Abstract
Background
Despite high projected burden, hypertension incidence data are lacking in South Asian population. We measured hypertension prevalence and incidence in the Center for cArdio-metabolic Risk Reduction in South Asia (CARRS) adult cohort.
Methods
The CARRS Study recruited representative samples of Chennai, Delhi, and Karachi in 2010/11, and socio-demographic and risk factor data were obtained using a standard common protocol. Blood pressure (BP) was measured in the sitting position using electronic sphygmomanometer both at baseline and two year follow-up. Hypertension and control were defined by JNC 7 criteria.
Results
In total, 16,287 participants were recruited (response rate = 94.3%) and two year follow-up was completed in 12,504 (follow-up rate = 79.2%). Hypertension was present in 30.1% men (95% CI: 28.7–31.5) and 26.8% women (25.7–27.9) at baseline. BP was controlled in 1 in 7 subjects with hypertension. At two years, among non-hypertensive adults, average systolic BP increased 2.6 mm Hg (95% CI: 2.1–3.1), diastolic BP 0.7 mm Hg (95% CI: 0.4–1.0), and 1 in 6 developed hypertension (82.6 per 1000 person years, 95% CI: 80.8–84.4). Risk for developing hypertension was associated with age, low socio-economic status, current alcohol use, overweight, pre-hypertension, and dysglycemia. Risk of incident hypertension was highest (RR = 2.95, 95% CI: 2.53–3.45) in individuals with pre-hypertension compared to normal BP. Collectively, 4 modifiable risk factors (pre-hypertension, overweight, dysglycemia, and alcohol use) accounted for 78% of the population attributable risk of incident hypertension.
Conclusion
High prevalence and poor control of hypertension, along with high incidence, in South Asian adult population call for urgent preventive measures.
Keywords: Hypertension, Prevalence, Incidence, South Asia, India
1. Introduction
Hypertension is a common risk factor for cardiovascular disease (CVD) and a major global public health problem [1]. Globally, hypertension affects approximately one in four adults [2] and results in over ten million deaths annually [3]. Furthermore, low- and middle-income countries (LMIC) contribute to nearly two-thirds of the mortality attributable to hypertension [4]. Although the average systolic blood pressure (SBP) is decreasing worldwide since 1980′s at the rate of 1 mmHg SBP per decade, it is increasing in LMICs, especially in the South Asian population [5]. There are several studies that document the prevalence of hypertension in South Asian countries [6], [7], [8], and numerous meta-analyses have unequivocally demonstrated that treating and effectively lowering blood pressure (BP) is associated with reductions in cardiovascular events and mortality [9], [10]. However, the treatment and control among prevalent hypertension cases are relatively poor in resource poor settings, though data are quite limited and little is known about hypertension management in South Asia [6].
Studies conducted in the Indian sub-continent suggest that hypertension onset occurs relatively early in life [11] and is often associated with clustering of multiple cardiovascular diseases risk factors [12]. However, there is paucity of data on the incidence and factors associated with progression to hypertension in this population. Reliable data on hypertension incidence is important to estimate the future burden of hypertension and to identify potential risk factors and subpopulations to target with preventive interventions.
In this report, we used data from a large, urban population cohort representative of three large cities in South Asia with the objective to examine prevalence, treatment and control, two year incidence, and factors associated with incident hypertension.
2. Methods
2.1. Population
The CARRS Study [13] recruited representative population cohorts of three metropolitan urban cities in south Asia with large, growing, and heterogeneous populations, namely Chennai, Delhi and Karachi. The cities were chosen based on convenience. The detailed methods including sample selection and measurements have been published elsewhere [13]. Briefly, households were selected in each of the three cities using a multi-stage cluster random sampling technique (selection of districts followed by random selection of municipal wards or census enumeration blocks and finally the selection of households within these sampling units) to ensure representativeness of the population. Two participants, one man and one woman, aged 20 years or older, were selected from each household based on “Kish method” as used in the WHO’s STEPS surveys [14].
2.2. Measurements
Trained field workers collected socio-demographic and risk factor data from all eligible participants using a structured questionnaire. Baseline assessment was done in year 2010–11. They also measured height, weight, waist circumference, systolic and diastolic BP from all participants using standardized equipment and measurement techniques. Blood pressure was recorded in the sitting position using electronic sphygmomanometer; Omron HEM-7080 and HEM-7080IT-E; Omron Corporation, Tokyo, Japan (certified by the British Hypertensive Society and the American association for Advancement of Medical Instrumentation [AAMI] protocols). A minimum of 2 measurements were taken, and 5 min apart. A third reading was also taken if the difference between first and second readings were ≥ 10 or ≥ 5 mm Hg for systolic and diastolic BP, respectively. The mean of the last two was used for analyses. Additionally, fasting blood samples were also collected for biochemistry analyses. Standard assay methods for assessment of diabetes (plasma glucose, haemoglobin A1c) and dyslipidemia (total cholesterol, VLDL-cholesterol, LDL- cholesterol, HDL-cholesterol and triglycerides) were used across the three sites. All laboratories participated in an external quality assurance program (RIQAS) from RANDOX for clinical chemistry, lipids and HbA1c. Performance of all participating labs were within the acceptable levels of <2 in the Cycle Average Standard Deviation Index (score close to zero indicates optimal performance) for all the parameters in the RIQAS. A summary of all surveillance indicators, measures, methods and instruments used in the study has been published in detail [13].
2.3. Annual follow-up
Trained field workers contacted all the study participants in the baseline survey annually and collected information on risk factors using a structured questionnaire. Additionally, anthropometry measurements and blood pressure readings were taken from all eligible participants during the second year follow-up. We used the same make of equipment for BP and anthropometric measurements, and standardization procedures for all study related measurements in the annual surveys as in the baseline survey. Year-2 follow-up was conducted in 2013-14.
2.4. Definitions
2.4.1. Hypertension prevalence, awareness, and treatment
Hypertension was defined as SBP of ≥140 mmHg and/or a diastolic blood pressure (DBP) of ≥90 mmHg and/or self-reported treatment for hypertension. Similarly, incident hypertension was defined as follow-up SBP of ≥140 mmHg and/or DBP of ≥90 mmHg and/or self-reported diagnosis of hypertension by a qualified physician among those who were not hypertensive at baseline [15]. Pre-hypertension was defined as SBP of ≥120 and/or DBP of ≥80 among individuals without hypertension. Incidence was estimated in individuals without hypertension at baseline.
To estimate awareness, treatment, and control of hypertension, we used the common denominator of total number of individuals with hypertension. Participants who had been told that they had hypertension by a healthcare professional and self-reported their status were categorised as ‘aware’, and those who reported current use of prescribed anti-hypertensive medication/s were categorised as ‘treated’. ‘Control of hypertension’ was defined as having an average of <140 and <90 mmHg for SBP and DBP, respectively, in hypertensive subjects at baseline. Parental history of hypertension was defined as self-reported status of treatment of hypertension in parents when they were <60 years old.
2.4.2. Socioeconomic Status (SES)
Household asset index and education were used to describe the SES of each participant. Principal components analysis was used to estimate cumulative household assets based on weighted scores for ownership of different household assets. Asset scores were then divided into tertiles of SES [16]. Education categories included in the analyses were ES1; “graduation & above”, ES2; “up to secondary”, ES3; “up to primary”, and ES4; “illiterates or individuals with no formal education”.
2.4.3. Others covariates
Physical activity was assessed using International Physical Activity Questionnaire (IPAQ). Waist circumference was used to define central obesity (men: ≥90 cm and women: ≥80 cm). A body mass index (BMI) of ≥25 kg/m2 was defined as overweight. Diabetes was defined as having either HbA1c value more than equal to 6.5% or fasting blood glucose more than equal to 126 mg/dL or self-reported glycemia-lowering medications. Prediabetes was defined as having HbA1c value between 5.7 to 6.5% and/or fasting glucose between 100 and 125 mg/dL. Dysglycemia was defined as either pre-diabetes or diabetes. Chronic kidney disease (CKD) status was derived from serum creatinine based estimated glomerular filtration rate (eGFR) measurements using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [17] study equation. An eGFR of less than 60 ml/min/1.73 m2 was defined as CKD. We did not have data from Karachi on serum creatinine.
2.4.4. Research ethics oversight
The Institutional Review Boards (IRBs) of the Public Health Foundation of India, New Delhi, All India Institute of Medical Sciences, New Delhi, Madras Diabetes Research Foundation, Chennai, India, Aga Khan University, Karachi, Pakistan, and Emory University, Atlanta, USA approved the CARRS study. All respondents gave written informed consent, themselves or through a next of kin/family member in the case of illiterate respondents, prior to enrolment and participation in the study.
2.4.5. Statistical analyses
The characteristics of the study population were summarized separately for men and women. The data were presented as mean with their standard deviation (SD) for continuous variables or as percentages for categorical variables. All estimates of mean BP, prevalence and incidence of hypertension were age-standardized to the 2010 World Bank regional population. Estimates were also adjusted based on survey weights to account for population representation due to sampling at different levels in each cluster. Prevalence estimates were calculated after accounting for the complex multi-stage survey design, stratification, and sampling weights.
Incidence of hypertension was estimated using follow-up data collected up to the second year among non-hypertensive individuals at baseline. Uneven response rate in the follow-up surveys in different risk groups was adjusted by inclusion of weights generated for non-response. Initially, logistic regression model coefficients were generated to estimate the probability of non-response after adjusting for baseline variables such as location (city), age, sex, education, tobacco use, BMI, pre-hypertension and diabetes. Further, we used an inverse propensity score as a weight in estimating the incidence of hypertension. Incidence rates were calculated per 1000 person years of follow-up and also as cumulative percentages over two year follow-up period with their 95% confidence intervals. Generalised linear model with Poisson family was employed to calculate the incidence relative risk ratio (RR) of potential risk factors of hypertension. Multivariable models included all baseline variables that were associated with incident hypertension in the bivariate models at 2nd year follow-up (p < 0.05). Parental history of blood pressure before age 60 was also included in the multivariable model. Analyses were repeated after imputing missing co-variates at baseline by using multiple imputation involving chained equations. The methods of multiple imputation have been explained elsewhere [18]. Proportion of data missing ranged from 1% for socio-demographic data to 23% for body weight. Multiple imputation was done using multiple imputation chained equations (MICE) approach for all missing observations in the exposure variables of interest at baseline. Ten imputed datasets were generated. Imputed values of missing continuous variables were modelled using linear regression and predictive mean matching, and imputed values of ordinal variables were modelled using ordinal logistic regression. Model convergence was checked, and diagnostics were performed on the imputed dataset. Population attributable fraction (PAF) of major modifiable risk factors were also estimated directly from the RR coefficients (Box 1, online supplement).
3. Results
3.1. Enrolment and response rate
We approached a total of 17,274 individuals in 10,002 households in the three study sites and 16,287 participants were recruited (the overall response rate was 94.3% at the participant level; 6906 Chennai [90.9%], 5364 Delhi [98.9%], and 4017 Karachi [94.3%]). There were 2393 households with single subjects (827 males and 1566 females). Fasting blood samples were collected at baseline from 13,720 of the participants (response rate = 84.2%). The response rate in the first, second and either of the initial two annual follow-up surveys were 78.6%, 79.2% and 93.2%, respectively. Individuals with elevated levels of CVD risk factors (for example; elevated BP in the prehypertension range) responded more than individuals with all optimal level risk factors. In the second annual follow-up survey the odds of participation among tobacco users [OR: 1.19; 1.04–1.36], and individuals with pre-hypertension [OR: 1.18; 1.02–1.36] were high as compared to non-users and normotensives, respectively.
3.2. General characteristics of the study population
The mean age (SD) of the population was 42 years (13.3) and 40 years (12.9) in men and women, respectively. Women were 52% of the study population. Nearly 55% of men and 41% women reported more than secondary education, and one of five individuals was either illiterate or had no formal education (Table 1). Men were relatively older in Karachi (mean age 43.2, SD = 16.2 years) as compared to Chennai (41.0, 13.1) and Delhi (42.2, 11.8). Less than primary school education among participants was more frequent in Karachi than Chennai or Delhi (Table 1).
Table 1.
General characteristics of the study population.
| Variables | Chennai |
Delhi |
Karachi |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| Men (N = 3188) | Women (N = 3718) | Men (N = 2680) | Women (N = 2684) | Men (N = 1892) | Women (N = 2125) | Men (N = 7760) | Women (N = 8527) | |
| Mean age (SD) | 41.04 (13.1) | 39.25 (11.9) | 42.20 (11.8) | 41.23 (11.1) | 43.16 (16.2) | 40.01 (12.9) | 41.96 (13.3) | 40.12 (12.9) |
| Education, % | ||||||||
| ES1 | 16.2 | 9.3 | 29.3 | 24.4 | 18.4 | 9.8 | 21.7 | 14.8 |
| ES2 | 35.7 | 27.3 | 31.8 | 24.4 | 33.0 | 26.2 | 33.6 | 26 |
| ES3 | 36.4 | 44.1 | 26.9 | 24 | 28.0 | 21.6 | 30.9 | 31.9 |
| ES4 | 11.8 | 19.2 | 12.0 | 27.2 | 20.7 | 42.5 | 13.8 | 27.3 |
| Socio economic status, % | ||||||||
| High | 18.4 | 18.8 | 44.7 | 45.3 | 37.1 | 32.9 | 32.7 | 31.3 |
| Medium | 37.6 | 35.2 | 24.9 | 23.5 | 47.1 | 48.1 | 34.8 | 34.0 |
| Low | 44.0 | 46.1 | 30.4 | 31.2 | 15.8 | 19.1 | 32.5 | 34.7 |
ES1 = educational status (above graduates), ES2 = secondary school education, ES3 = primary school and above, ES4 = lower than primary or no formal education or illiterates. High is third tertile, medium is second tertile, low is first tertile in the principal component analysis score.
3.3. Mean blood pressure levels
The mean SBP was highest in Delhi (men; 129 ± 17 and women; 121 ± 18mmHg) and lowest in Karachi (men; 123 ± 20 and women 117 ± 23mmHg). In older age groups, mean SBP was higher in both men (mean SBP in < = 24 and > = 65 years age group: 117.4 and 140.1 mmHg) and women (105.1 and 139.3 mmHg) (Table S1). DBP was also elevated in higher age groups until the age of 64 years and then started showing a decline especially in Delhi.
3.4. Prevalence, awareness, treatment and control of hypertension
Consistent with the mean blood pressure levels, the age adjusted prevalence of hypertension in men was highest in Delhi (37%) and lowest in Karachi (24%). Twenty eight percent of women in Delhi and Karachi were hypertensive, and in Chennai, 29% men and 25% women were so (Fig. S1). The prevalence was higher with age in both men and women (Table S2). Hypertension prevalence was particularly high in men ≤24 years in Delhi in comparison to adults of same age category in other cities (Table S2). Prehypertension was prevalent in nearly one third (30.3%) of the study population (men: 36%, and women: 25.2%) and the overall prevalence was highest in Delhi (33.3%) and lowest in Karachi (26.5%) (Fig. S1).
Among those with hypertension at baseline, awareness levels were highest in Karachi (men; 27% and women; 57%). They were 24 and 38% respectively, in Chennai and 22 and 36% respectively, in Delhi. Overall, treatment and control levels of hypertension, respectively, were very low in Delhi (men; 18 and 7%, women; 33 and 16%) and Chennai (men; 22 and 10%, women; 37 and 20%). More than half of women (55%) in Karachi were treated for hypertension, while BP control status was observed in 27% (Fig. 1 and Table S3).
Fig. 1.
Prevalence, awareness, treatment and control of hypertension in adults over 20-years of age in three South Asian cities. The denominator for awareness, treatment and control are all individuals with hypertension at baseline.
Although hypertension control rates were higher in individuals with established disease conditions such as diabetes (22.9%), chronic kidney disease (22.4%), heart disease (38.2%), and stroke (32.2%), they were still far less than optimal level (Table S4). Based on self-reported data at the time of the survey, more than two third (68.3%) of individuals with known hypertension were taking drugs regularly.
Hypertension awareness, treatment, and control showed a positive linear relationship with educational status (the rates were lowest in illiterate or participants with no formal education and highest in participants with more than graduate level education) only in men (Fig. S2). However, hypertension prevalence was not associated with educational status.
3.5. Incidence of hypertension
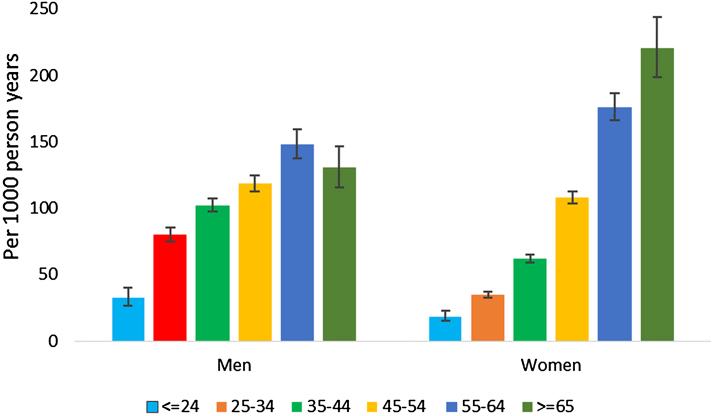
The mean SBP and DBP each increased in both men and women without hypertension at baseline during the two year follow-up period (Fig. S3). The highest secular increase in SBP was in women in the older age groups ( > 55 years) (Table S5). On average SBP increased by 2.6 mm Hg (95% CI: 2.1–3.1) and DBP by 0.7 mm Hg (95% CI: 0.4–1.0) over a mean follow up of 2 years. One of six participants without hypertension (16.2%) at baseline developed hypertension during the 2 year follow-up period. The overall age and non-response rate adjusted incidence rate was 82.6 per 1000 person-years (95% CI: 80.8–84.4), whereas the incidence rate adjusted only for non-response rate was 74.6 per 1000 person-years (95% CI: 70.5–79.1) (Table S6 and Table 2). Age adjusted incidence was highest in Delhi (94, 72 and 69 per 1000 person years of follow-up in Delhi, Chennai and Karachi, respectively). Nearly 9 of 10 incident cases (87.9%) were detected at the time of second year survey. Overall, there were no differences in the incidence of hypertension in men and women (Fig. 2). The incidence of hypertension, however, was lower in women than in men in the younger age group ( < 55 years), but was higher in women than men in the older age group (≥55 years). Incidence of hypertension was more than two times higher in participants with pre-hypertension in all age groups at baseline than those with normal blood pressure (Fig. S4).
Table 2.
Incidence and risk factors for hypertension.
| Variables | N | Un-adjusted incidence rate per 1000 p-y (95% CI) | Adjusted incidence rate per 1000 p-y (95% CI)∏ | Unadjusted RR, CI | Adjusted RR,CI (n = 6250) |
Adjusted RR,CI (without Karachi)Ⱡ (n = 4680) | Adjusted RR, CI (Missing co-variates imputed at baseline) |
|---|---|---|---|---|---|---|---|
| Overall | 8146 | 80.5 [76.3,85.0] | 74.6[70.5,79.1] | ||||
| City | |||||||
| Chennai | 3528 | 76.0[69.6,83.1] | 71.5[65.4,78.2] | 1 | 1 | 1 | 1 |
| Delhi | 2408 | 95.7[87.6,104.6] | 93.6[85.8,102.2] | 1.54‡[1.36,1.74] | 1.27** [1.09,1.47] | 1.21*[1.04,1.41] | 1.28***[1.12,1.47] |
| Karachi | 2210 | 70.4[63.3,78.3] | 69.0[62.0,76.9] | 1.16* [1.01,1.33] | 1.20 [1.00,1.44] | NA | 1.23* [1.05, 1.45] |
| Age groups, years | |||||||
| 20–24 | 718 | 28.6[21.1,38.7] | 26.8[19.2,38.6] | 1 | 1 | 1 | 1 |
| 25–34 | 2346 | 50.1[44.1,56.9] | 45.1[39.3,52.0] | 1.63**[1.12,2.36] | 1.38 [0.89,2.13] | 1.33 [0.76,2.35] | 1.37 [0.94, 2.00] |
| 35–44 | 2588 | 74.8[67.7,82.6] | 65.2[58.6,72.8] | 2.38ǂ[1.66,3.41] | 1.62*[1.05,2.48] | 1.65 [0.95,2.88] | 1.64** [1.13, 2.37] |
| 45–54 | 1530 | 113.9[102.5,126.5] | 110.1[98.9,122.8] | 3.99‡[2.78,5.71] | 2.35‡ [1.52,3.63] | 2.32‡ [1.33,4.06] | 2.42‡ [1.66,3.52] |
| 55–64 | 670 | 160.9[140.8,183.8] | 162.1 [143.2,184.3] | 5.97‡[4.15,8.61] | 3.44‡ [2.21,5.35] | 3.45‡ [1.95,6.09] | 3.46‡ [2.36,5.07] |
| 65−65 | 294 | 147.2[119.5,181.5] | 152.9[125.9,187.7] | 5.71‡[3.84,8.48] | 3.15‡ [1.96,5.06] | 3.38‡ [1.86,6.16] | 3.08‡ [2.04,4.65] |
| Sex | |||||||
| Female | 4627 | 70.9[65.7,76.5] | 65.4[60.3,71.1] | 1 | 1 | 1 | 1 |
| Male | 3519 | 93.1[86.3,100.5] | 92.3[85.3,99.9] | 1.43‡[1.28,1.60] | 1.08 [0.92,1.26] | 1.17 [0.98,1.40] | 1.05 [0.92, 1.21] |
| Education | |||||||
| ES1 | 1385 | 75.7[66.3,86.5] | 67.0[58.3,77.3] | 1 | 1 | 1 | 1 |
| ES2 | 2442 | 77.8[70.4,86.0] | 71.6[64.5,79.8] | 1.02 [0.85,1.22] | 1.15 [0.95,1.39] | 1.15 [0.93,1.44] | 1.13 [0.95, 1.34] |
| ES3 | 2672 | 80.8[73.4,88.8] | 75.1[68.0,83.2] | 1.04[0.87,1.24] | 1.19 [0.98,1.45] | 1.23 [0.99,1.54] | 1.14 [0.95, 1.36] |
| ES4 | 1647 | 88.3[78.8,99.0] | 85.8[76.3,97.0] | 1.24*[1.03,1.49] | 1.29*[1.05,1.60] | 1.25*[0.97,1.61] | 1.23* [1.02, 1.49] |
| BMI, kg/m2 | |||||||
| < 18.00 | 454 | 40.5[29.5,55.7] | 34.0[24.3,49.0] | 0.65*[0.45,0.94] | 0.73 [0.50,1.07] | 0.82[0.53,1.25] | 0.82 [0.60, 1.12] |
| 18.00−22.99 | 2010 | 59.1[52.1,67.1] | 54.3[47.6,62.2] | 1 | 1 | 1 | 1 |
| 23.00−24.99 | 1031 | 79.7[68.3,93.0] | 72.5[62.1,85.2] | 1.31**[1.07,1.61] | 1.14 [0.92,1.42] | 1.05 [0.82,1.34] | 1.10 [0.91, 1.34] |
| ≥25.00 | 3272 | 98.6[91.2,106.6] | 87.7[81.1,95.0] | 1.57‡[1.34,1.83] | 1.28*[1.04,1.59] | 1.18 [0.91,1.52] | 1.25* [1.03, 1.52] |
| Waist circumference, cm | |||||||
| Non obese | 3917 | 60.0[54.8,65.7] | 55.2[50.1,61.1] | 1 | 1 | 1 | 1 |
| Obese | 4229 | 99.4[92.9,106.3] | 89.5[83.4,96.1] | 1.63‡[1.44,1.84] | 1.04 [0.86,1.26] | 1.05 [0.84,1.32] | 1.06 [0.90, 1.27] |
| Tobacco use | |||||||
| Never users | 6326 | 75.8[71.2,80.8] | 70.4[65.8,75.3] | 1 | 1 | 1 | 1 |
| Ever users | 1820 | 96.6[87.1,107.1] | 96.5[86.9,107.5] | 1.41‡[1.24,1.59] | 1.08 [0.92,1.27] | 1.04 [0.86,1.26] | 1.09 [0.95, 1.26] |
| Current alcohol use | |||||||
| No | 7231 | 75.8[71.4,80.4] | 70.8[66.5,75.5] | 1 | 1 | 1 | 1 |
| Yes | 915 | 119.3[104.3,136.4] | 113.2[98.8,130.3] | 1.55‡[1.33,1.80] | 1.34** [1.10,1.62] | 1.25**[1.02,1.54] | 1.37‡ [1.15,1.63] |
| Physical Activity | |||||||
| Low | 811 | 92.9[79.6,108.5] | 85.0[72.8,100.0] | 1 | 1 | 1 | 1 |
| Medium | 2181 | 84.7[76.7,93.6] | 77.6[69.8,86.5] | 0.88[0.73,1.07] | 0.94 [0.76,1.16] | 1.01 [0.77,1.33] | 0.96 [0.80, 1.15] |
| High | 5154 | 76.5[71.3,82.1] | 72.3[67.1,78.0] | 0.76**[0.63,0.90] | 1.03 [0.85,1.26] | 1.00 [0.77,1.30] | 1.03 [0.86, 1.23] |
| Glycemic status | |||||||
| Normal | 2892 | 123.9[111.0,138.4] | 49.7[44.3,55.8] | 1 | 1 | 1 | 1 |
| Diabetes | 1282 | 83.5[76.4,91.1] | 119.2[106.9,133.2] | 2.39‡[2.04,2.80] | 1.27*[1.05,1.53] | 1.32* [1.06,1.64] | 1.22* [1.03, 1.44] |
| Pre-diabetes | 2977 | 54.4[48.7,60.8 | 77.3[70.7,84.8] | 1.56‡[1.34,1.80] | 1.12 [0.96,1.32] | 1.14 [0.94,1.39] | 1.09 [0.95, 1.26] |
| Parental history of hypertension | |||||||
| No | 6896 | 82.1[77.5,87.0] | 74.9[70.4,79.8] | 1 | 1 | 1 | 1 |
| Yes | 1250 | 71.9[62.2,83.2] | 73.2[62.9,85.6] | 0.97 [0.83,1.15] | 1.11 [0.93,1.32] | 1.20 [0.99,1.47] | 1.10 [0.93, 1.29] |
| eGFR, ml/min/1.73 m2 | |||||||
| > = 60 | 7137 | 77.8[73.3,82.5] | 73.3[69.0,78.0] | 1 | NA | 1 | NA |
| < 60 | 121 | 142.4[103.2,196.5] | 128.7[95.5,177.4] | 1.94‡[1.42,2.66] | 1.10 [0.69,1.74] | ||
| Baseline blood pressure levels, mmHg | |||||||
| Normal BP | 4420 | 38.0[34.1,42.3] | 35.7[31.8,40.3] | 1 | 1 | 1 | 1 |
| Pre-hypertension | 3726 | 131.1[123.1,139.6] | 129.4 [121.5,137.9] | 3.62‡ [3.17,4.14] | 2.95ǂ [2.53,3.45] | 3.3ǂ[2.74,4.04] | 2.90‡ [2.52,3.34] |
| Years of follow-up | 1.22**[1.08, 1.38] | 1.23** [1.06, 1.41] | 1.22* [1.04, 1.41] | 1.18* [1.04, 1.35] | |||
*p < 0.05, **P < 0.01, ‡p < 0.001, RR=relative risk ratio.
∏Adjusted for uneven response rate in different risk groups, Ⱡmodel includes serum creatinine based eGFR measurements from Delhi and Chennai (data are not available in Karachi). p-y = person years, ci = confidence interval, eGFR = estimated glomerular filtration rate, BMI = body mass index, ES1 = educational status (above graduates), ES2 = secondary school education, ES3 = primary school and above, ES4 = lower than primary or no formal education or illiterates.
Fig. 2.
Incidence of hypertension stratified by age groups in men and women. Incidence rate is given per 1000 person years of follow-up.
3.6. Predictors of incident hypertension
Hypertension incidence was similar in men and women (adjusted RR = 1.08, 95% CI: 0.92–1.26) in the multi-variable regression model. Hypertension incidence rate was positively and linearly associated with age, and inversely and linearly associated with educational status (Table 2). Overweight (BMI ≥ 25.0 kg/m2) was associated with 28% higher incidence of hypertension than those with BMI 18–23 kg/m2 (RR = 1.28; 95% CI: 1.04-1.59). Current alcohol use was associated with a 34% higher risk of hypertension relative to non-drinkers (RR = 1.34, 95% CI: 1.10–1.62). Presence of dysglycemia at baseline was associated with higher incidence of hypertension (RR for diabetes = 1.27, 95% CI: 1.05–1.53 and RR for pre-diabetes = 1.12; 95% CI: 0.96–1.32) in comparison to participants with normal glycemic levels. Incident hypertension was three times higher (RR = 2.95, 95% CI: 2.53–3.45) in individuals with pre-hypertension at baseline in comparison to individuals with normal BP. Regression results were comparable in the complete case analyses and in the analyses with imputed missing covariates at baseline (Table 2). Collectively, 4 modifiable risk factors (pre-hypertension, overweight, dysglycemia, and alcohol use) accounted for 78% of the population attributable risk of incident hypertension (Table S7).
4. Discussion
Based on population-based data from adults over 20 years of age from three large cities in South Asia, we estimated that on an average, one of three men and one of four women have hypertension. Hypertension awareness, treatment, and control are alarmingly low. Among non-hypertensive subjects, one of six adults developed hypertension over a two year period, probably the highest incidence reported in the world. Propensity to develop hypertension was higher among older, low socio-economic status participants, current alcohol users, and individuals characterized as overweight, pre-hypertensive and dysglycemic. The rate of progression from pre-hypertension to hypertension is three times higher than that of individuals with normal BP.
Our study findings on prevalence, awareness, and treatment of hypertension are consistent with previously reported data from the Indian sub-continent [6], [19], [20]. The incidence of hypertension among one of six adults over a two year period is a great cause of concern. It was significantly higher than the incidence data reported from developed countries [21], [22]. In absolute terms, this translates to more than doubling of the prevalence of hypertension (assuming that the same rate continues for a decade) in a span of ten years with a corresponding 243% and 271% increase among men and women, respectively. Our findings imply that the previous estimates by Kearney and colleagues on the prevalence of hypertension by 2025 (25% increase) is probably an under-estimate [2]. The anticipated increase in hypertension prevalence, in concurrence with a projected increase in prevalence of diabetes in this population [23], will lead to dramatic rises in the incidence of cardiovascular diseases.
We report that the risk for progression to hypertension in this population is associated with several socio-demographic (age, and educational status) and biological factors (overweight, blood pressure levels, and dysglycemia). Unlike the previous studies where they had used prevalent hypertension [24], [25], the outcome variable in our analyses was incident hypertension, confirming temporality of association. Some findings of public health significance are that the incidence of hypertension in individuals with pre-hypertension is more than three times than in those with normal BP, and furthermore, it is considerably higher in older age groups. Even at younger age groups, hypertension incidence risk is significantly higher in individuals with pre-hypertension than in those with normal BP. Risk stratification and targeted preventive strategies among non-hypertensive persons who are at greatest risk for progression to hypertension may help prevent the rapid rise in prevalence of hypertension.
Although the overall incidence of hypertension was similar in men and women, the pattern was distinctly different in older and younger age groups. The advantage women had in the younger age group is completely offset by higher incidence of hypertension in the older age group in comparison to men. This may be due to changes in the level of endogenous sex hormones in the post-menopausal age group as they are associated with greater longitudinal rise in BP [26]. The predilection of hypertension was 28% higher in participants above the BMI of 25 kg/m2, in comparison to individuals with BMI of 18–22.99 kg/m2, after adjustment of the effect of waist circumference and other potential confounding variables. However, it was similar in individuals with BMI of 23–24.99 kg/m2 and in individuals with BMI of 18–22.99 kg/m2. This implies that the overweight cut-off of BMI > 23 kg/m2 as suggested by some of the authors [27] are probably not relevant for hypertension risk stratification in South Asian settings.
The susceptibility to develop hypertension is higher in lower educated groups. These findings affirm our previous observations [28], [29] and is in contrast to the opinion expressed by a selected group of authors that non-communicable diseases and their risk factors are not a problem in poor communities [30]. The social gradient in hypertension has profound implications for the countries and the health care system in south Asia. As described in our study, a large majority of the incident hypertension remain undiagnosed in the absence of regular surveillance. They are more likely to result in complications of hypertension. Even if they are identified earlier, the probability of receiving treatment will be relatively low especially among individuals in the low socio-economic strata. Further it is well-established that majority of patients with hypertension will require two or more drugs to achieve BP control [31]. In this context, the high incidence rate of hypertension will also have huge financial implications for drug requirements. With families and individuals spending a significant proportion of their income for health care in South Asian countries especially in the lower socio-economic strata, the impact of the rising prevalence of hypertension on household economy is substantial.
4.1. Strengths and limitations
Prevalence and incidence estimates based on representative population-based sample from three large cities in South Asia, standardized measurement techniques, uniform study protocol and estimates after accounting for the complex study design are the major strengths of the study. The response rate in the baseline and follow-up surveys are very high. The incidence estimates are also adjusted for relatively lower non-response rate in the follow-up surveys. Finally, generalizability of our findings is limited to adult men and women living in metropolitan cities in the Indian sub-continent.
Sources of funding
This project has been funded in part by with Federal funds from the United States the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under contract no. HHSN268200900026C; and the United Health Group, Minneapolis, MN, USA. Several members of the research team at PHFI, Emory University, All India Institute of Medical Sciences, Aga Khan University and Madras Diabetes Research Foundation were/are supported by D43 NCDs in India Training Program through Award Number D43HD05249 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and Fogarty International Center; and the Wellcome Trust (Grant No: 096735/B/11/Z). Panniyammakal Jeemon is currently supported by a Wellcome Trust-DBT India Alliance Clinical and Public Health Intermediate Fellowship.
Disclosures
None.
Potential conflicts of interests
None to declare.
Acknowledgements
This project has been funded in part by with Federal funds from the United States the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under contract no. HHSN268200900026C; and the UnitedHealth Group, Minneapolis, MN, USA.
Footnotes
The authors’ takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ihj.2017.05.021.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Poulter N.R., Prabhakaran D., Caulfield M. Hypertension. Lancet. 2015;386(9995):801–812. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 2.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Collaborators GBDRF, Forouzanfar M.H., Alexander L. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(10010):2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arima H., Barzi F., Chalmers J. Mortality patterns in hypertension. J Hypertens. 2011;29(Suppl. 1):S3–7. doi: 10.1097/01.hjh.0000410246.59221.b1. [DOI] [PubMed] [Google Scholar]
- 5.Danaei G., Finucane M.M., Lin J.K. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377(9765):568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 6.Anchala R., Kannuri N.K., Pant H. Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32(6):1170–1177. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodani S., Mistry R., Khwaja A., Farooqi M., Qureshi R., Kazmi K. Prevalence and awareness of risk factors and behaviours of coronary heart disease in an urban population of Karachi, the largest city of Pakistan: a community survey. J Public Health (Oxf) 2004;26(3):245–249. doi: 10.1093/pubmed/fdh154. [DOI] [PubMed] [Google Scholar]
- 8.Rahman M.M., Gilmour S., Akter S., Abe S.K., Saito E., Shibuya K. Prevalence and control of hypertension in Bangladesh: a multilevel analysis of a nationwide population-based survey. J Hypertens. 2015;33(3):465–472. doi: 10.1097/HJH.0000000000000421. discussion 472. [DOI] [PubMed] [Google Scholar]
- 9.Ettehad D., Emdin C.A., Kiran A. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 10.Law M.R., Morris J.K., Wald N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta R., Misra A., Vikram N.K. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc Disord. 2009;9:28. doi: 10.1186/1471-2261-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeemon P., Prabhakaran D., Goenka S. Impact of comprehensive cardiovascular risk reduction programme on risk factor clustering associated with elevated blood pressure in an Indian industrial population. Indian J Med Res. 2012;135(4):485–493. [PMC free article] [PubMed] [Google Scholar]
- 13.Nair M., Ali M.K., Ajay V.S. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC public health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS). Geneva World Health Organization, 2002.
- 15.James P.A., Oparil S., Carter B.L. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 16.Ali M.K., Bhaskarapillai B., Shivashankar R. Socioeconomic status and cardiovascular risk in urban South Asia: The CARRS Study. Eur J Prev Cardiol. 2016;23(4):408–419. doi: 10.1177/2047487315580891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deepa M., Grace M., Binukumar B. High burden of prediabetes and diabetes in three large cities in South Asia: the Center for cArdio-metabolic Risk Reduction in South Asia (CARRS) Study. Diabetes Res Clin Pract. 2015;110(2):172–182. doi: 10.1016/j.diabres.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhansali A., Dhandania V.K., Deepa M. Prevalence of and risk factors for hypertension in urban and rural India: the ICMR-INDIAB study. J Hum Hypertens. 2015;29(3):204–209. doi: 10.1038/jhh.2014.57. [DOI] [PubMed] [Google Scholar]
- 20.Mohan V., Deepa M., Farooq S., Datta M., Deepa R. Prevalence, awareness and control of hypertension in Chennai – the Chennai urban rural epidemiology study (CURES-52) J Assoc Physicians India. 2007;55:326–332. [PubMed] [Google Scholar]
- 21.Tu K., Chen Z., Lipscombe L.L. Canadian hypertension education program outcomes research T: prevalence and incidence of hypertension from 1995 to 2005: a population-based study. CMAJ. 2008;178(11):1429–1435. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajjar I., Kotchen J.M., Kotchen T.A. Hypertension: trends in prevalence, incidence, and control. Annu Rev Public Health. 2006;27:465–490. doi: 10.1146/annurev.publhealth.27.021405.102132. [DOI] [PubMed] [Google Scholar]
- 23.Jayawardena R., Ranasinghe P., Byrne N.M., Soares M.J., Katulanda P., Hills A.P. Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC Public Health. 2012;12:380. doi: 10.1186/1471-2458-12-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah S.M., Loney T., Sheek-Hussein M. Hypertension prevalence, awareness, treatment, and control, in male South Asians immigrants in the United Arab Emirates: a cross-sectional study. BMC Cardiovasc Disord. 2015;15(1):30. doi: 10.1186/s12872-015-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta R., Sharma K.K., Gupta A. Persistent high prevalence of cardiovascular risk factors in the urban middle class in India: Jaipur Heart Watch-5. J Assoc Physicians India. 2012;60:11–16. [PubMed] [Google Scholar]
- 26.Wang L., Szklo M., Folsom A.R., Cook N.R., Gapstur S.M., Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224(1):228–234. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra A. Ethnic-Specific criteria for classification of body mass index: a perspective for asian indians and american diabetes association position statement. Diabetes Technol Ther. 2015;17(9):667–671. doi: 10.1089/dia.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prabhakaran D., Jeemon P., Reddy K.S. Commentary Poverty and cardiovascular disease in India: do we need more evidence for action? Int J Epidemiol. 2013;42(5):1431–1435. doi: 10.1093/ije/dyt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeemon P. Socio-economic status and cardiovascular risk among Indians. Prev Med. 2011;52(6):471–472. doi: 10.1016/j.ypmed.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian S.V., Corsi D.J., Subramanyam M.A., Smith G.D. Jumping the gun: the problematic discourse on socioeconomic status and cardiovascular health in India. Int J Epidemiol. 2013;42(5):1410–1426. doi: 10.1093/ije/dyt017. [DOI] [PubMed] [Google Scholar]
- 31.McManus R.J., Caulfield M., Williams B. National institute for H, clinical E: NICE hypertension guideline 2011: evidence based evolution. BMJ. 2012;344:e181. doi: 10.1136/bmj.e181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.