Abstract
Background & Aims
Cancer cells rely on metabolic alterations to enhance proliferation and survival. Metabolic gene alterations that repeatedly occur in liver cancer are largely unknown. We aimed to identify metabolic genes that are consistently deregulated, and are of potential clinical significance in human hepatocellular carcinoma (HCC).
Methods
We studied the expression of 2,761 metabolic genes in 8 microarray datasets comprising 521 human HCC tissues. Genes exclusively up-regulated or down-regulated in 6 or more datasets were defined as consistently deregulated. The consistent genes that correlated with tumor progression markers (ECM2 and MMP9) (Pearson correlation P < .05) were used for Kaplan-Meier overall survival analysis in a patient cohort. We further compared proteomic expression of metabolic genes in 19 tumors vs adjacent normal liver tissues.
Results
We identified 634 consistent metabolic genes, ∼60% of which are not yet described in HCC. The down-regulated genes (n = 350) are mostly involved in physiologic hepatocyte metabolic functions (eg, xenobiotic, fatty acid, and amino acid metabolism). In contrast, among consistently up-regulated metabolic genes (n = 284) are those involved in glycolysis, pentose phosphate pathway, nucleotide biosynthesis, tricarboxylic acid cycle, oxidative phosphorylation, proton transport, membrane lipid, and glycan metabolism. Several metabolic genes (n = 434) correlated with progression markers, and of these, 201 predicted overall survival outcome in the patient cohort analyzed. Over 90% of the metabolic targets significantly altered at the protein level were similarly up- or down-regulated as in genomic profile.
Conclusions
We provide the first exposition of the consistently altered metabolic genes in HCC and show that these genes are potentially relevant targets for onward studies in preclinical and clinical contexts.
Keywords: Liver Cancer, HCC, Tumor Metabolism
Abbreviations used in this paper: EMT, epithelial to mesenchymal transition; FA, fatty acid; HCC, hepatocellular carcinoma; logFC, log of fold change; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NB, nucleotide biosynthesis; OXPHOS, oxidative phosphorylation; PPP, pentose phosphate pathway; TCA, tricarboxylic acid; TCGA, The Cancer Genome Atlas; XM, xenobiotics metabolism
Graphical abstract
See editorial on page 283.
Summary.
We have identified metabolic targets that are consistently altered in human hepatocellular carcinoma, and are of potential clinical significance. This study exposes profound genomic dysregulation that could shed new light on how metabolism influences hepatocellular carcinogenesis.
Metabolism is an indispensable process in normal and cancer cells. In the early 20th century, Otto Warburg discovered an alteration in tumor metabolic phenotype. He observed that cancer cells highly depend on aerobic glycolysis for energy production even when oxygen is abundantly available.1, 2, 3 This discovery, complemented later by the dawn of the -omics era, has inspired several novel insights in cancer cell metabolism. Today, metabolic alteration is a recognized hallmark of cancer.4 It is now known that in addition to relying on glucose (often called the Warburg effect), cancer cells also depend on other metabolites such as glutamine, serine, and fatty acids.5, 6, 7, 8, 9 Furthermore, the accumulation of oncometabolites (eg, 2-hydroxyglutarate and fumarate), deregulated nutrient transporters (eg, glucose and monocarboxylate transporters), transcriptional regulators, epigenetic factors, and signaling molecules all prominently contribute to altered cancer metabolism.2, 10, 11 In line with the rapidly evolving insights on tumor metabolism, a recent review has grouped the emerging alterations into 6 hallmarks, among which are deregulated uptake of glucose and amino acids, increased demand for nitrogen, and altered gene regulation caused by buildup of metabolites such as acetyl coenzyme A and 2-hydroxyglutarate.12 Several molecular mediators of aberrant metabolism (eg, carnitine palmitoyltranferase 1, hexokinases, glucose transporter 1, glutaminase, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, and isocitrate dehydrogenases) have been studied in preclinical and clinical trials as potential cancer drug targets.13, 14 However, the extent and relevance of altered metabolism in cancer cells is still unclear.12, 14 This is partly due to the complex regulation of biochemical pathways as well as molecular heterogeneity within and across tumor entities. In addition, many studies have so far focused on the Warburg effect, thus narrowing the opportunities to identify novel and perhaps more relevant biochemical changes in cancer. Thus, as concluded by Pavlova and Thompson,12 a detailed understanding of tumor metabolic features, especially for individual tumor types, will assist in better tumor classification and improve the prospects of exploiting metabolism in cancer therapy.
Liver cancer poses a global health challenge due to its rising incidence coupled with a low survival rate, especially in the developing world.15, 16 Hepatocellular carcinoma (HCC) accounts for over 80% of liver cancer cases, and is highly malignant, recurrent, drug resistant, and often diagnosed at the advanced stage.17, 18 For these reasons, the need to identify molecular features that uniquely define or contribute to HCC progression remains clinically urgent. To exploit metabolic alterations in HCC as diagnostic and prognostic indicators or as therapeutic targets, the alterations that distinguish cancerous liver cells from functionally normal hepatocytes must be known. Therapeutic interventions also need to consider that the liver is responsible for systemic metabolism and detoxification—functions that must not be compromised in an attempt to modulate pathways in adjoining cancerous liver. It is known that metabolic gene networks are heterogeneous in cancer (HCC inclusive).19 Nevertheless, there are strong evidences that metabolic alterations have translational relevance in HCC. For instance, differences in acetate utilization have been reported as a possible phenotype for stratifying HCC patients.20 Low betaine and propionylcarnitine have been proposed as combinatorial serum biomarkers in HCC.21 Several metabolic targets are detectable by proteomic methods, and thus could serve as biomarkers in HCC.22 Furthermore, all liver function parameters currently in clinical use reflect changes in either metabolic activities or enzymes. One notable liver function enzyme, aspartate transaminase, has also recently been shown to predict future risk of HCC development from primary biliary cirrhosis.23 Therefore, identification of the consistently deregulated metabolic genes in HCC will accelerate future mechanistic studies aimed at exploiting specific candidates or pathways in diagnostic, prognostic or therapeutic contexts. In this study, we zoomed into the genomic landscape of human HCC with the aim of exposing consistently altered metabolic genes (hereafter also called targets) of potential clinical relevance. Across 8 datasets published in the last decade, we found that many metabolic genes are consistently deregulated regardless of the etiological background of the different patient cohorts. Many metabolic genes correlated with known markers of cancer progression, predicted survival outcome, and were similarly up- or down-regulated at the protein level in our analysis and other prior studies. We have revealed robust changes in metabolic gene expression in HCC to the extent that, to our knowledge, has not been previously acknowledged.
Methods
Collection of Liver Cancer Microarray Datasets, Processing and Identification of Consistently Altered Metabolic Genes
Eight liver cancer microarray datasets that have accompanying scientific publications (Table 1)24, 25, 26, 27, 28, 29, 30, 31 were assembled via online databases, namely ArrayExpress32 and the Gene Expression Omnibus (GEO) of the National Center for Biotechnology Information (NCBI).33 To eliminate analytical bias that might arise from data reprocessing, the NCBI GEO2R tool was used to directly determine the differentially expressed genes between healthy or adjacent liver tissue control samples and HCC samples in each dataset. In total, 521 human HCC gene expression profiles were compared with 420 control liver samples (Table 1). Thereafter, the GEO2R outputs were downloaded, and all genes differentially regulated at P < .05 were selected. Next, a previously published list of 2,752 metabolism-annotated genes7 was updated with 9 additional genes (Supplementary Table 1), and used to extract only the deregulated metabolic genes in each of the 8 datasets (Table 2). For this, the COUNTIF function was applied in Microsoft Excel (Microsoft Corp, Redmond, WA), followed by the removal of duplicate probes (eg, whereby a gene has 4 up-regulated probes, the one with the highest expression value was retained). Furthermore, the average log of fold change (logFC) of all differentially expressed genes as determined by GEO2R was calculated, and used as reference to set cutoff threshold values for each dataset. This step ensured the exclusion of metabolic gene probes with very small expression changes—also including duplicate probes of genes that in the same dataset are already among the top differentially regulated. For onward analyses, metabolic genes with +logFC at or above the cutoff value in the respective datasets were selected as up-regulated, whereas those with –logFC at or below cutoff value were selected as down-regulated. Few genes that had 2 probes with strongly opposite expression patterns in the same dataset (ie, one probe is up-regulated and the other down-regulated) were left in the gene list and used to test for consistent alteration across datasets. Following these prior steps, a metabolic gene was identified as consistently altered if it has the same expression pattern (ie, exclusively in the up-regulated or down-regulated category) in at least 6 of the 8 HCC datasets.
Table 1.
Microarray Data Analyzed to Identify Altered Metabolic Targets in HCC Patients
| Accession number | Data compareda | Number of samples |
Main etiology reported | Reference | |
|---|---|---|---|---|---|
| Control | HCC | ||||
| GSE14520b | Paired NT vs HCC | 220 | 225 | HBV | [24] |
| GSE39791 | Matched NT vs HCC | 72 | 72 | HBV | [25] |
| GSE57957 | Adjacent NT vs HCC | 39 | 39 | HBV | [26] |
| GSE36376 | AJCC Stage 3: Adjacent NT vs HCC | 32 | 38 | HBV | [27] |
| GSE60502 | Adjacent NT vs HCC | 18 | 18 | NA | [28] |
| GSE14323c | Normal liver vs HCC | 19 | 38 | HCV | [29] |
| GSE6764 | Normal liver vs very advanced HCC | 10 | 10 | HCV | [30] |
| GSE62232 | Normal liver vs HCC | 10 | 81 | Mixed: alcohol, HBV, HCV, etc.d | [31] |
| Total arrays | 420 | 521 | |||
HBV/HCV, hepatitis B/C virus; HCC, hepatocellular carcinoma; NA, detail could not be accessed; NT, nontumor.
Description of the data compared as documented in the National Center for Biotechnology Information Gene Expression Omnibus. Differential expression was analyzed with GEO2R tool. The overall design for each dataset can be found at https://www.ncbi.nlm.nih.gov/geo/.
Data platform analyzed was GPL3921.
Data platform analyzed was GPL571.
Includes unknown etiology, hemochromatosis, metabolic syndrome and combinations of alcohol with the other etiologic factors.
Table 2.
Selection of Metabolic Targets From the List of Deregulated Gene Probes in Each HCC Dataset Used in This Study
| HCC microarrays | logFC generated via NCBI GEO2R (P < .05) |
Number of metabolic genes selected |
|||
|---|---|---|---|---|---|
| Mean | SD | T used for selecting metabolic genes | Up-regulated (≥ +T) | Down-regulated (≤ -T) | |
| GSE14520 | 0.0295 | 0.598 | 0.2 | 542 | 654 |
| GSE39791 | 0.0102 | 0.3772 | 0.15 | 551 | 653 |
| GSE57957 | 0.0225 | 0.415 | 0.15 | 623 | 650 |
| GSE36376 | 0.275 | 0.484 | 0.1 | 934 | 404 |
| GSE60502 | 0.000321 | 0.976 | 0.4 | 340 | 597 |
| GSE14323 | –0.015 | 0.563 | 0.15 | 473 | 683 |
| GSE6764 | 0.0196 | 1.00129 | 0.45 | 437 | 628 |
| GSE62232 | 0.0104 | 0.6383 | 0.25 | 552 | 814 |
Mean and SD were calculated from all probe sets with logFC values at P < .05 (including metabolic and other genes). Metabolic genes with +logFC at and above threshold (T) were selected as up-regulated targets; those with –logFC at or below T selected as down-regulated targets.
HCC, hepatocellular carcinoma; logFC, log of fold change; NCBI, National Center for Biotechnology Information.
Selection of Progression Markers
Known markers of tumor invasion or metastasis, specifically extracellular matrix proteins and matrix metalloproteinases as well as epithelial-to-mesenchymal (EMT) markers (eg, SNAIs, TWIST, ZEBs, cadherins, vimentin) were manually curated from literature.34, 35 The expression of these genes was compared across 8 liver cancer microarrays in Oncomine—an online repository of curated cancer transcriptomics data.36 In the Oncomine platform, parameters were set as follows— Analysis type: Liver Cancer vs Normal Analysis, Threshold by: P = .05, Fold Change = All, and Gene Rank = All. Of the markers mentioned earlier, ECM2, CDH1, VIM, and MMP9 were the most consistently deregulated. Differential regulation of ECM2, MMP9, CDH1, and VIM as observed in Oncomine was also confirmed in the GEO2R output from the HCC datasets used to identify the metabolic targets. Besides GSE6764 and GSE14323, the microarrays in Oncomine include The Cancer Genome Atlas (TCGA) and GSE14520 liver cancer data used in this study for correlation with progression markers and overall survival analyses, respectively. Based on their consistent expression, ECM2, MMP9, CDH1, and VIM were selected as progression markers for correlation analyses with the metabolic genes.
Correlation of Metabolic Genes With Progression Markers
Liver cancer gene expression data from TCGA was used as a reference for the correlation of metabolic genes with the selected progression markers (ie, ECM2, MMP9, CDH1, and VIM). The messenger RNA expression data for each metabolic gene and the progression markers were obtained for the completed tumor analysis (n = 190 patients) via the cBioPortal platform (http://www.cbioportal.org). The data were log transformed and each metabolic gene was correlated with each of the progression markers found to be down-regulated in HCC (ie, ECM2 and CDH1) and those that are up-regulated (ie, MMP9 and VIM). To be included for further analysis, up-regulated metabolic genes were expected to correlate inversely with down-regulated progression markers, and directly with those up-regulated—the reverse being the case for down-regulated metabolic genes. Subsequently, a metabolic gene was selected if its Pearson correlation with at least ECM2 and MMP9 was statistically significant (P < .05).
Kaplan-Meier Overall Survival Analyses
For each metabolic gene that correlated with the progression markers (n = 434), Kaplan-Meier overall survival analysis was performed with log-rank (Mantel-Cox) test in GraphPad Prism. The dataset GSE14520, which is the largest of the cohorts analyzed (Table 1), is available with published clinical data, and so was used for the survival analysis. Prior to the analysis, the expression pattern of a given gene was confirmed to be the same in GSE14520 as generally described (ie, whether also up- or down-regulated in GSE14520 as in the other datasets). Only 6 genes were excluded from the survival analysis due to 1) lack of expression data (CAD and CES3), 2) duplicate probes that were strongly regulated in opposite directions (SLC16A3 and SMOX), 3) expression pattern that is not as generally described (BCAT1), or 4) probe identification issue (eg, CYP4A22 was excluded because the probe, 217319_x_at, is identified as LOC654164///CYP4A22///CYP4A11). For all other genes, the range of their expression from patients with the lowest to those with highest values varied markedly, and was very narrow for some genes. Specifically, for some genes, several patients had expression values that were the same or different by a slight margin, especially in intermediate range, and yet had different survival outcomes. Therefore, to ensure that the analyzed overall survival can be attributed to a difference in the expression of a given gene, its expression values were used to rank the patients into lower, intermediate, and higher groups. Subsequently, patients with lower (n = 75) and higher (n = 75) expression values for a given gene were adopted as a uniform inclusion criterion for survival prediction. Based on this criterion, overall survival was assessed using a total of 150 patients for each gene separately analyzed, and a statistical significance was accepted at P < .05.
Proteomics Analysis
To assess protein level alterations, our recently published proteomics data were reanalyzed focusing on the candidates corresponding to the consistent metabolic genes. The data contained 2736 proteins derived from mass spectrometric analyses of 19 fresh-frozen HCC samples and adjacent liver tissue samples. For the current analyses, paired comparisons of tumor and liver tissue samples were conducted irrespective of tumor stage and grade (n = 19), or according to the tumor stages T1 (n = 11) and T2-3 (n = 8) as well as the histological gradings G1 (n = 5), G2 (n = 8), and G3 (n = 6). Statistical evaluation was performed as recently described using a 1-way analysis of variance. Further details regarding patient characteristics, sample preparation, mass spectrometry, and proteomic data analysis have been extensively described in our prior publications.37, 38
Pathway Analysis
The Database for Annotation, Visualization and Integrated Discovery39 was used to perform functional annotation analysis of the top differentially expressed genes (in metabolism and other processes) for each of the 8 datasets. For this analysis, the gene lists from each dataset were first ranked by logFC. Thereafter, the top 1,500 up-regulated and down-regulated genes per dataset were used for a functional annotation with reference to pathway database of the Kyoto Encyclopedia of Genes and Genome.
Other Analyses
GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA) was used for Pearson correlation, overall survival analysis, and analyzing the expression of the genes relative to tumor size. For the latter, a multiple t test, 1 per row, was used. P < .05 was accepted as statistical significance throughout the study. Targets were highlighted as novel based on results from searching PubMed database for each of the consistent metabolic gene (total n = 634). The search terms used were official gene symbol plus HCC or Liver cancer or Cancer. All authors had access to the study data and had reviewed and approved the final manuscript.
Results
Metabolic Genes Are Consistently Altered in Human HCC
To gain a holistic insight on metabolic gene alterations in clinical HCC (Figure 1A, Tables 1 and 2), we assessed the expression pattern of almost all known human metabolic genes and transporters previously compiled by Possemato et al.7 In the HCC patient cohorts, the main reported etiologies were hepatitis B and C, alcohol, metabolic syndrome, mixed etiologies, or unknown (Table 1). With the exception of GSE62232, none of the other cohorts included data on metabolic syndrome, which is associated with nonalcoholic fatty liver disease (NAFLD) that predisposes to HCC. We reasoned that regardless of etiology, the expression of certain metabolic genes could be a consistent feature of liver cancer. Accordingly, we identified 634 metabolic genes that were deregulated in 6 or more datasets investigated (Supplementary Table 1). A total of 350 of the genes were down-regulated, of which 107, 158, and 85 were present in 8, 7, and 6 datasets, respectively. Assortment of the genes by their associated biochemical pathways revealed a predominant suppression of candidates involved in gluconeogenesis, urea cycle, ketogenesis, and xenobiotic, glutathione, amino acid, and fatty acid (FA) metabolism (Figure 1B). Several of these pathways also emerged in functional annotation analyses of topmost down-regulated genes (involved in metabolism and other processes) in each of the 8 datasets (Figure 2). Similarly, we found 284 consistently up-regulated metabolic genes comprising of 53, 120, and 111 hits in 8, 7, and 6 datasets, respectively. The up-regulated metabolic genes in HCC notably belonged to processes such as glycolysis, pentose phosphate pathway (PPP), tricarboxylic acid (TCA) cycle, glycan metabolism, nucleotide biosynthesis (NB), membrane lipid biochemistry, and several transporters (Figure 1B). NB and valine, leucine, and isoleucine biosynthesis were the metabolic processes that emerged in the functional annotation analysis of topmost up-regulated genes in each of the datasets.
Figure 1.
Summary of the study strategy used to identify the consistently altered metabolic targets in human HCC. (A) Flowchart of the steps adopted to identify metabolic targets of clinical relevance. Progression markers used are ECM2, MMP9, CDH1, and VIM. Genes considered to correlate with progression markers are only those that had significant Pearson correlation with at least ECM2 and MMP9 using The Cancer Genome Atlas liver cancer data as reference. *In the accompanying clinical data from GSE14520. Statistical significance were accepted at P < .05. See Supplementary Tables 1 and 2 for the metabolic genes and proteomics data, respectively. (B) Number of genes in each metabolic process that were consistently altered in 6 or more datasets. *Amino acid processes besides those already displayed in the graph (eg, glutamine). “Others” are mostly metabolic genes with other general functions beyond those displayed (see Supplementary Table 1). (C) Heatmap showing genes consistently among topmost 50 metabolic targets in 6 or more datasets. Blank means genes are not expressed at P < .05 or not expressed within the top 25 (up or down). ABC, adenosine triphosphate–binding cassette; HCC, hepatocellular carcinoma; NAD, nicotinamide adenine dinucleotide; NCBI GEO, National Center for Biotechnology Information Gene Expression Omnibus; OXPHOS, oxidative phosphorylation; PPP, pentose phosphate pathway; REDOX, reduction–oxidation reaction; S.M., small molecule; TCA, tricarboxylic acid.
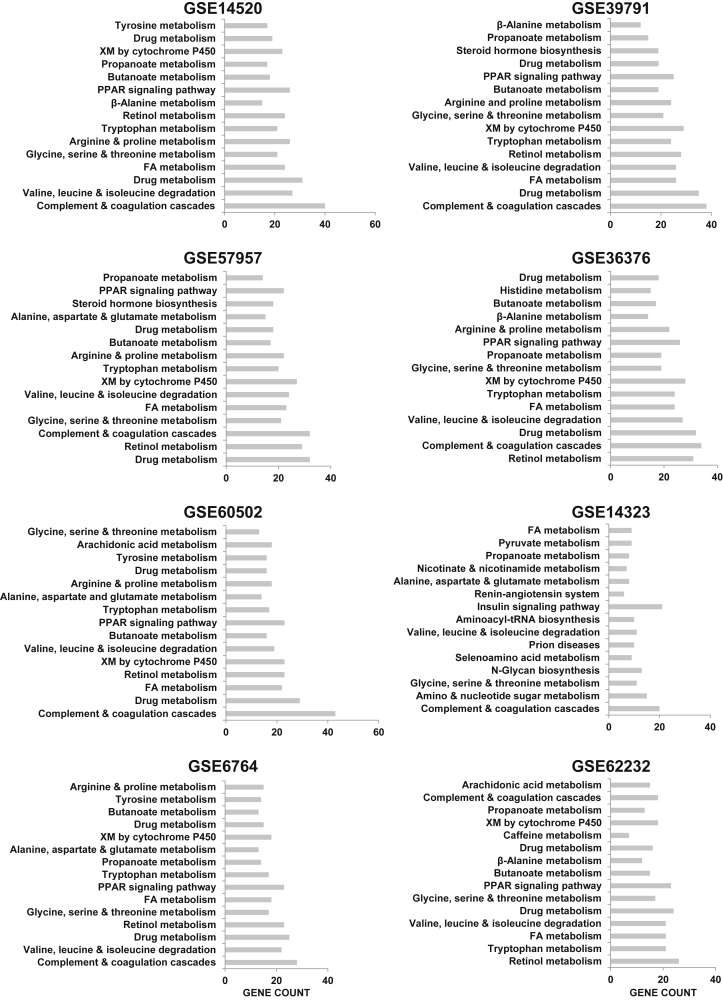
Figure 2.
Functional annotation of the top 1500 down-regulated genes (whether in metabolic or other processes) in each hepatocellular carcinoma dataset. FA, fatty acid metabolism; PPAR, peroxisome proliferator-activated receptor; XM, xenobiotics metabolism.
We observed that with the exception of few pathways (eg, proton transport, ketogenesis, gluconeogenesis, urea cycle), most others had a mixture of both consistently up-regulated as well as down-regulated genes (Figure 1B). To further highlight the consistency of metabolic gene alterations, we sought to identify the genes ranked in the top or bottom 25 in at least 6 datasets. Consequently, we found that the topmost down-regulated genes in HCC were SLCO1B3, an organic anion transporter of bilirubin; CYPA12, CYP2A6, CYP2C8, and CYP3A4 all of which are involved in xenobiotics metabolism (XM); FBP1 and PCK1 involved in gluconeogenesis, GLS2, among others (Figure 1C). On the other hand, aldo-keto reductase family 1 member B10, AKR1B10, emerged as the topmost up-regulated metabolic gene, ranking first in all but 1 dataset (GSE60502). Other topmost up-regulated genes were TKT, SQLE, ACLY, LYZ, TYMS, TXNRD, ACSL4, NQO1, FADS1, PLCB1, and muscle isoform of PKM (Figure 1C). Of the 8 datasets analyzed, GSE14323 showed a slightly divergent gene expression pattern (Figure 1C). In this dataset, SQLE and TKT were not differentially expressed (ie, P > .05); FADS1 was down-regulated, whereas CYP3A4, CYP2A6, and GLS2 were all up-regulated instead of being suppressed as in other datasets (Figure 1C). Gene enrichment analysis also identified XM and drug metabolism in the up-regulated gene category only in the GSE14323 dataset (Figure 3). Nevertheless, topmost deregulated targets, including AKR1B10, ACSL4, NNMT, and SLCO1B3 were also top hits in GSE14323 and were expressed in the same direction as in the other datasets (Figure 1C). Altogether, independent datasets reveal a strong deregulation of several metabolic genes in human HCC, and show that these alterations are broadly consistent across clinical cohorts.
Figure 3.
Functional annotation of the top 1500 up-regulated genes (whether in metabolic or other processes) in each hepatocellular carcinoma dataset. *Enriched among pathway annotations derived with down-regulated genes in the other datasets (see Figure 2). ECM, extracellular matrix; XM, xenobiotics metabolism.
Altered Metabolic Genes Show Similar Expression Patterns at Protein Level
We assessed the expression of metabolic genes at protein level in 19 human HCC tissue samples. A considerable number of the targets (n = 350), corresponding to 55% of the consistent metabolic genes, could be detected and quantified in the proteomics data (Figure 1A, Supplementary Table 2). Of those quantified, more than 90% (n = 252) were significantly expressed in the same direction as found at the gene level. For instance, of 207 down-regulated proteins, 167 were significantly decreased. Of these, 99% were also down-regulated at gene level with the exemption of BPGM and ATP5H, both of which are up-regulated in gene datasets. On the other hand, 143 targets were elevated at protein level—85 being significantly up. Of these, 79 (93%) were also consistently up-regulated at the gene level, with the exemption of PRG2, ST6GAL1, ACOX3, DHODH, TF, ABCD3 whose corresponding genes are consistently down-regulated in HCC. Next, based on common knowledge of biochemical pathways, we attempted to map the portrait of liver cancer metabolism using the consistently altered genes or their corresponding proteins where detected in our analysis. The snapshot clearly depicted the suppression of serine biosynthetic pathway, urea cycle, and transamination as striking features of HCC (Figure 4). Also represented were up-regulated targets in TCA cycle and mainly in NB, most of which were detected at protein level. In glycolysis, we found the novel hexokinase isoform, HKDC1 to be up-regulated at gene and protein levels. Consistently, most other glycolytic targets were similarly expressed at gene and protein level, and have been identified and or mechanistically investigated in previous HCC studies (Supplementary Table 1). We show that notable genes that encode enzymes at the initial and terminal steps in commonly studied biochemical pathways are deregulated in HCC, and most reflected in our proteomics analysis. These include HK2 and PKM (in glycolysis), GLS and GLUD1 (in glutaminolysis), CPS1 and ASL (in urea cycle), ACACA and FASN (in lipogenesis), HMGCS2 and SQLE (in cholesterogenesis), and PCK1 and FBP1 in gluconeogenesis (Figure 4, Supplementary Tables 1 and 2). Furthermore, we uncovered about 40 family of metabolic targets (mostly paralogues), whose members are frequently expressed in the opposite direction in HCC (Table 3). Examples include ALDO1 and ALDO3, ENO1 and ENO3, and ACACA and ACACB, which were also detected at protein level. Besides strongly overlapping with genomic data, the protein level expression of several metabolic targets varied significantly with tumor stage and grade (Supplementary Table 2). Taken together, metabolic gene expression changes in HCC reflect at the protein level, and putting them in the contexts of biochemical pathways could enhance the understanding of their functional relevance.
Figure 4.
Schematic representation of biochemical pathways in HCC using consistently altered metabolic genes or their encoded proteins. Metabolic targets that are down-regulated are presented in green, those up-regulated are in red; metabolites are in black. Those we detected to be significantly altered at protein level are not in italics. *Predicted overall survival. **Predicted overall survival and varied with tumor size. ***Previously identified to be of clinical significance in HCC either as a drug target, biomarker, or prognostic indicator (see Supplementary Table 1). •Differentially expressed in 6 datasets, but did not reach selection threshold in 1 of the datasets. ••Within this axis are nucleotide biosynthesis targets PPAT, PAICS, ADSL, and GART, PFAS, and ATIC, all of which are up-regulated (the latter three were also detected to be up at protein level). 3-PG, 3-phoshoglycerate; 6-PGL, 6-phosphogluconolactone; α-KG, alpha ketoglutarate; AA, amino acids; ADS, adenylosuccinate; AMP, adenosine monophosphate; F-6-P, fructose-6-bisphosphate; G-3-P, glyceraldehyde-3-phosphate; G-6-P, glucose-6-phosphate; CTP, cytidine triphosphate; GMP, guanosine monophosphate; IMP, inosine monophosphate; NADPH, nicotinamide adenine dinucleotide phosphate; OAA, oxaloacetate; OXPHOS, oxidative phosphorylation; P, phosphate; PEP, phosphoenolpyruvate; pPYR, phosphohydroxypyruvate; PRPP, phosphoribosyl pyrophosphate; pSER, phosphoserine; R-5-P, ribose-5-phosphate; TCA, tricarboxylic acid cycle; THF, tetrahydrofolate; UTP, uridine triphosphate; XMP, xanthosine monophosphate.
Table 3.
Family of Metabolic Targets Consistently Expressed in the Opposite Direction in HCC
| Metabolic processes | Up-regulated | Down-regulated |
|---|---|---|
| ABC transporters | ABCC4, ABCC5, ABCC10 | ABCC6, ABCC9 |
| Cholesterol trafficking | NPC1, NPC2 | NPC1L1 |
| Fatty acid biosynthesis | ACACAa | ACACBa |
| √ | ACSL3a, ACSL4a | ACSL1a, ACSL5a |
| √ | ELOVL5 | ELOVL6 |
| Fatty acid/phospholipids | PLA2G4C, PLA2G7 | PLA2G16 |
| Folate metabolism | MTHFR | MTHFD1a, MTHFSa |
| Glutaminolysis | GLS | GLS2a |
| Glutathione metabolism | GSTA4 | GSTA1, GSTA3, GSTZ1 |
| Glycerophospholipid biosynthesis | ABHD4 | ABHD2, ABHD6, ABHD10 |
| Glycogenesis | G6PC3 | G6PC |
| √ | GYG1a | GYG2 |
| Glycolysis | ALDOAa | ALDOBa |
| √ | ENO1a | ENO3a |
| √ | PFKP | PFKFB1 |
| Glycolysis-TCA cycle junction | MPC2 | MPC1 |
| Glycoprotein/glycolipids Metabolism | B4GALT3, B4GALT7 | B4GALT1 |
| Glycosaminoglycan metabolism | B3GAT3 | B3GAT1 |
| √ | NDST1 | NDST3 |
| √ | PAPSS1 | PAPSS2a |
| Lysophosphatidic acid synthesis | ENPP2, ENPP4 | ENPP1 |
| Oxidative stress | PON2 | PON1a, PON3a |
| Phospholipid | AGPAT1 | AGPAT2 |
| Purine biosynthesis | NUDT1, NUDT2 | NUDT7 |
| S-adenosylmethionine | MAT1Aa | MAT2A |
| Sphingolipid metabolism | PPAP2A | PPAP2B |
| Steroid biosynthesis | ACBD3a | ACBD4 |
| Second messenger molecule synthesis | PDE6D | PDE2A, PDE7B |
| √ | PLCG1 | PLCG2 |
| Xenobiotics | NAT9, NAT10 | NAT2 |
| √ | SULT1C2 | SULT1A1a, SULT1A2, SULT2A1a |
| Transporters | SLC16A3 | SLC16A2, SLC16A10, SLC16A4 |
| √ | SLC22A5 | SLC22A1 |
| √ | SLC25A6a, SLC25A3a |
SLC25A15, SLC25A16 SLC25A20a, SLC25A37 |
| Glucose transporters | SLC2A5, SLC2A6 | SLC2A2 |
| Transporters | SLC38A1, SLC38A6 | SLC38A2, SLC38A4 |
| √ | SLC39A1, SLC39A6 | SLC39A14, SLC39A8 |
| √ | SLC4A2, SLC4A7 | SLC4A4 |
| √ | SLC6A8 | SLC6A12, SLC6A13, SLC6A16 |
| √ | SLC7A1, SLC7A6, SLC7A11 | SLC7A2, SLC7A8 |
| Organic anion transport | SLCO2A1 | SLCO2B1 |
| Lipid binding/unclear | STARD7 | STARD5 |
ABC, adenosine triphosphate–binding cassette; HCC, hepatocellular carcinoma; TCA, tricarboxylic acid.
Also detected to be significantly deregulated at protein level in our analysis.
Consistently Altered Metabolic Genes Correlate With Progression Markers and Predict Survival Outcome in HCC Patients
Correlation of metabolic targets with mediators of other cancer hallmarks can help uncover a mutual relationship. Through such analysis, Hu et al19 observed a high mutual relationship between hypoxia inducible factor 1A and oxidative phosphorylation (OXPHOS) in cancer. Invasion or metastasis and EMT are crucial processes in tumor progression.34, 35, 40 Whether metabolic alterations have association with tumor progression is largely unexplored in HCC. To reveal metabolic targets that may play a role in HCC progression, we correlated each of the 634 identified metabolic genes with 4 consistent progression markers, namely ECM2 and MMP9, which are related to invasion or metastasis, and CDH1 and VIM, which are related to EMT processes (Figure 5A). We selected metabolic targets that correlated at least with ECM2 and MMP9, leading to the identification of 285 consistently down-regulated genes, and 149 hits in the up-regulated category (Pearson correlation P < .05) (Figure 1A, Supplementary Table 1). Genes that performed best in the correlation analysis were those in gluconeogenesis, metabolism of glutamine and other amino acids, ketogenesis, urea cycle, adenosine triphosphate–binding cassette transporters, PPP and XM (Figure 5B), suggesting metabolic processes most likely associated with cancer progression. On the other hand, fewer genes in proton transport and OXPHOS correlated with the progression markers. Next, we wondered whether genes that correlated with the progression markers could also predict overall survival of liver cancer patients. We searched for currently available literature on each correlated gene, and found that although many of the genes are not yet described in HCC, about 20 candidates were previously reported to predict prognostic outcome (Supplementary Table 1). We analyzed patient overall survival data from 1 of the HCC cohorts, and identified 201 genes whose expression predicted survival outcome (Tables 4 and 5). Of these genes, 61% were also among those whose expression significantly varied with tumor size (n = 186), implying that consistently altered metabolic genes are strongly associated with clinicopathological variables. In terms of the genes associated with overall survival and tumor size, fewer targets in OXPHOS, glycan metabolism as well as adenosine triphosphate–binding cassette transporters were statistically significant— none were significant among proton transport genes (ATP5SL, ATP6V1E1, ATP5E, ATP5G2, ATP6AP1, ATP6V0B, and ATP6V1F) (Figure 5C, Supplementary Table 1). In contrast, the metabolic processes with the best-performing candidates were notably gluconeogenesis, urea cycle, detoxification, amino acid metabolism, FA metabolism, PPP, and small molecule transport (Figure 5C, Supplementary Table 1). Taken together, our study reveals that altered expression of metabolic genes are broadly consistent in HCC, correlate with clinical parameters, and hold yet untapped prospects in liver cancer research.
Figure 5.
The proportion of genes that correlated with the progression markers, predicted overall survival, and varied with tumor size in the respective metabolic processes or pathways. (A, top) Expression of the progression markers in HCC datasets as observed in Oncomine database (https://www.oncomine.org/). “Hepatocellular carcinoma vs Normal” were compared in Oncomine. Mas, Roessler 2, and Wurmbach are represented in our analysis by GSE14323, GSE14520, and GSE6764, respectively. (A, bottom) Expression of the progression marker in the datasets used for identifying altered metabolic targets. (B) The proportion of consistent metabolic genes that correlated with ECM2 and MMP9 in The Cancer Genome Atlas liver cancer data, and were selected for subsequent analysis. (C) The proportion of selected targets that predicted survival outcome and varied with tumor size in the clinical data associated with GSE14520. *Amino acid processes besides those listed. ABC, adenosine triphosphate–binding cassette; NAD, nicotinamide adenine dinucleotide; PPP, pentose phosphate pathway; S.M., small molecule; TCA, tricarboxylic acid.
Table 4.
Consistently Down-Regulated Genes Associated With Hepatocyte Metabolic Functions Correlate With Expression of Progression Markers and Predict Survival Outcome in HCC Patients
| Metabolic process | Gene symbol | # of datasets | Expression | Correlationa |
Predicts overall survivalb | |
|---|---|---|---|---|---|---|
| CDH1 | VIM | |||||
| Xenobiotics | CYP4A11 | 7 | Down | c | .0001 | |
| CYP4F3 | 8 | Down | c | c | .0007 | |
| CYP3A4 | 7 | Down | c | .001 | ||
| CYP2J2 | 7 | Down | c | .0012 | ||
| ▪HSD17B6 | 8 | Down | c | .0018 | ||
| CYP2C8 | 8 | Down | c | .0022 | ||
| ▪SRD5A1 | 7 | Down | c | .0038 | ||
| CYP2A6 | 7 | Down | c | .0051 | ||
| HSD11B1 | 7 | Down | c | .0307 | ||
| CYP2A7 | 6 | Down | .0346 | |||
| CYP3A43 | 7 | Down | c | <.0001 | ||
| Detoxification | ▪FMO4 | 6 | Down | c | .0011 | |
| ▪EPHX2 | 7 | Down | c | c | .0032 | |
| ▪TPMT | 8 | Down | c | .0032 | ||
| CAT | 7 | Down | c | .0053 | ||
| FMO3 | 6 | Down | c | .0065 | ||
| Urea cycle | OTC | 7 | Down | c | .0055 | |
| ASL | 6 | Down | c | c | .0074 | |
| CPS1 | 7 | Down | c | .011 | ||
| Redox | ▪FDX1 | 7 | Down | c | .0081 | |
| ▪DHRS12 | 6 | Down | c | .0141 | ||
| ▪DHTKD1 | 6 | Down | c | .0167 | ||
| ▪DHRS1 | 7 | Down | c | <.0001 | ||
| CYB5A | 8 | Down | c | .0026 | ||
| Glutathione | ▪HAGH | 7 | Down | c | .0015 | |
| ▪MGST2 | 6 | Down | c | .0493 | ||
| Fatty acid | ACOX2 | 7 | Down | c | .0003 | |
| ▪ECHDC2 | 8 | Down | c | .0004 | ||
| ▪ACADM | 7 | Down | c | .0007 | ||
| ▪ACBD4 | 8 | Down | c | .0012 | ||
| ▪FAAH | 6 | Down | c | .0015 | ||
| ▪ACAA2 | 7 | Down | c | .0018 | ||
| ▪ACSM3 | 7 | Down | c | .002 | ||
| ▪PECR | 6 | Down | c | .0022 | ||
| ▪ETFDH | 8 | Down | c | .0028 | ||
| ▪ACSM5 | 7 | Down | c | .0104 | ||
| EHHADH | 7 | Down | c | .0115 | ||
| ACADVL | 6 | Down | .0127 | |||
| MTTP | 6 | Down | c | .0134 | ||
| ▪ACADSB | 8 | Down | c | .0212 | ||
| ▪SCP2 | 8 | Down | c | .0227 | ||
| ▪HADH | 7 | Down | c | .0228 | ||
| ▪ACAT1 | 8 | Down | .0264 | |||
| ▪PHYH | 7 | Down | c | c | .0302 | |
| ▪ACSL3 | 6 | Up | .0426 | |||
| ▪MLYCD | 8 | Down | c | <.0001 | ||
| Gluconeogenesis | G6PC | 6 | Down | c | c | .0015 |
| PCK1 | 7 | Down | c | .0245 | ||
| PCK2 | 7 | Down | c | <.0001 | ||
| FBP1 | 7 | Down | c | .0013 | ||
| Ketogenesis | ▪BDH1 | 7 | Down | c | .0082 | |
| Amino acid | ▪GCDH | 8 | Down | c | .0005 | |
| ▪PIPOX | 7 | Down | c | .0007 | ||
| ▪AGXT | 8 | Down | c | .0011 | ||
| CDO1 | 7 | Down | c | .0014 | ||
| ▪FAHD2A | 8 | Down | .0015 | |||
| ▪AASS | 7 | Down | c | .0018 | ||
| FTCD | 7 | Down | c | .002 | ||
| ADI1 | 6 | Down | c | .0029 | ||
| DAO | 6 | Down | c | .0038 | ||
| CTH | 7 | Down | c | .0039 | ||
| ▪HAAO | 7 | Down | c | .0039 | ||
| HPD | 7 | Down | c | .0052 | ||
| HGD | 7 | Down | c | .0052 | ||
| BHMT | 7 | Down | c | .0057d | ||
| SARDH | 8 | Down | c | .0079 | ||
| ▪BCKDHA | 7 | Down | .0081 | |||
| MSRA | 8 | Down | .0086 | |||
| ▪HIBCH | 7 | Down | c | .009 | ||
| ▪BHMT2 | 6 | Down | c | .0128 | ||
| ▪BCKDHB | 8 | Down | c | .0135 | ||
| CBS | 8 | Down | c | .0141d | ||
| MAT1A | 8 | Down | c | .0167d | ||
| PAH | 7 | Down | c | .0205 | ||
| ▪THNSL1 | 6 | Down | c | .0256 | ||
| FAH | 7 | Down | c | .0444 | ||
| SUOX | 7 | Down | c | <.0001 | ||
| ▪ALDH18A1 | 7 | Up | c | .02 | ||
| ▪ASNS | 7 | Up | c | .0103d | ||
| ABC transporter | ▪ABCA6 | 7 | Down | c | .0015 | |
| ▪ABCC6 | 8 | Down | c | c | .0106 | |
| Ion transport | ▪KCNJ8 | 7 | Down | c | .0109 | |
| ▪CNGA1 | 7 | Down | c | c | .0002 | |
| ▪ATP1B3 | 7 | Up | c | .0008 | ||
| ▪P2RX4 | 7 | Up | c | .0466 | ||
| ▪SLC39A1 | 7 | Up | .0235 | |||
| CLIC1 | 8 | Up | c | <.0001 | ||
| S.M. transport | ▪SLC25A15 | 7 | Down | c | c | .0003 |
| ▪SLC27A5 | 7 | Down | c | .0003 | ||
| ▪SLC16A2 | 8 | Down | c | .0007 | ||
| ▪SLC25A20 | 8 | Down | c | .001 | ||
| ▪AQP7 | 7 | Down | .0013 | |||
| ▪SLC6A12 | 7 | Down | c | .0015 | ||
| ▪STARD5 | 8 | Down | .0023 | |||
| ▪SLCO2B1 | 7 | Down | c | .0042 | ||
| ▪SLC46A3 | 8 | Down | .0057 | |||
| ▪SLC1A1 | 6 | Down | c | .0266 | ||
| ▪SLC27A2 | 8 | Down | c | .0421 | ||
| ▪SLC47A1 | 7 | Down | c | .0467 | ||
| SLC38A4 | 8 | Down | c | .0066 | ||
| SLC28A1 | 6 | Down | c | .0467 | ||
| AQP9 | 6 | Down | c | .0001 | ||
| SLC2A2 | 7 | Down | c | .0002 | ||
| SLC10A1 | 7 | Down | c | .0005 | ||
| SLC22A7 | 7 | Down | c | c | .0008 | |
| SLC23A2 | 7 | Down | c | .0096 | ||
| SLC22A1 | 8 | Down | .0129d | |||
| ▪SLC2A6 | 6 | Up | c | .0094 | ||
| ▪SLC36A1 | 7 | Up | c | .0338 | ||
| SLC38A1 | 6 | Up | c | .0047 | ||
| SLC29A2 | 6 | Up | .0142 | |||
| SLC7A1 | 8 | Up | c | .0163 | ||
| Multipurpose | ▪ADH6 | 7 | Down | c | .0001 | |
| ▪GOT2 | 7 | Down | c | .0001 | ||
| ▪MAOB | 6 | Down | c | c | .001 | |
| ▪ALDH7A1 | 6 | Down | c | .0011 | ||
| ▪CBR4 | 8 | Down | .004 | |||
| ALDH2 | 8 | Down | c | .0062 | ||
| ▪ALDH9A1 | 7 | Down | c | .0477 | ||
| ADH1B | 7 | Down | c | .0008 | ||
| ALDH6A1 | 8 | Down | c | .0062 | ||
| ADH1C | 6 | Down | c | .0071 | ||
| AKR7A3 | 7 | Down | c | .0169 | ||
| ▪CA12 | 8 | Up | c | .0027 | ||
| Others | ▪SORD | 7 | Down | c | .0003 | |
| ▪MMACHC | 6 | Down | c | .0006 | ||
| ▪HAO1 | 7 | Down | c | .0009 | ||
| ▪DCXR | 7 | Down | c | .002 | ||
| ▪QDPR | 7 | Down | c | .0035 | ||
| ▪PCCB | 6 | Down | c | .0058 | ||
| ▪GFOD2 | 7 | Down | .0059 | |||
| ▪AGL | 8 | Down | c | .009 | ||
| ▪GNE | 8 | Down | c | .0238 | ||
| ▪MTHFD1 | 7 | Down | c | .0254 | ||
| ▪RBKS | 7 | Down | c | .0351 | ||
| ▪ADCY1 | 8 | Down | c | .0375 | ||
| ▪GAMT | 8 | Down | c | .0386 | ||
| UGP2 | 7 | Down | c | .0014 | ||
| ALAS1 | 6 | Down | c | .0027 | ||
| PON3 | 7 | Down | c | .0047 | ||
| RDH16 | 7 | Down | c | .0089 | ||
| ABAT | 8 | Down | c | .0093 | ||
| HAO2 | 8 | Down | .0152d | |||
| GRHPR | 7 | Down | c | <.0001 | ||
| MTHFS | 8 | Down | c | c | .0028 | |
| ▪NNT | 6 | Down | c | .0046 | ||
| ▪MUT | 7 | Down | c | c | .0067 | |
| GLYAT | 7 | Down | c | .0002 | ||
| SULT2A1 | 6 | Down | c | .0002 | ||
| CRYL1 | 7 | Down | c | .0045 | ||
| GNMT | 8 | Down | c | .0009 | ||
| PON1 | 7 | Down | c | .0072 | ||
| ▪NANS | 6 | Up | c | .0009 | ||
| ▪DDAH2 | 8 | Up | c | .001 | ||
| ▪MFSD10 | 7 | Up | c | .0057 | ||
| ▪LTA4H | 8 | Up | .0087 | |||
| ▪GYG1 | 7 | Up | c | .0267 | ||
| SULT1C2 | 8 | Up | .0064 | |||
| AACS | 7 | Up | c | .0069 | ||
| NOX4 | 7 | Up | c | .0422 | ||
| SMS | 6 | Up | c | c | .0328 | |
| SRM | 7 | Up | c | .0006 | ||
Genes categorized as other or multipurpose are those with functions either not yet well defined or not directly connected to a particular metabolic process. Kaplan-Meier overall survival was calculated for each gene and statistical significance analyzed by log-rank (Mantel-Cox) test. Square (▪) indicates novel targets in liver cancer as determined by searching PubMed.
#, number of datasets in which each gene is expressed at P < .05.
All listed metabolic genes correlated with ECM2 and MMP9.
Denotes P value.
Indicates those that also correlated with CDH1 and or VIM (Pearson correlation was considered significant at P < .05).
Previously identified as a survival predictor in hepatocellular carcinoma (HCC) (see the referenced study and other similarly described targets in Supplementary Table 1).
Table 5.
Genes in Mainly Up-Regulated Pathways That Correlated With the Expression of Progression Markers and Predicted Survival Outcome in HCC Patients
| Metabolic process | Gene symbol | # of datasets | Expression | Correlationa |
Predicts overall survivalb | |
|---|---|---|---|---|---|---|
| CDH1 | VIM | |||||
| Glycolysis | ▪PFKFB1 | 6 | Down | c | .0202 | |
| ALDOB | 8 | Down | c | c | .0022d | |
| ALDOA | 8 | Up | c | .0001d | ||
| HK2 | 6 | Up | c | .0001d | ||
| PKM | 8 | Up | c | .0003d | ||
| PDK4 | 7 | Down | .0177 | |||
| PPP | ▪DERA | 7 | Down | c | .0075 | |
| TKT | 7 | Up | c | .0132 | ||
| G6PD | 7 | Up | .0021d | |||
| TCA cycle | ▪ACO1 | 7 | Down | c | c | .0211 |
| ACLY | 7 | Up | .0054 | |||
| ME2 | 7 | Up | c | .0257 | ||
| OXPHOS | NDUFA4L2 | 7 | Up | .0003 | ||
| Nucleotide | ▪DPYS | 7 | Down | c | .0455 | |
| XDH | 7 | Down | c | c | .0047 | |
| UPB1 | 7 | Down | c | .0053 | ||
| ▪GMPS | 7 | Up | .0056 | |||
| ▪ADSL | 7 | Up | c | .0183 | ||
| ▪IMPDH2 | 8 | Up | c | .0085 | ||
| ▪NT5DC2 | 8 | Up | c | .0206 | ||
| RRM2 | 8 | Up | .0422 | |||
| UCK2 | 7 | Up | c | .0005 | ||
| ADA | 6 | Up | c | .0328 | ||
| Membrane lipid | ▪PLCB1 | 8 | Up | .0006 | ||
| ▪GPD1L | 8 | Up | c | .0029 | ||
| ▪LPIN2 | 7 | Down | c | .0154 | ||
| ▪PLCE1 | 8 | Up | c | .0459 | ||
| LPCAT1 | 7 | Up | c | .0001 | ||
| PTDSS2 | 6 | Up | .0005 | |||
| Glycan | ▪CTBS | 8 | Down | .0203 | ||
| ▪GAL3ST1 | 6 | Up | c | .0026 | ||
| ▪B3GALNT1 | 8 | Up | c | .013 | ||
| ▪NAGPA | 7 | Up | c | .0325 | ||
| ▪DDOST | 7 | Up | .0401 | |||
| SULF1 | 7 | Up | c | .0291 | ||
| Cholesterol | ▪HMGCS2 | 7 | Down | c | .0002 | |
| LCAT | 8 | Down | .0165 | |||
| ▪LBR | 7 | Up | .0269 | |||
Kaplan-Meier overall survival was calculated for each gene and statistical significance analyzed by log-rank (Mantel-Cox) test. Square (▪) indicates novel targets in liver cancer as determined by PubMed search.
#, number of datasets in which each gene is expressed at P < .05.
All listed metabolic genes correlated with ECM2 and MMP9.
Denotes P value.
Indicates those that also correlated with CDH1 and or VIM (Pearson correlation was considered significant at P < .05).
Previously identified as a survival predictor in hepatocellular carcinoma (HCC) (see the referenced study and other similarly described targets in Supplementary Table 1).
Discussion
Identification of the consistently altered metabolic targets is an indispensable step toward exploiting metabolism in basic, translational, and clinical cancer studies. We have exposed for the first time, metabolic genes that are consistently deregulated in human HCC. The metabolic genes, when put in the context of their associated biochemical pathways, reveal the suppression of well-known hepatocyte metabolic functions (eg, XM), and the up-regulation of energy-yielding processes (eg, glycolysis), as consistent features of HCC. XM genes are among the topmost down-regulated candidates in HCC and prominently emerged in pathway annotation analysis that took into account nonmetabolic genes in each dataset. Previous genomic study reported down-regulation of XM genes across 22 cancers, HCC inclusive, and suggested it may be associated with sensitivity to chemotherapies.19 Therapeutic resistance is currently an intractable problem in cancer, and has contributed to the failure of several drug trials in HCC.18, 41, 42 Although it is still unclear how down-regulated XM genes may influence drug sensitivity, studies suggest they are induced by therapy and cause a depletion of the systemic drug level. One example is cytochrome P450 3A4 (CYP3A4), which is down-regulated in HCC. In non–small cell lung cancer, it has been suggested that to ensure bioavailability, Erlotinib should not be used in combination with inducers of CYP3A4.43 In a xenograft model of HCC, treatment with Sorafenib caused the induction of CYP3A4, which coincided with reduced systemic level of the drug and the onset of resistance.44 Thus, our study could help in further identification of targets in XM or other metabolic processes that are down-regulated, but are prone to be re-expressed to mediate resistance.
Besides XM, the predominant down-regulation of genes in urea cycle, glutathione, FA, amino acid, gluconeogenesis, ketogenesis, and transamination are also consistent features of HCC (Figure 2). Notably, genes in urea cycle and gluconeogenesis scored very high in their correlation with progression markers, variation with tumor size, and prediction of overall survival. Reasons for the profound down-regulation of critical biochemical pathway targets could be multifactorial, including, among others, 1) lack of key pathway substrates; 2) products that are detrimental to HCC cell proliferation or survival, hence warranting pathway inhibition; 3) diversion of substrates into other pathways of higher priority for the tumor; or 4) transcriptional and epigenetic controls, or mutations that repress gene expression. Using urea cycle as an example, the supplementation of HCC cells with recombinant arginase, which hydrolyzes arginine to produce ornithine and urea, inhibited proliferation and induced cell cycle arrest.45 This implies a possible availability of substrate (in this case, arginine), but a lack of the enzymatic machinery for urea production. It also offers hint that intracellular urea as a product is detrimental to HCC cells. However, detrimental products may not explain why gluconeogenesis genes are suppressed given that cancer cells rely on its end product (glucose). As such, there is currently no molecular information to sufficiently explain why the down-regulation of these genes is crucial for orchestrating the global metabolic activities of HCC. Regarding FA biosynthesis, it is known that conditions such as nonalcoholic steatohepatitis (NASH) arise from FA accumulation and can lead to HCC. In line with our finding in human HCC, the down-regulation of FA genes has been reported in mice exposed to chronic choline-deficient high-fat diet in which NASH transited to HCC.46 Thus, impaired FA metabolism may represent an early event in HCC development that is consistent even across species, but yet unappreciated. In amino acid metabolism, we uncovered a striking suppression of serine pathway genes (PHGDH, PSAT1, SHMT1, SHMT2, GLDC) in HCC —with the exception of PSPH. The serine pathway branches from glycolysis at the level of 3-phosphoglycerate, with PHGDH catalyzing the first step.6, 7 Serine deficiency, and the down-regulation of the serine pathway genes, has been reported in patients with NAFLD.47 Thus, similar to FA metabolism discussed above, the down-regulation of serine pathway may also represent an early event in HCC development or progression. Suppressed serine pathway in HCC could also expose interesting contrasts when compared with breast cancer, where up-regulated serine pathway via PHGDH is essential in tumorigenesis.6, 7 PSPH, the only member of serine pathway we found to be up-regulated in HCC, has been reported to be critical for cMyc-driven cancer progression upon nutrient deprivation.48 Interestingly, the serine pathway genes mentioned earlier—except GLDC that was not shown—were all induced in HCC cell lines upon glucose or glutamine deprivation.48 Downstream of glycolysis, the down-regulation of the mitochondrial pyruvate carrier MPC1 is also notable in HCC. MPC1 has been reported to be down-regulated in NASH patients,47 and has been identified as a repressor of Warburg effect in cancer.49 MPC1 was also previously shown to be consistently down-regulated in HCC and several other cancers, and is induced upon glutamine deprivation as observed in colon cancer cells.49 These evidences strongly support that HCC have a predominant down-regulation of metabolic genes involved in physiologically relevant biochemical pathways. It further offers hint that several of the down-regulated targets could be re-induced to mediate resistance or stress response when cellular homeostasis is challenged, thus highlighting novel aspects of liver cancer metabolism for further investigation.
The consistently up-regulated metabolic genes in HCC mostly belong to processes such as glycolysis, PPP, NB, TCA cycle, OXPHOS, proton transport, membrane lipid, glycan metabolism, and small molecule transport. Whereas the prospects of novel insights still abound in known alterations (eg, glycolysis, TCA, OXPHOS, NB),12 similar opportunities also exist in processes least studied in HCC (eg, NB, proton transport, membrane lipid, glycan metabolism). For instance, nucleoside transporters and metabolizing enzymes (eg, uridine-cytidine kinases) modulate sensitivity to nucleoside analogues in leukemia.50, 51, 52, 53 UCK2 is up-regulated in HCC, but there are currently no data on its function. In addition, we identified other NB genes with yet unclear role in HCC (eg, TYMS [up-regulated], CDA and DPYD [both down-regulated]). In colorectal cancer, sequence variants of TYMS, CDA, and DPYD were found to be clinically relevant predictors of toxicity to fluoropyrimidine drugs (eg, 5-fluorouracil) and the prodrug capecitabine.53 Therefore, it might be interesting to investigate the role of NB targets in drug sensitivity in HCC. Similarly, the up-regulation of genes in glycan metabolism could broaden the chances of finding new drug targets, given that glycans have been considered prospective agents in cancer therapy.54 In addition, given their strategic expression on cell membranes, the proteins encoded by genes in glycan metabolism may represent important biomarkers in HCC. Altogether, the consistently up-regulated targets and their associated pathways could shed light on drug resistance mechanisms as well as the molecular mechanisms of metabolic reprogramming. Indeed, as was previously noticed in cancer,19 we observed a mixture of both up-regulated and down-regulated genes in most of the metabolic pathways. For example, although glycolytic targets are predominantly up-regulated, ALDOB, ENO3, and PFKFB1 are down-regulated, as are some glucose transporters (Figure 4). Although it is unclear if HCC actually require the down-regulation of these genes for optimal glycolysis, their suppressed expression seem to be beneficial for cancer cells. For instance, previous study show that low ALDOB expression influence aggressiveness and predict patients survival outcome in HCC.55 In our analysis, low expression of ALDOB and also PFKFB1 predicted overall survival. We detected ALDOB at protein level and its expression varied with tumor size. Therefore, the relevance of the up-regulated genes in metabolic pathways with predominantly down-regulated targets and vice versa cannot be overlooked. Similarly, isoforms of metabolic genes that are consistently expressed in the opposite direction (eg, GLS and GLS2, MPC1 and MPC2, MAT1A and MAT2A) (see Table 3, Supplementary Table 1) represent alterations that could be of clinical importance in liver cancer.
Conclusions
We have revealed metabolic targets that are consistently deregulated and so can be further studied as potential clinical biomarkers, therapeutic targets, or prognostic indicators in liver cancer. Several of the targets reflect at protein level, correlate with the progression markers, vary with tumor size, and predicted patient overall survival. Moreover, 54% (n = 343) (Supplementary Figure 1) were represented in the recent list of gene mutations identified in HCC by exome sequencing analysis.31 We believe these metabolic targets are broadly of promising clinical relevance in HCC. Consistent with this notion, most of the identified metabolic targets already described in the literature were proposed as biomarkers, therapeutic targets, or prognostic indicators in HCC. Examples include AKR1B10, CLIC1, PKM, ASNS, GLS, LPCAT1, NDUFA4L2, SLC39A6, and VDAC1, which are all up-regulated, and CYP1A2, ASS1, MAT1A, GLS2, and ALDH1L1, which are all down-regulated in HCC (see Supplementary Table 1). Hence, our findings are in agreement with several independent studies that have relied on various HCC patient cohorts. It gives strong impetus for detailed mechanistic studies on metabolic targets and their associated pathways in HCC. It is worthy to note that, a potential limitation of our work is the probability that some metabolic genes were not captured, for instance, due to gene probes not currently annotated. Also, although several targets are well known for their involvement in specific biochemical pathways, our assortment of some others to pathways should be used as a guide especially for those with yet unknown biological roles. Furthermore, due to technical limitations, we did not detect all proteins corresponding to the metabolic genes. Nevertheless, given the consistency of the genes we identified from several independent HCC datasets, and their correspondingly similar expression pattern where detected at proteomic level, this study is to date the most extensive exposition of the metabolic genes often deregulated in human HCC. Whether these alterations are specific to liver cancer or also present in other liver diseases, especially those related to metabolism such as NASH, should be an interesting subject of future investigation contingent on the accumulation of a comparable amount of genomic data for the clinical disease in question. For such study, the targets herein reported will serve as useful reference.
Supplementary Figure 1.
Consistently Altered Metabolic Targets Among List of Mutations in Published Exome Sequencing Analysis of Hepatocellular Carcinoma.
Our findings are also important in other aspects of liver cancer metabolism. For example, it will assist future studies in deciding on specific metabolic pathways to modulate therapeutically, and could increase the chances of identifying alternative metabolic pathways or targets that are used by HCC to evade therapy. It will also assist in identifying unique metabolic gene pattern in liver cancer compared with other cancers. In addition, the consistently altered targets represent a powerful tool for determining the in vitro or in vivo experimental HCC models that best depict the human HCC situation, especially from metabolism perspective—this knowledge is currently lacking and if obtained can help fine-tune future prospects of understanding liver cancer metabolism. In the context of personalized medicine, we hope that the consistently altered targets, including those most deregulated, as shown in Figure 1C, will be relevant for the identification of patients whose liver tumors have divergent expression patterns that might warrant individualized interventions.
Acknowledgments
The published datasets that were analyzed in this study are freely accessible from the National Center for Biotechnology Information Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/) under the Accession numbers GSE6764, GSE14323, GSE14520, GSE36376, GSE39791, GSE57957, GSE60502, and GSE62232. In addition, The Cancer Genome Atlas liver cancer data were accessed via cBioPortal database (http://www.cbioportal.org/).
Footnotes
Author contributions ZCN conceived the study, performed data analyses and wrote manuscript. SH and CM performed PubMed Search for the identification of novel metabolic targets and also discussed manuscript outline. SR discussed method and presentation of data. DAM and BS performed proteomics analysis. MPE performed critical revision of the manuscript. SD provided supervisory support and corrected manuscript. All authors read the final version of the paper.
Conflicts of interest The authors declare no conflicts.
Funding Z.C.N. is a recipient of PhD Scholarship from the Niger, Delta Development Commission, Nigeria, and appreciates the generous support from the Graduate School (HBIGS), University of Heidelberg, Germany. C.M. receives support through a grant from the Deutsche Forschungsgemeinschaft (DFG) (Me4532/1-1). S.D. is supported by funds from the DFG (Do373/13-1), the BMBF program LiSyM (Grant PTJ-FKZ: 031 L0043), and the Marie Curie Actions of the European Union’s Seventh Framework Programme (FP7/2007-2013) Grant PITN-GA-2012-316549 (IT LIVER: Inhibiting TGF-beta in liver diseases). The funding bodies did not influence the content of this article.
Supplementary Material
Consistently Altered Metabolic Targets in Human Hepatocellular Carcinoma
Proteomic Quantification of the Metabolic Targets in Tumor vs Adjacent Normal Liver Tissues
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koppenol W.H., Bounds P.L., Dang C.V. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locasale J.W., Grassian A.R., Melman T., Lyssiotis C.A., Mattaini K.R., Bass A.J., Heffron G., Metallo C.M., Muranen T., Sharfi H., Sasaki A.T., Anastasiou D., Mullarky E., Vokes N.I., Sasaki M., Beroukhim R., Stephanopoulos G., Ligon A.H., Meyerson M., Richardson A.L., Chin L., Wagner G., Asara J.M., Brugge J.S., Cantley L.C., Vander Heiden M.G. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Possemato R., Marks K.M., Shaul Y.D., Pacold M.E., Kim D., Birsoy K., Sethumadhavan S., Woo H.K., Jang H.G., Jha A.K., Chen W.W., Barrett F.G., Stransky N., Tsun Z.Y., Cowley G.S., Barretina J., Kalaany N.Y., Hsu P.P., Ottina K., Chan A.M., Yuan B., Garraway L.A., Root D.E., Mino-Kenudson M., Brachtel E.F., Driggers E.M., Sabatini D.M. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le A., Lane A.N., Hamaker M., Bose S., Gouw A., Barbi J., Tsukamoto T., Rojas C.J., Slusher B.S., Zhang H., Zimmerman L.J., Liebler D.C., Slebos R.J., Lorkiewicz P.K., Higashi R.M., Fan T.W., Dang C.V. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carracedo A., Cantley L.C., Pandolfi P.P. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschey M.D., DeBerardinis R.J., Diehl A.M., Drew J.E., Frezza C., Green M.F., Jones L.W., Ko Y.H., Le A., Lea M.A., Locasale J.W., Longo V.D., Lyssiotis C.A., McDonnell E., Mehrmohamadi M., Michelotti G., Muralidhar V., Murphy M.P., Pedersen P.L., Poore B., Raffaghello L., Rathmell J.C., Sivanand S., Vander Heiden M.G., Wellen K.E., Target Validation Team Dysregulated metabolism contributes to oncogenesis. Semin Cancer Biol. 2015;35(Suppl):S129–S150. doi: 10.1016/j.semcancer.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowicki S., Gottlieb E. Oncometabolites: tailoring our genes. FEBS J. 2015;282:2796–2805. doi: 10.1111/febs.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galluzzi L., Kepp O., Vander Heiden M.G., Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 14.Vander Heiden M.G., DeBerardinis R.J. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168:657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzmaurice C., Dicker D., Pain A., Hamavid H., Moradi-Lakeh M., MacIntyre M.F., Allen C., Hansen G., Woodbrook R., Wolfe C., Hamadeh R.R., Moore A., Werdecker A., Gessner B.D., Te Ao B., McMahon B., Karimkhani C., Yu C., Cooke G.S., Schwebel D.C., Carpenter D.O., Pereira D.M., Nash D., Kazi D.S., De Leo D., Plass D., Ukwaja K.N., Thurston G.D., Yun Jin K., Simard E.P., Mills E., Park E.K., Catalá-López F., deVeber G., Gotay C., Khan G., Hosgood H.D., 3rd, Santos I.S., Leasher J.L., Singh J., Leigh J., Jonas J.B., Sanabria J., Beardsley J., Jacobsen K.H., Takahashi K., Franklin R.C., Ronfani L., Montico M., Naldi L., Tonelli M., Geleijnse J., Petzold M., Shrime M.G., Younis M., Yonemoto N., Breitborde N., Yip P., Pourmalek F., Lotufo P.A., Esteghamati A., Hankey G.J., Ali R., Lunevicius R., Malekzadeh R., Dellavalle R., Weintraub R., Lucas R., Hay R., Rojas-Rueda D., Westerman R., Sepanlou S.G., Nolte S., Patten S., Weichenthal S., Abera S.F., Fereshtehnejad S.M., Shiue I., Driscoll T., Vasankari T., Alsharif U., Rahimi-Movaghar V., Vlassov V.V., Marcenes W.S., Mekonnen W., Melaku Y.A., Yano Y., Artaman A., Campos I., MacLachlan J., Mueller U., Kim D., Trillini M., Eshrati B., Williams H.C., Shibuya K., Dandona R., Murthy K., Cowie B., Amare A.T., Antonio C.A., Castañeda-Orjuela C., van Gool C.H., Violante F., Oh I.H., Deribe K., Soreide K., Knibbs L., Kereselidze M., Green M., Cardenas R., Roy N., Tillmann T., Li Y., Krueger H., Monasta L., Dey S., Sheikhbahaei S., Hafezi-Nejad N., Kumar G.A., Sreeramareddy C.T., Dandona L., Wang H., Vollset S.E., Mokdad A., Salomon J.A., Lozano R., Vos T., Forouzanfar M., Lopez A., Murray C., Naghavi M. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allemani C., Weir H.K., Carreira H., Harewood R., Spika D., Wang X.S., Bannon F., Ahn J.V., Johnson C.J., Bonaventure A., Marcos-Gragera R., Stiller C., Azevedo e Silva G., Chen W.Q., Ogunbiyi O.J., Rachet B., Soeberg M.J., You H., Matsuda T., Bielska-Lasota M., Storm H., Tucker T.C., Coleman M.P., CONCORD Working Group Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekervel J., van Pelt J., Verslype C. Advanced unresectable hepatocellular carcinoma: new biologics as fresh ammunition or clues to disease understanding? Curr Opin Oncol. 2013;25:409–416. doi: 10.1097/CCO.0b013e3283621074. [DOI] [PubMed] [Google Scholar]
- 18.Llovet J.M., Villanueva A., Lachenmayer A., Finn R.S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–424. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 19.Hu J., Locasale J.W., Bielas J.H., O'Sullivan J., Sheahan K., Cantley L.C., Vander Heiden M.G., Vitkup D. Heterogeneity of tumor-induced gene expression changes in the human metabolic network. Nat Biotechnol. 2013;31:522–529. doi: 10.1038/nbt.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björnson E., Mukhopadhyay B., Asplund A., Pristovsek N., Cinar R., Romeo S., Uhlen M., Kunos G., Nielsen J., Mardinoglu A. Stratification of hepatocellular carcinoma patients based on acetate utilization. Cell Rep. 2015;13:2014–2026. doi: 10.1016/j.celrep.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q., Tan Y., Yin P., Ye G., Gao P., Lu X., Wang H., Xu G. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 2013;73:4992–5002. doi: 10.1158/0008-5472.CAN-13-0308. [DOI] [PubMed] [Google Scholar]
- 22.Megger D.A., Bracht T., Kohl M., Ahrens M., Naboulsi W., Weber F., Hoffmann A.C., Stephan C., Kuhlmann K., Eisenacher M., Schlaak J.F., Baba H.A., Meyer H.E., Sitek B. Proteomic differences between hepatocellular carcinoma and nontumorous liver tissue investigated by a combined gel-based and label-free quantitative proteomics study. Mol Cell Proteomics. 2013;12:2006–2020. doi: 10.1074/mcp.M113.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trivedi P.J., Lammers W.J., van Buuren H.R., Parés A., Floreani A., Janssen H.L., Invernizzi P., Battezzati P.M., Ponsioen C.Y., Corpechot C., Poupon R., Mayo M.J., Burroughs A.K., Nevens F., Mason A.L., Kowdley K.V., Lleo A., Caballeria L., Lindor K.D., Hansen B.E., Hirschfield G.M., Global PBC Study Group Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut. 2016;65:321–329. doi: 10.1136/gutjnl-2014-308351. [DOI] [PubMed] [Google Scholar]
- 24.Roessler S., Jia H.L., Budhu A., Forgues M., Ye Q.H., Lee J.S., Thorgeirsson S.S., Sun Z., Tang Z.Y., Qin L.X., Wang X.W. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202–10212. doi: 10.1158/0008-5472.CAN-10-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J.H., Sohn B.H., Lee H.S., Kim S.B., Yoo J.E., Park Y.Y., Jeong W., Lee S.S., Park E.S., Kaseb A., Kim B.H., Kim W.B., Yeon J.E., Byun K.S., Chu I.S., Kim S.S., Wang X.W., Thorgeirsson S.S., Luk J.M., Kang K.J., Heo J., Park Y.N., Lee J.S. Genomic predictors for recurrence patterns of hepatocellular carcinoma: model derivation and validation. PLoS Med. 2014;11:e1001770. doi: 10.1371/journal.pmed.1001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mah W.C., Thurnherr T., Chow P.K., Chung A.Y., Ooi L.L., Toh H.C., Teh B.T., Saunthararajah Y., Lee C.G. Methylation profiles reveal distinct subgroup of hepatocellular carcinoma patients with poor prognosis. PLoS One. 2014;9:e104158. doi: 10.1371/journal.pone.0104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim H.Y., Sohn I., Deng S., Lee J., Jung S.H., Mao M., Xu J., Wang K., Shi S., Joh J.W., Choi Y.L., Park C.K. Prediction of disease-free survival in hepatocellular carcinoma by gene expression profiling. Ann Surg Oncol. 2013;20:3747–3753. doi: 10.1245/s10434-013-3070-y. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y.H., Cheng T.Y., Chen T.Y., Chang K.M., Chuang V.P., Kao K.J. Plasmalemmal Vesicle Associated Protein (PLVAP) as a therapeutic target for treatment of hepatocellular carcinoma. BMC Cancer. 2014;14:815. doi: 10.1186/1471-2407-14-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mas V.R., Maluf D.G., Archer K.J., Yanek K., Kong X., Kulik L., Freise C.E., Olthoff K.M., Ghobrial R.M., McIver P., Fisher R. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med. 2009;15:85–94. doi: 10.2119/molmed.2008.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wurmbach E., Chen Y.B., Khitrov G., Zhang W., Roayaie S., Schwartz M., Fiel I., Thung S., Mazzaferro V., Bruix J., Bottinger E., Friedman S., Waxman S., Llovet J.M. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- 31.Schulze K., Imbeaud S., Letouzé E., Alexandrov L.B., Calderaro J., Rebouissou S., Couchy G., Meiller C., Shinde J., Soysouvanh F., Calatayud A.L., Pinyol R., Pelletier L., Balabaud C., Laurent A., Blanc J.F., Mazzaferro V., Calvo F., Villanueva A., Nault J.C., Bioulac-Sage P., Stratton M.R., Llovet J.M., Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkinson H., Sarkans U., Kolesnikov N., Abeygunawardena N., Burdett T., Dylag M., Emam I., Farne A., Hastings E., Holloway E., Kurbatova N., Lukk M., Malone J., Mani R., Pilicheva E., Rustici G., Sharma A., Williams E., Adamusiak T., Brandizi M., Sklyar N., Brazma A. ArrayExpress update–an archive of microarray and high-throughput sequencing-based functional genomics experiments. Nucleic Acids Res. 2011;39:D1002–D1004. doi: 10.1093/nar/gkq1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M., Yefanov A., Lee H., Zhang N., Robertson C.L., Serova N., Davis S., Soboleva A. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan L., Pantel K., Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes D.R., Kalyana-Sundaram S., Mahavisno V., Varambally R., Yu J., Briggs B.B., Barrette T.R., Anstet M.J., Kincead-Beal C., Kulkarni P., Varambally S., Ghosh D., Chinnaiyan A.M. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naboulsi W., Bracht T., Megger D.A., Reis H., Ahrens M., Turewicz M., Eisenacher M., Tautges S., Canbay A.E., Meyer H.E., Weber F., Baba H.A., Sitek B. Quantitative proteome analysis reveals the correlation between endocytosis-associated proteins and hepatocellular carcinoma dedifferentiation. Biochim Biophys Acta. 2016;1864:1579–1585. doi: 10.1016/j.bbapap.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Naboulsi W., Megger D.A., Bracht T., Kohl M., Turewicz M., Eisenacher M., Voss D.M., Schlaak J.F., Hoffmann A.C., Weber F., Baba H.A., Meyer H.E., Sitek B. Quantitative tissue proteomics analysis reveals versican as potential biomarker for early-stage hepatocellular carcinoma. J Proteome Res. 2016;15:38–47. doi: 10.1021/acs.jproteome.5b00420. [DOI] [PubMed] [Google Scholar]
- 39.Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Meyer C., Dzieran J., Liu Y., Schindler F., Munker S., Müller A., Coulouarn C., Dooley S. Distinct dedifferentiation processes affect caveolin-1 expression in hepatocytes. Cell Commun Signal. 2013;11:6. doi: 10.1186/1478-811X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llovet J.M., Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–2079. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 42.Zhu A.X., Rosmorduc O., Evans T.R., Ross P.J., Santoro A., Carrilho F.J., Bruix J., Qin S., Thuluvath P.J., Llovet J.M., Leberre M.A., Jensen M., Meinhardt G., Kang Y.K. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 43.Cataldo V.D., Gibbons D.L., Pérez-Soler R., Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364:947–955. doi: 10.1056/NEJMct0807960. [DOI] [PubMed] [Google Scholar]
- 44.Kuczynski E.A., Lee C.R., Man S., Chen E., Kerbel R.S. Effects of sorafenib dose on acquired reversible resistance and toxicity in hepatocellular carcinoma. Cancer Res. 2015;75:2510–2519. doi: 10.1158/0008-5472.CAN-14-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam T.L., Wong G.K., Chong H.C., Cheng P.N., Choi S.C., Chow T.L., Kwok S.Y., Poon R.T., Wheatley D.N., Lo W.H., Leung Y.C. Recombinant human arginase inhibits proliferation of human hepatocellular carcinoma by inducing cell cycle arrest. Cancer Lett. 2009;277:91–100. doi: 10.1016/j.canlet.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 46.Wolf M.J., Adili A., Piotrowitz K., Abdullah Z., Boege Y., Stemmer K., Ringelhan M., Simonavicius N., Egger M., Wohlleber D., Lorentzen A., Einer C., Schulz S., Clavel T., Protzer U., Thiele C., Zischka H., Moch H., Tschöp M., Tumanov A.V., Haller D., Unger K., Karin M., Kopf M., Knolle P., Weber A., Heikenwalder M. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Mardinoglu A., Agren R., Kampf C., Asplund A., Uhlen M., Nielsen J. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:3083. doi: 10.1038/ncomms4083. [DOI] [PubMed] [Google Scholar]
- 48.Sun L., Song L., Wan Q., Wu G., Li X., Wang Y., Wang J., Liu Z., Zhong X., He X., Shen S., Pan X., Li A., Wang Y., Gao P., Tang H., Zhang H. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–444. doi: 10.1038/cr.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schell J.C., Olson K.A., Jiang L., Hawkins A.J., Van Vranken J.G., Xie J., Egnatchik R.A., Earl E.G., DeBerardinis R.J., Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400–413. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galmarini C.M., Mackey J.R., Dumontet C. Nucleoside analogues: mechanisms of drug resistance and reversal strategies. Leukemia. 2001;15:875–890. doi: 10.1038/sj.leu.2402114. [DOI] [PubMed] [Google Scholar]
- 51.Damaraju V.L., Damaraju S., Young J.D., Baldwin S.A., Mackey J., Sawyer M.B., Cass C.E. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene. 2003;22:7524–7536. doi: 10.1038/sj.onc.1206952. [DOI] [PubMed] [Google Scholar]
- 52.Valencia A., Masala E., Rossi A., Martino A., Sanna A., Buchi F., Canzian F., Cilloni D., Gaidano V., Voso M.T., Kosmider O., Fontenay M., Gozzini A., Bosi A., Santini V. Expression of nucleoside-metabolizing enzymes in myelodysplastic syndromes and modulation of response to azacitidine. Leukemia. 2014;28:621–628. doi: 10.1038/leu.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loganayagam A., Arenas Hernandez M., Corrigan A., Fairbanks L., Lewis C.M., Harper P., Maisey N., Ross P., Sanderson J.D., Marinaki A.M. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br J Cancer. 2013;108:2505–2515. doi: 10.1038/bjc.2013.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shriver Z., Raguram S., Sasisekharan R. Glycomics: a pathway to a class of new and improved therapeutics. Nat Rev Drug Discov. 2004;3:863–873. doi: 10.1038/nrd1521. [DOI] [PubMed] [Google Scholar]
- 55.Tao Q.F., Yuan S.X., Yang F., Yang S., Yang Y., Yuan J.H., Wang Z.G., Xu Q.G., Lin K.Y., Cai J., Yu J., Huang W.L., Teng X.L., Zhou C.C., Wang F., Sun S.H., Zhou W.P. Aldolase B inhibits metastasis through Ten-Eleven Translocation 1 and serves as a prognostic biomarker in hepatocellular carcinoma. Mol Cancer. 2015;14:170. doi: 10.1186/s12943-015-0437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consistently Altered Metabolic Targets in Human Hepatocellular Carcinoma
Proteomic Quantification of the Metabolic Targets in Tumor vs Adjacent Normal Liver Tissues