Abstract
Study Design
A retrospective database study.
Objective
The goal of this study was to (1) evaluate the trends in the use of intraoperative neuromonitoring (ION) for anterior cervical discectomy and fusion (ACDF) surgery in the United States and (2) assess the incidence of neurological injuries after ACDFs with and without ION.
Summary of Background Data
Somatosensory-evoked potentials (SSEPs) and motor-evoked potentials (MEPs) are the commonly used ION modalities for ACDFs. Controversy exists on the routine use of ION for ACDFs and there is limited literature on national practice patterns of its use.
Methods
A retrospective review was performed using the PearlDiver Patient Record Database to identify cases of spondylotic myelopathy and radiculopathy that underwent ACDF from 2007 to 2014. The type of ION modality used and the rates of neurological injury after surgery were assessed.
Results
During the study period, 15,395 patients underwent an ACDF. Overall, ION was used in 2627 (17.1%) of these cases. There was a decrease in the use of ION for ACDFs from 22.8% in 2007 to 4.3% use in 2014 (P < 0.0001). The ION modalities used for these ACDFs were quite variable: SSEPs only (48.7%), MMEPs only (5.3%), and combined SSEPs and MMEPs (46.1%). Neurological injuries occurred in 0.23% and 0.27% of patients with and without ION, respectively (P = 0.84). Younger age was associated with a higher utility of ION (<45: 20.3%, 45–54: 19.3%, 55–64: 16.6%, 65–74: 14.3%, and >75: 13.6%, P < 0.0001). Significant regional variability was observed in the utility of ION for ACDFs across the country (West; 21.9%, Midwest; 12.9% (P < 0.0001).
Conclusion
There has been a significant decrease in the use of ION for ACDFs. Furthermore, there was significant age and regional variability in the use of ION for ACDFs. Use of ION does not further prevent the rate of postoperative neurological complications for ACDFs as compared with the cases without ION. The utility of routine ION for ACDFs is questionable.
Keywords: anterior cervical discectomy and fusion, cervical radiculopathy, cervical spine, cervical spondylosis, cervical spondylotic myelopathy, intraoperative monitoring, intraoperative neuromonitoring, motor evoked potential, neurological injury, somatosensory evoked potential
Neurological injury is a known risk with cervical spine surgery. Intraoperative neuromonitoring (ION) is often used during cervical spine surgery to detect early neurological injury. Somatosensory-evoked potentials (SSEPs) is the most commonly used ION.1 It monitors spinal cord function through the ascending sensory pathway and may not detect motor tract injuries. Therefore, SSEPs are often supplemented with motor-evoked potentials (MEPs). MEPs provide a direct measure of the corticospinal motor tract function. MEPs can be especially useful in anterior cervical spine surgery when motor tracts are particularly at risk.
Controversy exists in the utility of routine use of ION for anterior cervical spine surgeries for degenerative conditions without deformity.2 The decision to use ION for ACDFs is often guided by surgeon choice and experience and there is no consensus on the optimal ION modality to use. Proponents of ION for ACDFs claim that it improves patient safety and functional outcome, while opponents refute this claim by citing increased cost and the lack of correlation between ION abnormalities and postoperative neurological deficits.3–6 To date, little has been written on the national practice patterns of ION for ACDFs in patients with degenerative cervical spine diseases. The goal of this study is to (1) evaluate the trends in the use of ION for ACDFs in the United States and (2) assess neurological risk after ACDFs with and without ION.
MATERIALS AND METHODS
A retrospective review was performed using the PearlDiver Patient Record Database (www.pearldiver.com; PearlDiver, Inc., Warsaw, IN) to search through the patient records within the Humana private insurance databases. The Pearl-Diver database is commercially available and contains de-identified patient data that are Health Insurance Portability and Accountability Act (HIPAA) compliant and allow researchers to construct queries with billing codes to identify patient groupings that meet specified criteria of interest. The raw datasets are filtered by characteristics such as demographic and clinical information. The dataset used in this study spans 2007 through 2014.
Data Collection
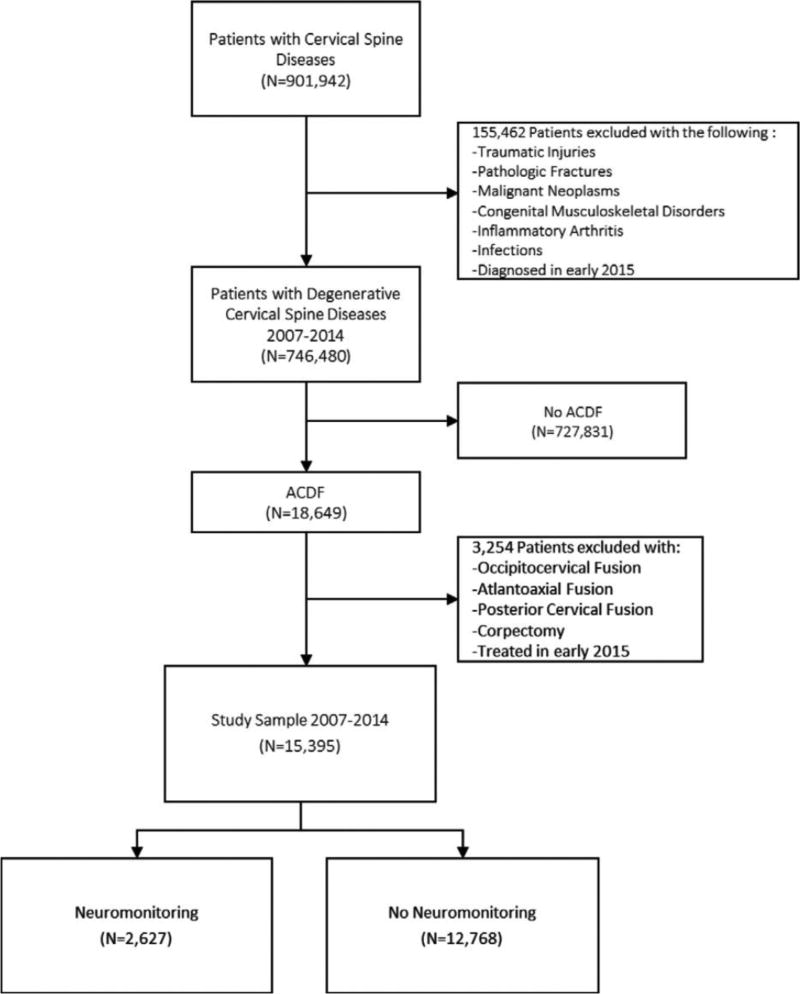
The database was used to identify cases of cervical degenerative diseases undergoing ACDF surgery with and without neuromonitoring from years 2007 to 2014 using both current procedural terminology (CPT) and international classification of diseases, ninth revision (ICD-9) codes (see Appendix, http://links.lww.com/BRS/B152). Patients with potentially confounding diagnoses such as traumatic injuries, pathologic fractures, malignant neoplasms, congenital musculoskeletal disorders, inflammatory arthridities, and infections were excluded (see Appendix, http://links.lww.com/BRS/B152). Patients undergoing concomitant posterior cervical fusion/instrumentation or corpectomy were also excluded (see Appendix, http://links.lww.com/BRS/B152) (Figure 1).
Figure 1.
Inclusion and exclusion criteria.
Statistical Analysis
Chi-square hypothesis testing was used. The STATA statistical software version 11.0 (STATACorp, College Station, TX) was used to perform the analyses. The significance level was set at P < 0.05.
RESULTS
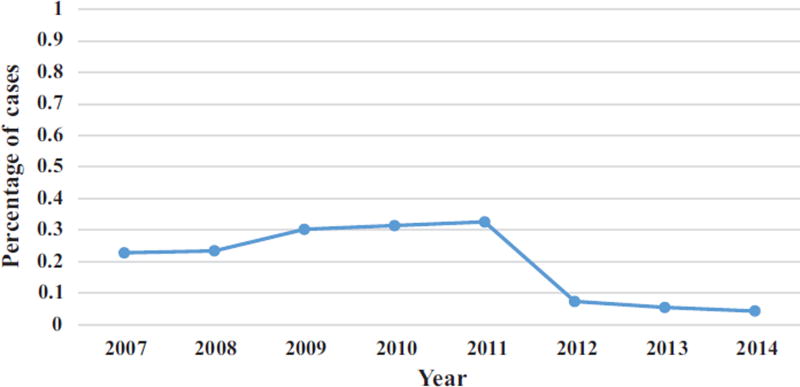
During the study period, 2007 to 2014, 15,395 patients underwent ACDF surgery for spondylotic myelopathy and/or radiculopathy. Overall, ION was used in 2627 (17.1%) of these cases. There was a steady increase in the utility of ION for ACDFs from 22.8% in 2007 to a peak of 32.6% in 2011. However, this was followed by a steady decline to 4.3% in 2014 (P < 0.0001) (Figure 2).
Figure 2.
Percentage of ACDF surgery performed with neuromonitoring during the study period.
Type of Neuromonitoring Modality
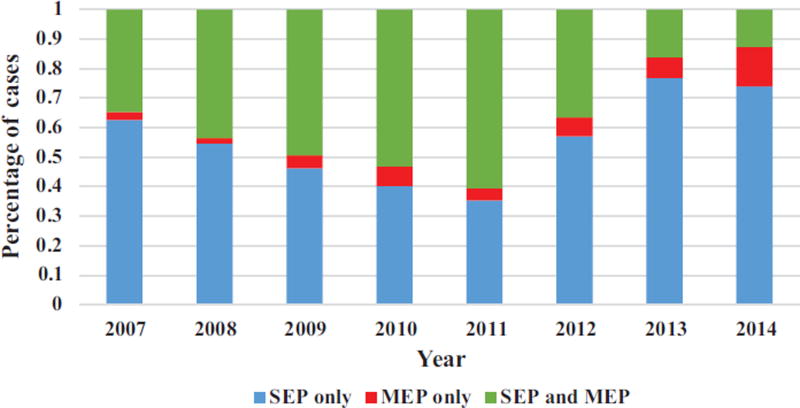
Out of a total of 2627 patients who had an ACDF with ION, the combination of SSEPs and MEPs was used in 46.1% (1213/2627) of cases; SSEPs alone: 48.7%%; MEPs alone: 5.3%. Among cases that utilized ION, there was a steady decrease in the combined use of SSEPs and MEPs over the duration of study period. Combined SSEPs and MEPs: 34.7% (in 2007) to 12.7% (in 2014) (P < 0.0001) (Figure 3).
Figure 3.
Percentage of ACDF surgery performed based on specific neuromonitoring modalities during the study period.
Neurological Injury
The overall rate of postoperative neurological injuries within 30 days of the index surgery was 0.27% (41/15395). The rate was 0.23% in patients who had an ACDF with ION compared with 0.27% in patients without ION (P = 0.84).
Age
Among patients who had ACDFs, ION was used in 20.3% (408/2009) of patients <45 years of age, compared with 19.3%, 16.6%, 14.3%, and 13.6% of patients in age groups 45 to 54, 55 to 64, 65 to 74, and >75 years, respectively (P < 0.0001) (Table 1).
TABLE 1.
Demographic Information of Patients from 2007 to 2014
| Total Number of ACDFs With Neuromonitoring |
Total Number of ACDFs | P | |
|---|---|---|---|
| Age, yrs* | <0.0001 | ||
| <45 | 408 | 2009 | |
| 45–54 | 752 | 3889 | |
| 55–64 | 648 | 3893 | |
| 65–74 | 656 | 4571 | |
| >75 | 155 | 1137 | |
| Gender | 0.55 | ||
| Female | 1376 | 7981 | |
| Male | 1251 | 7414 | |
| Region | <0.0001 | ||
| Midwest | 449 | 3483 | |
| Northeast | 35 | 216 | |
| South | 1859 | 10,398 | |
| West | 284 | 1299 | |
| Total* | 2627 | 15,395 |
The discrepancy between total value and summation of values in the age category is attributed to the transfer of patients between subgroups.
Gender
ION was used in 17.2% (1376/7981) of females compared with 16.9% (1251/7414) of males who had ACDFs (P = 0.55) (Table 1).
Region
ION was used in 21.9% (284/1299) of the ACDFs in the West compared with 17.9% (1859/10,398) in the South, 16.2% (35/216) in the Northeast, and 12.9% (449/3483) in the Midwest (P < 0.0001) (Table 1).
DISCUSSION
ION has been used since the 1970s for spinal surgeries.3 ION with SSEPs and MEPs are the two reliable ways to monitor spinal cord function. Before their widespread use, the Stagnara wake-up test served as the only way to intraoperatively assess neurologic function.7 ION is now used in a variety of spinal operations, including deformity surgery, tumor resection, and trauma.1 For deformity surgery, a substantial body of literature shows that ION can aid with early detection of neurological injuries that occur with traction, compression, or ischemia of the spinal cord during the deformity correction.8–11 A survey showed that the majority of Scoliosis Research Society (SRS) members utilize ION routinely for their spinal deformity surgeries.12 For cervical spine surgeries such as ACDF, the routine use ION remains controversial and there is no consensus on the optimal neuromonitoring modality to use. In this study, we attempted to answer two questions. First, what is the national trend in the use of ION for ACDFs in the United States? Second, what is the incidence of neurological injuries after ACDFs with and without ION? Using a large administrative database of privately insured patients, we found an overall decrease in the use ION for ACDFs. SSEP is the most common ION modality of choice. Furthermore, age and regional variability exists in the use of ION for ACDFs. Lastly, despite ION having the theoretical advantage of early detection of neurologic injury, in cases of spondylotic myelopathy and/or radiculopathy, the routine use of ION for ACDFs does not decrease the rate of postoperative neurologic complications.
To the knowledge of the authors, there are no studies in the literature on the national trends of ION specifically for ACDFs in the treatment of spondylotic myelopathy/radiculopathy. In a retrospective study of 443,194 patients from the Nationwide Inpatient Sample (NIS) that had a variety of spine procedures, James et al13 reported that the overall utility of ION was 7.1%, which is lower than the 19.6% observed in this study. For nonspecified procedures involving the cervical spine, ION use increased from 0.6% of all cases in 2007 to 10.5% of cases in 2011, which differs from the decreased trend observed in the current study. There are several reasons that explain this discordance. This present study analyzed a specific patient sample, that is, patients with spondylotic myelopathy and/or radiculopathy who had an ACDF. This patient group is considered “low-risk” compared with patients with fractures, tumor, or deformities. There is a growing body of literature against the routine use of ION during “low-risk” procedures such as ACDFs. Increased health care cost and a lack of correlation between ION abnormalities and postoperative neurological deficits are two reasons often cited by opponents of ION for ACDFs. In an economic analysis study of 720 patients who had cervical decompression and reconstruction without ION, Traynelis et al6 reported no persistent postoperative neurological deficits in their series. On the basis of their estimate, the use of SSEPs and MEPs for ION would have cost $633.32 at the hourly rate and incurred a total of $1,024,754 in 2011 US dollars for reimbursement at the 2011 Medicare rate. Furthermore, the additional operating room time needed to set up ION increases resource utilization and ultimately cost. Although the study by James et al13 shows that there is a trend toward an increased use of ION for spine surgery as a whole because it is becoming more available and surgeons are adopting this technology into their practice, the decreased utility of ION specifically for ACDFs observed in the present study shows that surgeons are selective in their indications for ION.
The study by Epstein et al4 was one of the first to demonstrate better outcomes in cervical spinal surgery by using ION. In their series, 100 patients who had cervical spine surgery with continuous SSEPs were compared with 218 historical control patients who had no SSEPs. Four percent of the patients in the nonmonitored group developed quadriplegia compared with none of the patients in the monitored group. Improved outcomes with ION in the study were attributed to early detection of impending neurological injury by SSEPs, which allowed prompt reversal of hypotension, distraction, and manipulation of the spinal cord. Since the publication by Epstein et al,4 the use of ION has been found to be of limited value by several authors. Taunt et al5 reported on 163 patients who had ACDFs with continuous SSEPs. Three (1.8%) false positives were noted in which intraoperative SSEP alerts did not correlate with postoperative neurological deterioration and one (0.6%) false negative was noted in which a new postoperative neurological deficit developed without a SSEP alert. In a series comparing 577 monitored patients with 462 control patients who had ACDF surgery, Smith et al14 reported no new postoperative neurological deficits in the control group compared with one deficit in the monitored group despite normal SSEP signals. Due to the fact that SSEPs only monitor the ascending sensory pathways in the dorsal spinal cord, some authors have advocated for the additional use of MEPs to monitor the ventral corticospinal tract especially in anterior cervical spine surgery.
Bose et al15 published a study on a series of 119 patients who had anterior cervical spine surgery with combined MEPs and SSEPs. Three patients developed neurological deficits postoperatively—one of which was detected by MEPs. In the remaining two patients who developed a new postoperative neurological deficit (including one patient who developed quadriparesis), the authors reported that preexisting pathology and/or anesthetic effects compromised the detection of neurological changes by ION. Lee et al16 also published on a series of 1445 patients who had anterior cervical surgery with MEP, SEP, and electromyography (EMG). ION alerts were observed in 267 (18%) patients. Eight surgeries needed to be aborted due to persistent MEP/SSEP amplitude loss. However, none of these eight patients developed a new postoperative neurological deficit. This finding calls the utility of combined MEP/SSEP ION for anterior cervical spine surgery into question, as this modality may be too sensitive in detecting subclinical ION amplitude loss that may not necessarily manifest as a new postoperative neurologic deficit. This observation likely explains the findings in our study that shows that among cases that utilized ION, there was a steady decrease in the combined use of SSEPs and MEPs over the duration of the study period from 34.7% (in 2007) to 12.7% (in 2014).
The overall incidence of neurological injuries after ACDF surgery observed in this study is 0.27%, which is within the range of 0% to 4% reported in the literature.5,13,14,16,17 In this study, the use of ION did not decrease the rate of postoperative neurologic deficits. Similarly, Bose et al15 and Cole et al18 showed no difference in rate of neurological injury after ACDF with or without ION. These findings of low risk of neurological injuries after ACDF and the lack of consistent data demonstrating decreased rate of neurological injuries with ION further calls into question the utility of routine ION for ACDFs. In 2009, Resnick et al2 published a (class II) clinical guideline stating that although ION may aide in diagnosing potential neurological injuries, these intraoperative alerts may be subclinical and may not necessarily prevent neurological injuries and improve outcomes. It is interesting to note that the publication of this guideline in 2009 correlates with the steady decline of ION for ACDFs noted in this study from 2011 to 2014. This finding further highlights the importance of conducting large administrative national database studies, as it may serve as a way to evaluate the impact of clinical guidelines on national practice patterns.
Regional variation was observed in the use of ION for ACDFs, which is consistent with other published studies.13,18 However, this is the first study to report on age-related variation in the utility of ION. Some of the factors that may be responsible for these findings include availability of ION, concerns about the cost of ION, as well as litigation and medical malpractice claims in various parts of the United States.
LIMITATIONS
Although the Pearldiver database has been successfully utilized in the past to address research questions in a variety of orthopedic subspecialties, there are some limitations inherent in using this administrative database. Important clinical information such as disease severity, intraoperative events, surgery complexity, and neuromonitoring sensitivity, specificity, false-positive, and false-negative rates are not maintained in this database and therefore could not be assessed in this study. In addition, information on type and severity of neurological complications (i.e., spinal cord vs. nerve root injury, complete vs. incomplete neurological injury, transient vs. permanent neurological injury) are not recorded in this database. Selection bias may also have affected the validity of the results in this study, as a privately insured patient population may have inherent differences compared with patients with nonprivate insurance such as Medicare or Medicaid. To the best of our knowledge, there are no studies linking insurance status on ION availability and/or utility. Lastly, as with most studies utilizing large population-based administrative database, the accuracy of coding diagnosis and procedures by a medical coder ultimately depends on the coder’s training and experience and the quality of documentation by the physician. Despite these limitations, we believe that this study is valuable because of its large sample size and homogenous sample of ACDFs specifically for spondylotic myelopathy/radiculopathy. In addition, a large administrative national database study provides national trends for ACDF surgeries in the United States.
CONCLUSION
Using a large administrative database of privately insured patients, we found that only 17.1% of ACDFs for spondylotic radiculopathy/myelopathy in the United States are done with ION and there was a decline in the use ION over the past several years. Use of ION did not further prevent postoperative neurological complications for ACDFs as compared with the cases without ION. This study adds information to the growing body of literature that questions the value of ION for “low-risk” surgeries such as ACDF.
Supplementary Material
Key Points.
-
□
Controversy exists in the utility of routine use of intraoperative neuromonitoring (ION) for anterior cervical discectomy and fusion (ACDF) for degenerative conditions without deformity.
-
□
On a national level, there has been a significant decrease in the use of ION for ACDF.
-
□
The overall risk of neurological complications after ACDF is low (<1%).
-
□
The use of ION did not further prevent postoperative neurological complications for ACDFs as compared with the cases without ION.
-
□
The utility of routine ION for ACDFs is questionable.
Acknowledgments
No funds were received in support of this work.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
No relevant financial disclosures are associated with this work.
Supplemental digital content is available for this article. Direct URL citations appearing in the printed text are provided in the HTML and PDF version of this article on the journal’s Web site (www.spinejournal.com).
References
- 1.Magit DP, Hilibrand AS, Kirk J, et al. Questionnaire study of neuromonitoring availability and usage for spine surgery. J Spinal Disord Tech. 2007;20:282–9. doi: 10.1097/01.bsd.0000211286.98895.ea. [DOI] [PubMed] [Google Scholar]
- 2.Resnick DK, Anderson PA, Kaiser MG, et al. Electrophysiological monitoring during surgery for cervical degenerative myelopathy and radiculopathy. J Neurosurg Spine. 2009;11:245–52. doi: 10.3171/2009.2.SPINE08730. [DOI] [PubMed] [Google Scholar]
- 3.Engler GL, Spielholz NJ, Bernhard WN, et al. Somatosensory evoked potentials during Harrington instrumentation for scoliosis. J Bone Joint Surg Am. 1978;60:528–32. [PubMed] [Google Scholar]
- 4.Epstein NE, Danto J, Nardi D. Evaluation of intraoperative somatosensory-evoked potential monitoring during 100 cervical operations. Spine. 1993;18:737–47. doi: 10.1097/00007632-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Taunt CJ, Jr, Sidhu KS, Andrew SA. Somatosensory evoked potential monitoring during anterior cervical discectomy and fusion. Spine. 2005;30:1970–2. doi: 10.1097/01.brs.0000176321.02963.72. [DOI] [PubMed] [Google Scholar]
- 6.Traynelis VC, Abode-Iyamah KO, Leick KM, et al. Cervical decompression and reconstruction without intraoperative neurophysiological monitoring. J Neurosurg Spine. 2012;16:107–13. doi: 10.3171/2011.10.SPINE11199. [DOI] [PubMed] [Google Scholar]
- 7.Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res. 1973:173–8. doi: 10.1097/00003086-197306000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Dinner DS, Luders H, Lesser RP, et al. Intraoperative spinal somatosensory evoked potential monitoring. J Neurosurg. 1986;65:807–14. doi: 10.3171/jns.1986.65.6.0807. [DOI] [PubMed] [Google Scholar]
- 9.Bieber E, Tolo V, Uematsu S. Spinal cord monitoring during posterior spinal instrumentation and fusion. Clin Orthop Relat Res. 1988:121–4. [PubMed] [Google Scholar]
- 10.Jones SJ, Edgar MA, Ransford AO, Thomas NP. A system for the electrophysiological monitoring of the spinal cord during operations for scoliosis. J Bone Joint Surg Br. 1983;65:134–9. doi: 10.1302/0301-620X.65B2.6826615. [DOI] [PubMed] [Google Scholar]
- 11.Brown RH, Nash CL, Jr, Berilla JA, Amaddio MD. Cortical evoked potential monitoring. A system for intraoperative monitoring of spinal cord function. Spine. 1984;9:256–61. [PubMed] [Google Scholar]
- 12.Dawson EG, Sherman JE, Kanim LE, Nuwer MR. Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine. 1991;16(8 Suppl):S361–364. [PubMed] [Google Scholar]
- 13.James WS, Rughani AI, Dumont TM. A socioeconomic analysis of intraoperative neurophysiological monitoring during spine surgery: national use, regional variation, and patient outcomes. Neurosurg Focus. 2014;37:E10. doi: 10.3171/2014.8.FOCUS14449. [DOI] [PubMed] [Google Scholar]
- 14.Smith PN, Balzer JR, Khan MH, et al. Intraoperative somatosensory evoked potential monitoring during anterior cervical discectomy and fusion in nonmyelopathic patients: a review of 1,039 cases. Spine J. 2007;7:83–7. doi: 10.1016/j.spinee.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Bose B, Sestokas AK, Schwartz DM. Neurophysiological monitoring of spinal cord function during instrumented anterior cervical fusion. Spine J. 2004;4:202–7. doi: 10.1016/j.spinee.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Hilibrand AS, Lim MR, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine. 2006;31:1916–22. doi: 10.1097/01.brs.0000228724.01795.a2. [DOI] [PubMed] [Google Scholar]
- 17.Flynn TB. Neurologic complications of anterior cervical interbody fusion. Spine. 1982;7:536–9. doi: 10.1097/00007632-198211000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Cole T, Veeravagu A, Zhang M, et al. Intraoperative neuromonitoring in single-level spinal procedures: a retrospective propensity score-matched analysis in a national longitudinal database. Spine. 2014;39:1950–9. doi: 10.1097/BRS.0000000000000593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.