Abstract
Atrial fibrillation is the most common arrhythmia worldwide with increasing frequency noted with age. Hyperthyroidism is a well-known cause of atrial fibrillation with a 16%–60% prevalence of atrial fibrillation in patients with known hyperthyroidism Ross et al. (2016). While hyperthyroidism as a causative factor of atrial fibrillation is well established, this literature review aims to answer several questions on this topic including:
1. The relationship of atrial fibrillation to hyperthyroidism
2. Atrial fibrillation as a predictor of hyperthyroidism
3. The pathophysiology of thyrotoxic atrial fibrillation
4. Subclinical hyperthyroidism and the relationship with atrial fibrillation
5. Cardioversion and Catheter ablation of hyperthyroid patients with atrial fibrillation
6. Thrombotic risk of hyperthyroid patients with atrial fibrillation
7. Management of Thyrotoxic Atrial fibrillation
8. Pharmacological rhythm control in patients with hyperthyroidism and atrial fibrillation
9. Treatment of Hyperthyroidism to prevent atrial fibrillation
10. Clinical Implications of Hyperthyroidism and Atrial Fibrillation
Keywords: Atrial fibrillation, Hyperthyroidism, Pathogenesis
1. Introduction
Hyperthyroidism, or thyrotoxicosis occurs due to excess release of thyroid hormone due to an overactive thyroid gland or passive release of the stored hormone. Additionally, hyperthyroidism occurs from over treatment with thyroid hormone. Hyperthyroidism is generally considered overt or subclinical, depending on the biochemical severity of the hyperthyroidism. Overt hyperthyroidism is defined as suppressed (usually undetectable) thyrotropin (TSH) and elevated levels of triiodothyronine (T3) and/or estimated free thyroxine (free T4). Subclinical hyperthyroidism is defined as a low or undetectable serum TSH with values within the normal reference range for both T3 and free T4.1 Hyperthyroidism should be considered the potential illness whenever TSH level is subnormal.
The prevalence of hyperthyroidism in the United States is approximately 1.2% (0.5% overt and 0.7% subclinical).2 In an older community in Baltimore, the prevalence of low TSH was 9.6% for participants on thyroid hormone and 0.8% for untreated individuals.3 In a study performed by Krahn et al., the authors found that <1% of cases of atrial fibrillation are secondary to an acute hyperthyroid state.4 Despite hyperthyroidism being a relatively rare cause of atrial fibrillation, this review underscores the importance of identifying thyrotoxic atrial fibrillation, understanding differences in pathophysiology and management as well as illustrating the importance of screening for hyperthyroidism in those presenting with atrial fibrillation. We have compiled literature from PubMed, Scopus and Ovid to ensure a thorough and accurate literature review.
2. New onset hyperthyroidism and the relationship with atrial fibrillation
Hyperthyroidism is a well-known cause of atrial fibrillation. In a large population based study by frost and colleagues, all patients with new onset hyperthyroidism in the inpatient setting were followed ±30 days from the diagnosis of hyperthyroidism to observe for a new onset diagnosis of atrial fibrillation or atrial flutter. It was found that 8.3% of such patients had a new onset diagnosis of atrial fibrillation or atrial flutter.2 In patients with hyperthyroidism it was found that those who were male, advancing age, coronary artery disease, congestive heart failure and valvular heart disease were found to have a higher incidence of atrial fibrillation.5
2.1. Atrial fibrillation as a predictor of developing hyperthyroidism
In a large nationwide cohort study performed in Denmark by Selmer and colleagues, patients who were diagnosed with new onset atrial fibrillation were followed in the outpatient setting for 13 years to identify if they would develop hyperthyroidism.6 In the 13 year follow up there was a significantly higher incidence of hyperthyroidism being diagnosed particularly in the male population between the ages of 51–60 when compared to the general population of that age without a diagnosis of atrial fibrillation. Another Canadian study was performed testing this association on a smaller scale and failed to show an association.7 However, as the study performed by Selmer and colleagues was the most comprehensive study, its clinical application includes routine screening for hyperthyroidism in patients with new onset atrial fibrillation. While there are no studies on the incidence of subclinical hyperthyroidism after presenting with atrial fibrillation, routine monitoring of thyroid studies would identify this subgroup. The authors believed that these findings may have been secondary to:
-
1
Autoantibody formation against β1-adrenergic and M2-muscarinic receptors has been known to occur in hyperthyroidism and may trigger Atrial fibrillation prior to thyroid dysfunction.8
-
2
A genetic susceptibility to atrial fibrillation may be linked to hyperthyroidism9
-
3
Patients may have relative rises or falls in thyroid function testing, yet still remain within normal limits compared to reference values. Such variation may increase the risk of developing atrial fibrillation and will subsequently increase the risk of clinical hyperthyroidism in the future10, 11
Current guidelines are not available regarding frequency of screening and are per clinician discretion.
3. Pathophysiology
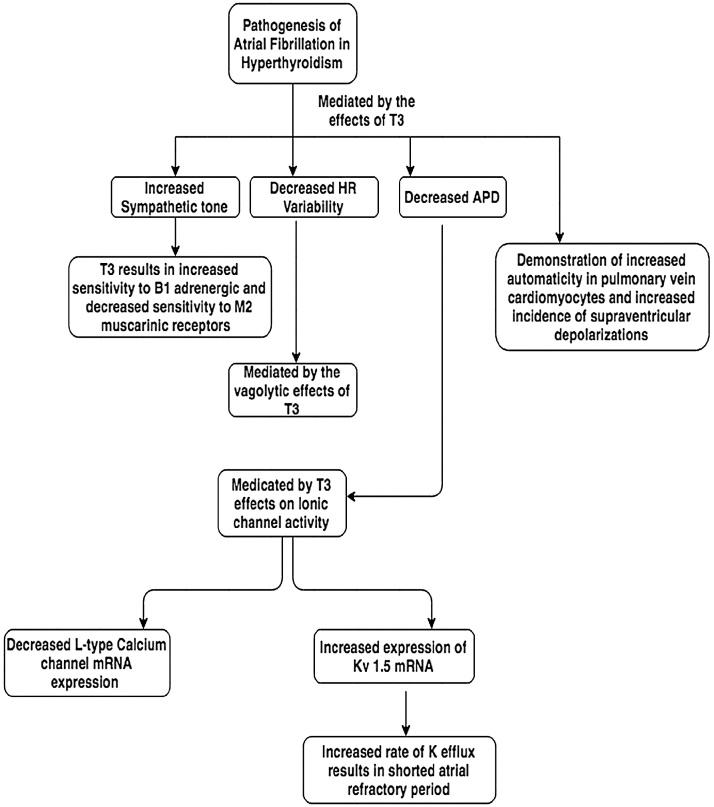
Atrial fibrillation irrespective of thyroid function is believed to be due to chaotic electrical activity resulting in a micro-reentrant tachycardia.12 The wavelength theory described by Allessie and co-workers describes a wavelength, which is a product of atrial refractoriness and conduction velocity. If the patient has a long wavelength as per this theory then re-entry will not be maintained and thus self terminate.13 In order for atrial fibrillation to be sustained the wavelength has to be short enough such that wavefronts can circulate the atrium without termination. As per this theory, atrial refractoriness, conduction velocity or both has to be sufficiently reduced to allow re-entry of wavefronts and sustainment of atrial fibrillatory waves. Other theories exist regarding the mechanism of atrial fibrillation including the presence of anatomical substrate and abnormal ectopic atrial firing. Because anyone of these abnormal findings can result in atrial fibrillation, it is important to note differences in pathophysiology between those who have atrial fibrillation with hyperthyroid and euthyroid functions. In those patients who were hyperthyroid it was found that elevated thyroid hormone altered the β1-adrenergic and M2-muscarinic receptors of the heart resulting in increased sympathetic function, tachycardia and decreased atrial refractory period. It is also known that thyroid hormone plays a role in altering ionic channels. In a study performed by Watanbe et al. the effects of thyroid hormone on mRNA expression and currents of major ionic channels were studied in murine atria. The authors found that thyroid hormone resulted in such major changes14:
-
1.)
Decreased L-type Calcium channel mRNA expression
-
2.)
Increased expression of Kv 1.5 mRNA
-
3.)
The above changes resulted in increased outward current and decreased inward current resulting in shorter action potential duration
In another study performed in rats, the authors compared action potential duration and whole cell currents in the right and left atria in both euthyroid and hyperthyroid mice. The authors found more significant shortening of APD and greater delayed rectifier potassium current increases in the right atrium than the left atrium in hyperthyroid rats which can further increase the risk for atrial arrhythmias.15 A separate study performed by Chen et al. studied the effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes in rabbits. The authors found that thyroid hormone had the following effects on arrhythmogenesis16:
-
1.)
Decreased APD
-
2.)
Increased spontaneous activity in pulmonary vein cardiomyocytes
-
3.)
Increased occurrence of delayed after-depolarizations in pulmonary vein beating and non-beating cardiomyocytes
-
4.)
Increased after-depolarizations in beating cardiomyocytes
With the following changes noted, the authors concluded that thyroid hormone plays a role on arrhythmogenesis with an increase of triggered activity or automaticity in pulmonary vein cardiomyocytes.16
Atrial fibrillation in hyperthyroid human patients, similar to the animal models as discussed is believed to be due to a decreased atrial refractory period and increased sympathetic tone with decreased heart-rate variability.17 Wustmann and colleagues noted that hyperthyroid patients with no known diagnosis of atrial fibrillation when compared to euthyroid patients without a diagnosis of atrial fibrillation were found to have an increased incidence of supraventricular depolarizations which normalized after treating the hyperthyroidism.18 While these supraventricular depolarizations have been linked to developing atrial fibrillation in patients who are euthyroid, Wustmann and colleagues did not attempt to prove any causal relationship in hyperthyroid patients. Another study performed by Komiya and colleagues showed that when invasive electrophysiological tests were performed on hyperthyroid patients with paroxysmal atrial fibrillation, in comparison to the control group, there was no increased incidence of abnormal atrial electrograms, whereas those with paroxysmal atrial fibrillation who were euthyroid had a significantly increased incidence of abnormal atrial electrograms when compared to the control. Komiya and colleagues also noted that in patients who were euthyroid, atrial refractory period was not reduced in comparison to controls.19 Interestingly, the only common finding noted between euthyroid and hyperthyroid patients in the pathogenesis of atrial fibrillation was a prolonged conduction delay and conduction zone. A flowchart illustrating the pathophysiology of atrial fibrillation in hyperthyroidism is shown in Fig. 1.
Fig. 1.
A figure illustrating the pathogenesis of atrial fibrillation in hyperthyroidism.
3.1. Subclinical hyperthyroidism and the association with atrial fibrillation
Sawin and his group reported a 2.8-fold increased risk of atrial fibrillation in subclinical hyperthyroid individuals over the age of 60.20 Subsequent studies reported similar results.21, 22 The Rotterdam study performed by Heeringa and colleagues, showed a graded response in patients with atrial fibrillation and thyroxine levels and lower TSH.11 The mechanism involved was hypothesized by Heeringa and colleagues and is believed to be threefold:
-
1
Active T 3 binds to T3 Nuclear receptors resulting in specific cardiac gene expression
-
2
T3 Reduces heart rate variability by reducing vagal tone increasing risk for arrhythmias
-
3
T3 causes peripheral vasodilation increasing cardiac preload and altering contraction
TSH has also been identified as an independent predictor of developing atrial fibrillation with lower limits of normal increasing risk regardless of free T4 levels in patients greater than age 60 within the next decade.20 While no guidelines are available regarding the frequency of monitoring thyroid function, the association noted prompts close monitoring of thyroid function status in the elderly per clinician discretion.
3.2. Cardioversion in those with atrial fibrillation and hyperthyroidism
Cardioversion may be an option in those who remain in atrial fibrillation after 8–10 weeks of remaining in a euthyroid state with anticoagulation for at least three weeks due to the concern of atrial stunning. In patients with atrial fibrillation who are of euthyroid or hyperthyroid status at the time of diagnosis, it is important to note that the chances of failed cardioversion dramatically increase with time. A large risk for recurrence is noted 1 year after diagnosis and even larger risk of recurrence 2 years after diagnosis.23 In a study performed by Gurdogan and colleagues, irrespective of thyroid status, prolonged duration of atrial fibrillation prior to cardioversion was a poor predictor for sustaining sinus rhythm.24 Further studies are required to identify those who are good candidates for cardioversion when there is a history of hyperthyroidism.
3.3. Catheter ablation of atrial fibrillation in those with hyperthyroidism
In patients with hyperthyroidism related atrial fibrillation, there is no clear consensus in the present literature regarding the efficacy of catheter ablation. Ma et al. studied the efficacy of circumferential pulmonary vein ablation in patients with thyrotoxic atrial fibrillation and found that it was a viable therapeutic option.25 A case-control study evaluating the efficacy of catheter ablation of paroxysmal atrial fibrillation in patients with a history of AIT found that a single ablation with pulmonary vein isolation had a lower efficacy when compared to patients with paroxysmal atrial fibrillation without a history of AIT. A history of AIT was also found to be an independent predictor of atrial tachycardia. After multiple ablations however, the risk of recurrence was noted to be equal in patients with and without AIT.26 A subsequent study performed by Wang et al. studying the safety and efficacy of early radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation complicated with AIT found that pulmonary vein isolation was safe and efficacious with a higher incidence of atrial tachyarrhythmias occurring up to three months post procedure but were not noted 12 months post procedure when matched with controls.27 In a separate study performed by Machino et al., the efficacy of radiofrequency ablation with pulmonary vein isolation was tested in patients who had a history of hyperthyroidism and were euthyroid for a period of three months and matched with a control group of patients with atrial fibrillation without a history of hyperthyroidism. The authors found that there was no difference in risk of occurrence of atrial fibrillation in both groups.28 Another study performed by Wongcharoen et al. showed that patients with a history of hyperthyroidism had more frequent ectopic events and a higher risk of occurrence of atrial fibrillation after a single ablation.29 In this study however, the risk of recurrence was not tested after multiple ablations. In the majority of studies, ablation was considered after restoring a euthyroid state for at least three months and was indicated for refractory atrial fibrillation. Thus we conclude that in those patients who have permenant, refractory atrial fibrillation despite restoration of a euthyroid state, ablation may be considered. However, owing to the higher risk of recurrence, multiple ablations are usually required.
3.4. Thrombotic risk of atrial fibrillation and hyperthyroidism
Atrial Fibrillation has been known to significantly increase the risk of developing stroke and thrombotic episodes. The current guidelines use the CHA2DS2-VASC scoring system to predict who is at increased risk for a thrombotic episode and would benefit from anticoagulation. Atrial Fibrillation with hyperthyroidism has been studied in various trials with varying results. As per an analysis performed by Presti and Hart regarding several older trials, hyperthyroidism and atrial fibrillation have been shown to independently increase the risk of a thrombotic episode regardless of CHA2DS2-VASC score, particularly embolic events to the central nervous system early in the course of the disease.30 In a large recent trial conducted in China by Chan et al., of 9727 Chinese patients with nonvalvular AF from July 1997 to December 2011, hyperthyroidism with atrial fibrillation was not known to independently increase the risk of thrombotic events with the risk for thrombosis based of the traditional risk factors outlined in the CHA2DS2-VASC score.31 Two major recommendations are present regarding anticoagulation in those with atrial fibrillation and hyperthyroidism. As per the American College of Chest Physicians, hyperthyroidism was not found to be an independent risk factor of thrombosis in those with atrial fibrillation and anticoagulation should be based of the traditional CHA2DS2-VASC score.31, 32 According to the American College of Cardiology however, hyperthyroidism independently increases the risk for development of stroke or thrombosis and patients should receive anticoagulation during the hyperthyroid phase regardless of CHA2DS2-VASC score.33 In a study performed by de Souza and colleagues, in patients with hyperthyroidism and atrial fibrillation, a traditional CHADS2 score was used to predict who would benefit from anticoagulation with a subsequent TEE performed to assess the efficacy of the score in identifying possible thrombogenic milieu. In this study it was found that only age was an accurate predictor of thrombogenic milieu with the remaining risk factors having a low yield.34 This study further delineates that the decision to initiate anticoagulation in patients who have hyperthyroidism and atrial fibrillation may ideally be done on an individual basis.
3.5. Management of atrial fibrillation secondary to hyperthyroidism
In the majority of cases, the cause of hyperthyroidism is either secondary to autoimmune (graves) disease, toxic multinodular goiter or toxic adenoma. The mainstay of treatment is B-blockade and treatment with an anti-thyroid agent (PTU or methimazole). While B-blocker therapy is first line to manage narrow complex tachyarrhythmias in the setting of thyrotoxicosis, in certain cases of tachycardia induced cardiomyopathy there may be a concern for hemodynamic compromise. In such cases a short acting B-blocker such as esmolol may be given to assess tolerability.23 Digoxin may also be considered in those with a tenuous hemodynamic status. However, due to a few factors; increased renal clearance, increased sympathetic tone in atrial fibrillation with reduced vagal tone and a large volume of distribution of digoxin, a larger than usual dose of digoxin is required, thus caution must be used to avoid digoxin toxicity.35, 36 In patient’s where B-blocker therapy is contraindicated, other management choices include calcium channel blockers such as diltiazem or verapamil. These agents should however be avoided in those with a reduced ejection fraction or hemodynamic instability due to a strong negative inotropic effect. Amiodarone may be used in the acute setting due to the benefit of converting the patient to normal sinus rhythm when combined with anti-thyroid medications such as PTU to reduce the chance of worsening thyrotoxicosis.37 Caution must be used however due to the risk of atrial stunning and thrombogenic phenomenon if not properly anticoagulated. Regarding anticoagulation, at present, there is no consensus on the recommendation for anticoagulation during thyroid storm. Elevated thyroxine levels in thyroid storm are known to theoretically increase the risk of a thrombotic event secondary to a metabolic change that occurs in these patients namely: increased levels of Factor VIII, Factor IX, fibrinogen, vWF, plasminogen activator-inhibitor-1 and deficiency of antithrombin III with increased clearance of circulating heparin.38, 39 The choice to anticoagulate patients in thyroid storm is per the clinician’s decision. Some clinicians choose to anticoagulate based of CHA2DS2-VASC score whereas others anticoagulate during the thyrotoxic phase regardless of other thrombotic risk factors.
3.6. Pharmacological rhythm control in patients with atrial fibrillation and hyperthyroidism
Rhythm control is usually not recommended in patients with hyperthyroidism and atrial fibrillation as nearly two-thirds of patients revert to normal sinus rhythm 8–10 weeks after achieving a euthyroid state.40 In those patients who continue to remain in atrial fibrillation after achieving a euthyroid state, rhythm control may be an option however like the general atrial fibrillation population, rate control is usually preferred initially. Options for rhythm control include class IA, IC as well as class III agents. The use of amiodarone may be indicated acutely as mentioned above during a thyroid storm to restore sinus rhythm or for chronic therapy in those with atrial fibrillation refractory to rate control. While the use of amiodarone has not been clearly studied in those with thyrotoxic atrial fibrillation, amiodarone is known to cause hyperthyroidism as well as hypothyroidism.
Hyperthyroidism secondary to amiodarone is known as amiodarone induced thyrotoxicosis (AIT) and has been further sub classified into two categories. AIT has an incidence of approximately 3% in North America with an incidence of 10% for those living in iodine-depleted areas.41 AIT Type I occurs in patients with underlying thyroid disease wherein the iodine load from amiodarone therapy (75 mg of iodine per 200 mg oral tablet) results in increased production of T3 and T4 known as the Jod- Basedow phenomenon.42 AIT Type 2 is secondary to an autoimmune destructive thyroiditis where in T3 and T4 is released from the thyroid gland. This is secondary to an elevated concentration of iodide from pharmacological therapy resulting in inhibition of thyroid hormone synthesis and release of stored hormone from the thyroid gland known as the Wolf-Chaikoff effect.43 AIT type 1 has a higher incidence in countries with iodine deficient regions whereas AIT type 2 is more common in iodine replete areas.44
In a study performed by Czarnywojtek et al., in patients who were previously hyperthyroid and underwent radioactive ablation of the thyroid gland (presently euthyroid), the efficacy of administering radioactive iodine was assessed prior to initiating amiodarone therapy despite being euthyroid at the time of administration. As per this study the authors concluded that the use of preventive radioactive iodine in euthyroid patients who were previously hyperthyroid prior to chronic amiodarone therapy was found to reduce the incidence of thyrotoxicosis and subsequently help sustain sinus rhythm. Of note 5 patients required an additional dose of radioactive iodine during this study.45 In another study performed by Kunii et al., the efficacy of Bepridil was tested in converting patients with atrial fibrillation to sinus rhythm in those with and without co-morbid hyperthyroidism. The authors found that Bepidril was equally efficacious irrespective of thyroid status.46 However, owing to the untoward effects of Bepidril such as the risk of Torsade de pointe, the drug has been banned in several countries and should be used cautiously.
3.7. Treatment of hyperthyroidism to prevent atrial fibrillation
In patients with overt hyperthyroidism without atrial fibrillation, thyroid treatment is warranted to reduce the risk of this potential complication. Treatment of subclinical hyperthyroidism should be done in individuals older than 65 and with a TSH level of <0.1 mU/L. If the patient is less than 65 year-old and TSH >0.1, treatment of subclinical hyperthyroidism should be done if the individual has symptoms or has other risk factors i.e. heart disease or bisphosphonate treatment.1, 47
3.8. Clinical implications
The prognosis of those with hyperthyroidism and atrial fibrillation has not been previously studied to our knowledge. However, identifying that hyperthyroidism is a causative factor of atrial fibrillation plays a crucial role in management as outlined above. Similarly, while the prevalence of hypothyroidism is significantly higher in those with atrial fibrillation when compared to hyperthyroidism, in a large study performed by Selmer et al. as mentioned above, routine follow up with patients after the diagnosis of atrial fibrillation showed that the incidence of hyperthyroidism was significantly higher when matched with those without a history of atrial fibrillation.6 Thus while no current guidelines are available, routine screening with TSH levels after the diagnosis of atrial fibrillation may help with early detection of hyperthyroidism and decrease the risk of metabolic and cardiovascular side effects.
4. Conclusion
Atrial fibrillation is the most common arrhythmia in the world with increasing prevalence with age. Causative factors are vast with differences in required management. In this review we focused on hyperthyroidism as a causative factor of atrial fibrillation as well as its prevalence in the population with atrial fibrillation. The goal of this review was to address several questions regarding the differences in management when co-morbid hyperthyroidism is present. The overall prognosis of having co-morbid hyperthyroidism and atrial fibrillation has not clearly been studied, however as the risk of recurrence is similar amongst the euthyroid and hyperthyroid groups, we are lead to believe the over all prognosis of hyperthyroidism and atrial fibrillation is similar to those with atrial fibrillation when matched for other variables. Further studies may be indicated to help guide management, however, this review aims to summarize the current major goals in the management of this population.
Conflicts of interest
None.
References
- 1.Ross D.S., Burch H.B., Cooper D.S. American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. doi: 10.1089/thy.2016.0229. [2016] [DOI] [PubMed] [Google Scholar]
- 2.Hollowell J.G., Staehling N.W., Flanders W.D. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III) J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.Mammen J.S., McGready J., Oxman R., Chia C.W., Ladenson P.W., Simonsick E.M. Thyroid hormone therapy and risk of thyrotoxicosis in community-resident older adults: findings from the baltimore longitudinal study of aging. Thyroid. 2015;25(9):979–986. doi: 10.1089/thy.2015.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krahn A.D., Klein G.J., Kerr C.R. How useful is thyroid function testing in patients with recent-onset atrial fibrillation? The Canadian Registry of Atrial Fibrillation Investigators. Arch Intern Med. 1996;156(19):2221–2224. [PubMed] [Google Scholar]
- 5.Frost L., Vestergaard P., Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2004;164(15):1675–1678. doi: 10.1001/archinte.164.15.1675. [DOI] [PubMed] [Google Scholar]
- 6.Selmer C., Hansen M.L., Olesen J.B. New-onset atrial fibrillation is a predictor of subsequent hyperthyroidism: a nationwide cohort study. PLoS One. 2013;8(2):e57893. doi: 10.1371/journal.pone.0057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr C.R., Boone J., Connolly S.J. The Canadian Registry of Atrial Fibrillation: a noninterventional follow-up of patients after the first diagnosis of atrial fibrillation. Am J Cardiol. 1998;82(8A):82N–85N. doi: 10.1016/s0002-9149(98)00589-x. [DOI] [PubMed] [Google Scholar]
- 8.Stavrakis S., Yu X., Patterson E. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves' hyperthyroidism. J Am Coll Cardiol. 2009;54(14):1309–1316. doi: 10.1016/j.jacc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purtell K., Roepke T.K., Abbott G.W. Cardiac arrhythmia and thyroid dysfunction: a novel genetic link. Int J Biochem Cell Biol. 2010;42(11):1767–1770. doi: 10.1016/j.biocel.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gammage M.D., Parle J.V., Holder R.L. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med. 2007;167(9):928–934. doi: 10.1001/archinte.167.9.928. [DOI] [PubMed] [Google Scholar]
- 11.Heeringa J., Hoogendoorn E.H., van der Deure W.M. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168(20):2219–2224. doi: 10.1001/archinte.168.20.2219. [DOI] [PubMed] [Google Scholar]
- 12.Markides V., Schilling R.J. Atrial fibrillation: classification, pathophysiology, mechanisms and drug treatment. Heart. 2003;89(8):939–943. doi: 10.1136/heart.89.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammers W.J., Allessie M.A. Pathophysiology of atrial fibrillation: current aspects. Herz. 1993;18(1):1–8. [PubMed] [Google Scholar]
- 14.Watanabe H., Ma M., Washizuka T. Thyroid hormone regulates mRNA expression and currents of ion channels in rat atrium. Biochem Biophys Res Commun. 2003;308(3):439–444. doi: 10.1016/s0006-291x(03)01420-7. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y., Jones S.V., Dillmann W.H. Effects of hyperthyroidism on delayed rectifier K+ currents in left and right murine atria. Am J Physiol Heart Circ Physiol. 2005;289(4):H1448–55. doi: 10.1152/ajpheart.00828.2004. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.C., Chen S.A., Chen Y.J., Chang M.S., Chan P., Lin C.I. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J Am Coll Cardiol. 2002;39(2):366–372. doi: 10.1016/s0735-1097(01)01731-4. [DOI] [PubMed] [Google Scholar]
- 17.Bielecka-Dabrowa A., Mikhailidis D.P., Rysz J., Banach M. The mechanisms of atrial fibrillation in hyperthyroidism. Thyroid Res. 2009;2(1):4. doi: 10.1186/1756-6614-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wustmann K., Kucera J.P., Zanchi A. Activation of electrical triggers of atrial fibrillation in hyperthyroidism. J Clin Endocrinol Metab. 2008;93(6):2104–2108. doi: 10.1210/jc.2008-0092. [DOI] [PubMed] [Google Scholar]
- 19.Komiya N., Isomoto S., Nakao K., Hayano M., Yano K. Electrophysiological abnormalities of the atrial muscle in patients with paroxysmal atrial fibrillation associated with hyperthyroidism. Clin Endocrinol (Oxf) 2002;56(1):39–44. doi: 10.1046/j.0300-0664.2001.01459.x. [DOI] [PubMed] [Google Scholar]
- 20.Sawin C.T., Geller A., Wolf P.A. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 21.Selmer C., Olesen J.B., Hansen M.L. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012;345:e7895. doi: 10.1136/bmj.e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappola A.R., Fried L.P., Arnold A.M. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295(9):1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Gelder I.C., Crijns H.J., Van Gilst W.H., Verwer R., Lie K.I. Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct-current electrical cardioversion of chronic atrial fibrillation and flutter. Am J Cardiol. 1991;68(1):41–46. doi: 10.1016/0002-9149(91)90707-r. [DOI] [PubMed] [Google Scholar]
- 24.Gurdogan M., Ari H., Tenekecioglu E. Predictors of atrial fibrillation recurrence in hyperthyroid and euthyroid patients. Arq Bras Cardiol. 2016;106(2):84–91. doi: 10.5935/abc.20160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma C.S., Liu X., Hu F.L. Catheter ablation of atrial fibrillation in patients with hyperthyroidism. J Interv Card Electrophysiol. 2007;18(2):137–142. doi: 10.1007/s10840-007-9088-y. [DOI] [PubMed] [Google Scholar]
- 26.Mikhaylov E.N., Orshanskaya V.S., Lebedev A.D., Szili-Torok T., Lebedev D.S. Catheter ablation of paroxysmal atrial fibrillation in patients with previous amiodarone-induced hyperthyroidism: a case-control study. J Cardiovasc Electrophysiol. 2013;24(8):888–893. doi: 10.1111/jce.12140. [DOI] [PubMed] [Google Scholar]
- 27.Wang M., Cai S., Sun L., Zhao Q., Feng W. Safety and efficacy of early radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation complicated with amiodarone-induced thyrotoxicosis. Cardiol J. 2016;23(4):416–421. doi: 10.5603/CJ.a2016.0029. [DOI] [PubMed] [Google Scholar]
- 28.Machino T., Tada H., Sekiguchi Y. Prevalence and influence of hyperthyroidism on the long-term outcome of catheter ablation for drug-refractory atrial fibrillation. Circ J. 2012;76(11):2546–2551. doi: 10.1253/circj.cj-12-0340. [DOI] [PubMed] [Google Scholar]
- 29.Wongcharoen W., Lin Y.J., Chang S.L. History of hyperthyroidism and long-term outcome of catheter ablation of drug-refractory atrial fibrillation. Heart Rhythm. 2015;12(9):1956–1962. doi: 10.1016/j.hrthm.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Presti C.F., Hart R.G. Thyrotoxicosis, atrial fibrillation, and embolism, revisited. Am Heart J. 1989;117(4):976–977. doi: 10.1016/0002-8703(89)90642-x. [DOI] [PubMed] [Google Scholar]
- 31.Chan P.H., Hai J., Yeung C.Y. Benefit of anticoagulation therapy in hyperthyroidism-related atrial fibrillation. Clin Cardiol. 2015;38(8):476–482. doi: 10.1002/clc.22427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singer D.E., Albers G.W., Dalen J.E. Antithrombotic therapy in atrial fibrillation: american college of chest physicians evidence-based clinical practice guideline (8th edition) Chest. 2008;133(Suppl. 6):546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 33.European Heart Rhythm A, Heart Rhythm S, Fuster V. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice Guidelines and the European Society of Cardiology Committee for practice guidelines (Writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2006;48(4):854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Souza M.V., Duarte M.M., Coeli C.M., Vaisman M. Atrial fibrillation and hyperthyroidism: relation between transoesophageal markers of a thrombogenic milieu and clinical risk factors for thromboembolism. Clin Endocrinol (Oxf) 2012;76(3):448–453. doi: 10.1111/j.1365-2265.2011.04232.x. [DOI] [PubMed] [Google Scholar]
- 35.Morrow D.H., Gaffney Thomas E., Braunwald E. Studies on digitalis VIII. Effect of autonomic innervation and of myocardial catecholamine stores upon the cardiac action of ouabain. J Pharmacol Exp Ther. 1963;140:236–245. [Google Scholar]
- 36.Shenfield G.M. Influence of thyroid dysfunction on drug pharmacokinetics. Clin Pharmacokinet. 1981;6(4):275–297. doi: 10.2165/00003088-198106040-00003. [DOI] [PubMed] [Google Scholar]
- 37.Parmar M.S. Thyrotoxic atrial fibrillation. MedGenMed. 2005;7(1):74. [PMC free article] [PubMed] [Google Scholar]
- 38.Horacek J., Maly J., Svilias I. Prothrombotic changes due to an increase in thyroid hormone levels. Eur J Endocrinol. 2015;172(5):537–542. doi: 10.1530/EJE-14-0801. [DOI] [PubMed] [Google Scholar]
- 39.Belchikov Y.G., Marotta S.E. Heparin management in a patient with thyroid storm. Pharmacotherapy. 2010;30(4):134e–138e. doi: 10.1592/phco.30.4.421. [DOI] [PubMed] [Google Scholar]
- 40.Nakazawa H.K., Sakurai K., Hamada N., Momotani N., Ito K. Management of atrial fibrillation in the post-thyrotoxic state. Am J Med. 1982;72(6):903–906. doi: 10.1016/0002-9343(82)90850-6. [DOI] [PubMed] [Google Scholar]
- 41.Martino E., Safran M., Aghini-Lombardi F. Environmental iodine intake and thyroid dysfunction during chronic amiodarone therapy. Ann Intern Med. 1984;101(1):28–34. doi: 10.7326/0003-4819-101-1-28. [DOI] [PubMed] [Google Scholar]
- 42.Newman C.M., Price A., Davies D.W., Gray T.A., Weetman A.P. Amiodarone and the thyroid: a practical guide to the management of thyroid dysfunction induced by amiodarone therapy. Heart. 1998;79(2):121–127. doi: 10.1136/hrt.79.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert M.J., Burger A.G., Galeazzi R.L., Engler D. Are selective increases in serum thyroxine (T4) due to iodinated inhibitors of T4 monodeiodination indicative of hyperthyroidism? J Clin Endocrinol Metab. 1982;55(6):1058–1065. doi: 10.1210/jcem-55-6-1058. [DOI] [PubMed] [Google Scholar]
- 44.Tsang W., Houlden R.L. Amiodarone-induced thyrotoxicosis: a review. Can J Cardiol. 2009;25(7):421–424. doi: 10.1016/s0828-282x(09)70512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czarnywojtek A., Zgorzalewicz-Stachowiak M., Wolinski K. Results of preventive radioiodine therapy in euthyroid patients with history of hyperthyroidism prior to administration of amiodarone with permanent atrial fibrillation-a preliminary study. Endokrynol Pol. 2014;65(4):269–274. doi: 10.5603/EP.2014.0036. [DOI] [PubMed] [Google Scholar]
- 46.Kunii Y., Uruno T., Matsumoto M. Pharmacological conversion of atrial fibrillation in the patients of Graves' disease. Tokai J Exp Clin Med. 2012;37(4):107–112. [PubMed] [Google Scholar]
- 47.Biondi B., Bartalena L., Cooper D.S., Hegedus L., Laurberg P., Kahaly G.J. The 2015 european thyroid association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. Eur Thyroid J. 2015;4(3):149–163. doi: 10.1159/000438750. [DOI] [PMC free article] [PubMed] [Google Scholar]