patients had HCC detected beyond TNM stage T1 (Figure 3). Nine (14.1 %) patients had absence of screening—three patients did not have an ultrasound or AFP and six patients only had an AFP without abdominal imaging. Among these patients, the median time from last surveillance testing to HCC diagnosis was 1.8 years. Sixteen (25.0 %) patients had absence of follow-up for positive surveillance tests, of whom eight had a suspicious mass on ultrasound, five had a positive AFP level (three with doubling of AFP and two with new elevation >20 ng/ml), and three patients had both a mass on ultrasound and positive AFP level (one with doubling of AFP and two with new elevation >20ng/ml). The remaining 39 (60.9 %) patients had an absence of detection, with tumors discovered beyond TNM stage T1 despite appropriate surveillance and follow-up. Of those with absence of detection, 14 patients had negative surveillance testing within 6 months of HCC diagnosis, 7 patients within 6 –9 months, and 18 patients had the tumors missed by surveillance testing 9–12 months before diagnosis. Of these 39 patients, four had an absence of detection despite consistent surveillance at 6-month intervals during the year before HCC diagnosis.
Figure 3.
Hepatocellular carcinoma (HCC) surveillance outcome failures for patients beyond tumor, node, and metastases (TNM) stage T1.
Rates of absence of detection did not differ by patient BMI (64.4 vs. 52.6 % for those with BMI 30 vs. >30, respectively, P=0.38) or the presence of cirrhosis (57.1 vs. 65.5 % for those with vs. without cirrhosis respectively, P=0.49). Cirrhosis was present in three (33.3%) of the patients with absence of screening, twelve (75 %) of the patients with absence of follow-up, and twenty (51.3 %) of the patients with absence of detection.
Surveillance process failures, including absence of screening and absence of follow-up, were significantly less common among patients discovered to have TNM stage 1 tumors (15.8 vs. 39.1 %, P=0.001). Of the 19 patients with TNM stage T1 tumors, only one (5.3%) had absence of screening and two (10.5%) had absence of follow-up.
DISCUSSION
In this retrospective analysis of HALT-C data, we found that even among patients closely followed by expert hepatologists in academic centers, nearly one-third of patients had inconsistent HCC surveillance. Only 20 % of patients who developed HCC were found at a very early stage (TNM stage T1) and over one-fourth of tumors were found beyond Milan criteria. This study is the first that describes the contribution of surveillance process failures to the occurrence of more advanced HCC stage. We found that patients with tumors beyond stage T1 were significantly more likely to have experienced an absence of screening or follow-up, and these surveillance process failures potentially contributed to more advanced tumors in one-third of patients.
The strongest predictor for receipt of consistent surveillance was study site, after adjusting for differences in patient characteristics. Although consistent surveillance was associated with patient-level factors including platelet count and clinic visit adherence, these factors explained a smaller proportion of the variance in surveillance rates. This implies that variations in physician- and system-level factors are more important than patient-level factors in determining surveillance rates, as suggested from prior studies (14,23). We examined several potential system-level factors, including general clinical research center support and the number of enrolled patients, but could not identify any factor correlated with consistent surveillance rates. Site-level variation in visit adherence did not explain inter-site difference in consistent surveillance rates. However, we were unable to fully analyze why study site was an important predictor of consistent surveillance given limited data on physician- and system-level factors. We did not have data regarding which study sites covered screening costs or the timing and location of ultrasonography (e.g., on-site on the day of the visit vs. performing locally on a different day). Finally, potentially relevant factors, such as number of study coordinators and study team commitment toward HCC screening, are difficult to measure and may vary during the study.
In clinical practice, inconsistent surveillance could be related to physician-level factors, including under-recognition of at-risk individuals or lack of provider knowledge, clinic time constraints, or physicians forgetting to order surveillance given competing clinical concerns. Several potential barriers, such as under-recognition of the at-risk population, were not present in this setting given that all patients had known advanced fibrosis related to HCV and were followed by hepatologists in academic centers with the aid of a clinical protocol. Furthermore, surveillance rates were similar among patients with and without cirrhosis, so inconsistent surveillance was not related to lower surveillance rates among those without cirrhosis. If physicians forgetting to order surveillance is an important determinant of surveillance underuse, intervention such as reminder systems may be a more effective means to increase HCC surveillance rates than physician education.
Although studies have suggested breakdowns in follow-up may contribute to advanced breast, cervical, and colon cancer, (9,10) our study is the first to examine this issue in HCC surveillance. The effectiveness of HCC surveillance is dependent on timely follow-up with cross-sectional imaging among patients with an abnormal surveillance test. We found that follow-up was not completed within 6 months of positive surveillance testing, and therefore may have contributed to more advanced tumor stage, in 25 % of patients diagnosed with HCC. Follow-up rates for positive surveillance testing may be even lower in clinical practice, given other potential barriers such as lack of provider knowledge about appropriate follow-up testing, financial barriers, and limited access to CT/MRI imaging. Further studies are necessary to determine specific barriers to follow-up in clinical practice and if there are subgroups of patients at higher risk for not receiving timely follow-up testing.
Although an absence of screening or follow-up was present in one-third of patients with HCC, the most common reason for detecting HCC at a late stage was an absence of detection. Surveillance failure was attributed to an absence of detection in nearly 70% of patients with tumors beyond Milan criteria despite use of both ultrasound and AFP. Ultrasound and AFP had a complementary role in surveillance, as there were several patients whose HCC diagnosis was triggered by AFP without a suspicious mass on ultrasound (19). An effectiveness study recently demonstrated that ultrasound only had a sensitivity of 32% for early stage tumors, which was significantly increased to 63 % when used in combination with AFP (24). The variable effectiveness of ultrasound may be related to differences in operator experience and technique, with many patients in the United States receiving their ultrasounds in local community centers instead of tertiary care centers. Furthermore, the ability of ultrasound to accurately visualize the liver in patients with morbid obesity or a very nodular liver may be impaired (25). Although we did not find a difference in detection rates according to BMI, we could not assess the impact of truncal obesity, which might be more important than BMI. Similarly, we did not find a difference in detection rates according to cirrhosis; however, some patients without cirrhosis on baseline biopsies might have had cirrhosis at the time of HCC related to progression of fibrosis or the initial liver biopsy being understaged because of sampling error. It is clear that better surveillance tools, including more accurate biomarkers or more cost-effective advanced imaging with lower radiation risk, are necessary to help improve the sensitivity of finding tumors at an early stage. Until that time, removal of AFP from the AASLD guidelines may decrease the sensitivity of surveillance to find HCC at an early stage.
Our study has several limitations. We used data from the HALT-C Trial, which followed highly compliant patients in a near optimal setting, so our findings may not be generalizable to other clinical settings. However, surveillance process failures are likely to be more prevalent in conventional clinical practice where additional barriers to care are present. Second, it is possible that some patients had imaging performed, for surveillance or follow-up purposes, without being recorded in the HALT-C database. Although HCC surveillance was not the primary focus of HALT-C, the development of HCC was an important study outcome and the protocol included a standardized HCC surveillance algorithm. Given the prospective nature of HALT-C and that HCC outcomes were a secondary aim, we believe this would account for a minority of surveillance process failures. Finally, our analysis was limited by missing data given that this was a secondary analysis of the HALT-C Trial. We were only able to determine follow-up rates for positive surveillance testing among patients with HCC given that data regarding CT or MRI imaging was not routinely collected on all patients. Our ability to examine why study site was an important predictor of consistent surveillance was restricted by limited data on physician- and system-level factors. The study’s strengths include its large well-characterized cohort and prospective data collection system, providing near optimal conditions for a surveillance study.
Although optimal surveillance protocols, including novel effective biomarkers, are still evolving, these data provide insights into the contribution of surveillance process failures to the occurrence of advanced HCC. The most common reason for finding HCC at a late stage was an absence of detection by ultrasound and AFP. However, patients with tumors beyond stage T1 were also significantly more likely to have experienced an absence of screening or follow-up, and these surveillance process failures potentially contributed to more advanced tumors in over one-third of patients under near-optimal conditions. Further studies are needed to determine the prevalence and impact of surveillance process failures in clinical practice.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Hepatocellular carcinoma is one of the leading causes of death among patients with cirrhosis.
A minority of hepatocellular carcinomas are diagnosed at an early stage in clinical practice.
The impact of screening process failures on hepatocellular carcinoma tumor stage at presentation has not been studied.
WHAT IS NEW HERE
A significant portion of patients have inconsistent HCC surveillance and/or delayed follow-up of abnormal screening tests.
Screening process failures potentially contribute to more advanced tumor stage at time of presentation in one-third of patients.
Better surveillance tools are necessary given that failure to detect lesions is the most common reason for late-stage tumor presentation.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Amit G. Singal, MD, MS.
Specific author contributions: Amit Singal was involved in planning and conducting the study, collecting the data, analyzing and interpreting the data, drafting the manuscript, and revising the manuscript for important intellectual content. Dr Singal approved the final draft submitted. Mahendra Nehra was involved in analyzing and interpreting the data. He approved the final draft submitted. Beverley Adams-Huet was involved in analyzing and interpreting the data and revising the manuscript for important intellectual content. Dr Adams-Huet approved the final draft submitted.
Adam Yopp was involved in interpreting the data and revising the manuscript for important intellectual content. Dr Yopp approved the final draft submitted. Jasmin Tiro was involved in planning and conducting the study, interpreting the data, and revising the manuscript for important intellectual content. Dr Tiro approved the final draft submitted. Jorge Marrero was involved in interpreting the data and revising the manuscript for important intellectual content. Dr Marrero approved the final draft submitted. Anna Lok was involved in planning and conducting the study, collecting the data, interpreting the data, and revising the manuscript for important intellectual content. Dr Lok approved the final draft submitted. William Lee was involved in planning and conducting the study, collecting the data, interpreting the data, and revising the manuscript for important intellectual content. Dr Lee approved the final draft submitted.
Financial disclosures: This work was conducted with support from UT-STAR, NIH/NCATS Grant Number KL2 TR000453, NIH/NCATS Grant UL1-TR000451, and the ACG Junior Faculty Development Award awarded to Dr Singal. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, the University of Texas Southwestern Medical Center and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
Potential competing interests: None.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Singal AG, Marrero JA. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2010;26:189–95. doi: 10.1097/MOG.0b013e3283383ca5. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated non-surgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zapka JG, Taplin SH, Solberg LI, et al. A framework for improving the quality of cancer care: The case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
- 9.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97:675–83. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 10.Taplin SH, Ichikawa L, Yood MU, et al. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst. 2004;96:1518–27. doi: 10.1093/jnci/djh284. [DOI] [PubMed] [Google Scholar]
- 11.Singal AG, Tiro JA, Gupta S. Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastro Hep. 2013 doi: 10.1016/j.cgh.2012.11.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis c virus-infected veterans in the united states. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 13.Davila JA, Morgan RO, Richardson PA, et al. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the united states. Hepatology. 2010;52:132–41. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singal A, Volk M, Rakoski M, et al. Patient involvement is correlated with higher hcc surveillance in patients with cirrhosis. J Clin Gastroenterol. 2011;45:727–32. doi: 10.1097/MCG.0b013e31820989d3. [DOI] [PubMed] [Google Scholar]
- 15.Singal AG, Yopp A, Skinner C, et al. Utilization of hepatocellular carcinoma surveillance among american patients: a systematic review. J Gen Intern Med. 2012 Jan 4;:861–7. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mant J. Process vs. outcome indicators in the assessment of quality of health care. Int J Qual Health Care. 2001;13:475–80. doi: 10.1093/intqhc/13.6.475. [DOI] [PubMed] [Google Scholar]
- 17.Di Bisceglie AM, Shiffman M, Everson GT. Prolonged therapy of advanced chronic hepatitis c with low-dose peginterferon. N Engl J Med. 2008;359:2429–41. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lok AS, Everhart JE, Wright EC, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis c. Gastroenterology. 2011;140:840–49. doi: 10.1053/j.gastro.2010.11.050. quiz e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 21.Bremner KE, Bayoumi AM, Sherman M, et al. Management of solitary 1 cm to 2 cm liver nodules in patients with compensated cirrhosis: a decision analysis. Can J Gastroenterol. 2007;21:491–500. doi: 10.1155/2007/182383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota K, Ina H, Okada Y, et al. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581–6. doi: 10.1023/a:1022505203786. [DOI] [PubMed] [Google Scholar]
- 23.Singal AG, Yopp A, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res. 2012;5:1124–30. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–9. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrero JA. Screening tests for hepatocellular carcinoma. Clin Liver Dis. 2005;9:235–51. vi. doi: 10.1016/j.cld.2004.12.006. [DOI] [PubMed] [Google Scholar]