Abstract
Background
Role of myeloperoxidase in chronic kidney disease (CKD) and its association with Coronary Artery Disease (CAD) is controversial. In this study, we compared myeloperoxidase and protein-bound 3-chlorotyrosine (ClY) levels in subjects with varying degrees of CKD and tested their associations with CAD.
Methods
From Clinical Phenotyping Resource and Biobank Core 111 patients were selected from CKD stage 1 to 5. Plasma myeloperoxidase level was measured using enzyme-linked-immunosorbent assay. Plasma protein-bound 3-ClY, a specific product of hypochlorous acid generated by myeloperoxidase was measured by liquid chromatography mass spectrometry.
Results
We selected 29, 20, 24, 22, and 16 patients from stage 1 to 5 CKD, respectively. In a sex adjusted general linear model, mean±SD of myeloperoxidase levels decreased from 18.1±12.3 pmol in stage 1 to 10.9±4.7 pmol in stage 5 (p=0.011). In patients with and without CAD the levels were 19.1±10.1 and 14.8±8.7 pmol (p=0.036). There was an increase in 3-ClY mean from 0.81±0.36 mmol/mol-tyrosine in stage 1 to 1.42±0.41 mmol/mol-tyrosine in stage 5 (p<0.001). Mean 3-ClY levels in patients with and without CAD were 1.25±0.44 and 1.04±0.42 mmol/mol-tyrosine (p=0.023). C-statistic of ClY when added to myeloperoxidase level to predict CKD stage 5 was 0.86 as compared to 0.79 for the myeloperoxidase level alone (p=0.0097).
Conclusion
Myeloperoxidase levels decrease from stage 1 to 5, whereas activity increases. In contrast, both myeloperoxidase and ClY levels rise in presence of CAD at various stages of CKD. Measuring both plasma myeloperoxidase and 3-CLY levels provide added value to determine burden of myeloperoxidase-mediated oxidative stress.
Keywords: Oxidative stress, Chronic renal failure, Cardiovascular, biomarkers, Inflammation
Introduction
Growing body of evidence reveals higher rate of cardiovascular morbidity and mortality in patients with chronic kidney disease (CKD) [1–8]. Although the traditional risk factors are prevalent in CKD patients, they cannot entirely explain the rate and risk of cardiovascular outcomes in CKD [9]. Atherosclerosis is an inflammatory disorder characterized by oxidative stress which leads to CAD [10,11]. This is likely of particular importance in diseases such as CKD where chronic inflammation initiates and propagates further oxidative stress. Myeloperoxidase, a phagocytic heme enzyme, is a major source of reactive oxidants in the human vasculature [11], and has been widely implicated in the development of atherosclerotic lesions. In an initial large epidemiologic study, the level of plasma myeloperoxidase was a strong independent predictor of CAD [12], and recent clinical studies have both supported and refuted the strength of this association [13–15]. Further, myeloperoxidase has been localized to atherosclerotic plaques, and oxidants produced by myeloperoxidase activate protease cascades and plaque rupture [16,17]. The mechanisms linking myeloperoxidase and CKD-atherosclerosis are incompletely defined, and how plasma levels of myeloperoxidase link with its activity are not entirely understood.
Myeloperoxidase is a highly abundant iron containing heme protein that is encoded by 14Kb gene located on the chromosome 17q23.1 [18]. The proteolytic form of the enzyme is 146 kDa and is most abundant in phagocytic cells [19,20]. Myeloperoxidase uses H2O2 and chloride to generate the powerful oxidant hypochlorous acid (HOCl).
The importance of this reaction is underscored by the presence of enzymatically active myeloperoxidase in human atherosclerotic lesions [21]. Although most oxidation products generated by HOCl are either nonspecific or yield uninformative compounds, in vitro and in vivo human and model system studies demonstrate that myeloperoxidase converts tyrosine into 3-ClY, a stable product [22–30]. Cumulative body of evidence suggests a higher rate of adverse cardiovascular outcomes in association with higher plasma levels of myeloperoxidase in non-CKD patient population which may reveal a role for its prognostic value in risk stratification [12,31,32]. In a prospective cohort of 885 selective coronary artery angiography followed for 13 years for cardiovascular mortality outcome, elevation of myeloperoxidase was associated with 5.3-fold higher mortality risk [31]. In a case-control observation nested in the European Prospective Investigation into Cancer and Nutrition study with 8 years of follow up, elevated myeloperoxidase levels predicted risk of future CAD in healthy individuals [32]. In another case-control study comparing angiography proven established CAD with individuals without CAD, both leukocyte and blood myeloperoxidase levels were higher in presence of CAD [12].
CKD is associated with substantial fatal and non-fatal cardiovascular morbidities, impacting people with and without previously known clinical cardiovascular diseases [33]. Importantly, as CKD severity increases, CAD risk markedly increases. It is unclear, if myeloperoxidase activity and level could in part explain the added cardiovascular burden in advancing CKD, as the association of myeloperoxidase level with different stages of CKD has not been systematically investigated. Although, studies suggest higher rate of mortality and CV outcomes in association with higher myeloperoxidase levels in dialysis patients [34,35], other studies show normal or even decreasing level of myeloperoxidase at higher stages of CKD [36,37]. Such a discrepancy in myeloperoxidase distribution by CKD stage may be a reflection of dissociation between myeloperoxidase level and its activity in non-dialysis CKD patients. We hypothesized that myeloperoxidase-specific protein-bound 3-ClY, a specific marker of myeloperoxidase activity, would predict severity of CKD and CAD risk better than myeloperoxidase levels alone. To our knowledge, such a study has not been conducted in a human CKD population.
Materials and Methods
Patients
This study utilizes the Clinical Phenotyping Resource and Biobank Core (CPROBE) of the George M. O’Brien Translational Core Center. CPROBE is a multicenter observational cohort of adult and pediatric participants with CKD stages 1 to 5 recruited from the University of Michigan (Ann Arbor, MI), Renaissance Renal Research Institute (Detroit, MI), Wayne State University (Detroit, MI), John H Stroger Hospital (Chicago, IL), Temple University (Philadelphia, PA), and Levine Children’s Hospital (Charlotte, NC). Patients’ demographic, socioeconomic and clinical information are collected, along with blood and urine biosamples, at enrollment and annually, thereafter. Biosamples, stored in a centralized biobank, are available for translational research. Overall, 111 C-PROBE participants with available clinical data and serum samples, and frequency matched by race were selected from 5 stages of CKD. Presence of CAD was defined as previous history of unstable angina, acute myocardial infarction or history of revascularization with angioplasty, stenting or coronary artery bypass grafting. Baseline clinical and laboratory data were used. CKD-EPI formula was used to calculate estimated glomerular filtration rate (eGFR). Stored plasma samples from enrolled patients were obtained for myeloperoxidase and oxidized amino acid measurements.
MPO Measurement
Myeloperoxidase was quantified in plasma samples with a commercial sandwich enzyme-linked immunosorbent assay (ELISA) kit (Biolegend, San Diego, California). All samples were run in duplicate and sample average value is expressed as pmol plasma.
Mass Spectrometry Analysis
Protein-bound 3-ClY a marker for myeloperoxidase activity was measured by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) as described previously [24]. Plasma protein was precipitated with ice-cold trichloroacetic acid (10% vol/vol) and diluted in 50mM phosphate buffer pH7.4. The protein precipitate was delipidated with water/methanol/water-washed diethyl ether (1:3:7; vol/vol/vol). Known concentrations of isotopically labeled internal standards 13C6 tyrosine, 13C915N1tyrosine, and 13C6 3-chlorotyroine were added, and samples were hydrolyzed for 24hrs in 4 N methane sulfonic acid treated with benzoic acid. Following extraction with Supelclean ENVI ChromP columns (3ml, Supelco Inc., Bellefonte, PA), ClY levels were quantified by LC-ESI-MS/MS with multiple reaction monitoring (MRM) positive ion acquisition mode as described previously utilizing an Agilent-6410 triple-quadrupole MS system equipped with an Agilent 1200 LC system [38,39]. Labeled precursor amino acid, 13C915N1tyrosine, was added to monitor potential internal artifact formation of 3-ClY and was noted to be negligible. ClY levels were normalized for precursor amino acid tyrosine and expressed as ClY mmol/mol Tyrosine.
Other laboratory values
For measuring plasma lipid profile Daytona chemistry analyzer series were used with the following details; for total plasma cholesterol enzymatic assay using Randox RX Series CH-3810; for total plasma triglyceride GPO-PAP method using RX Series TR-3823; for plasma LDL and HDL a two-step process including elimination of chylomicron and other lipoproteins followed by quantification using Randox RX Series CH-3841 and CH-3811, respectively. Colorimetric method was applied to measure serum creatinine, serum albumin (using bromocresol green), and urine protein (using pyrogallol red). WBC in blood was measured by Sysmex XN9000.
Quality Control
The myeloperoxidase levels were measured in duplicate and the overall intra-assay coefficient of variation (CV) was 2.47%. For ClY measurement and to assess the drift in mass spectrometer over time a pooled plasma sample was run at the beginning and then after run of every 10 samples. The intra-assay CV of MS spectra for tyrosine and ClY were 8.96% and 9.74%, respectively.
Statistical Analysis
Independent t-test and analysis of variance (ANOVA) were used to compare mean of continuous variables in 2 and more than 2 groups, respectively. Distribution of categorical variables by stages of CKD and CAD were tested using chi-square. Analysis of covariance (ANCOVA) was applied to evaluate the effect of covariates on mean of myeloperoxidase and ClY levels by stages of CKD, as well as by presence of CAD across stages of CKD. Logistic regression analysis was applied to estimate risk of CAD by change in myeloperoxidase and ClY levels in adjusted models. Probabilistic scores of myeloperoxidase and ClY levels and their combination to predict CAD and CKD stage 5 were calculated using logistic regression models. We generated receiver operating characteristic curves to predict CAD and CKD stage 5 from the aforementioned probabilistic scores and compared the corresponding c-statistic with each other. Study has 89% power to detect 8 pmol myeloperoxidase difference with SD=7 at alpha=0.05, using a two-sided ANOVA test. Similarly, study has over 95% power to detect 0.6 mmol/mol tyrosine difference in ClY level with SD=0.6 at alpha=0.05, using a two-sided ANOVA test.
Results
Baseline Characteristics
Overall 111 patients from different stages of CKD were selected, including 29, 20, 24, 22, and 16 patients from stage 1 to stage 5 of CKD, respectively. This participant sample had a mean age was 53 years (SD=19 and 32% were Black or African-Americans. Etiology of CKD was glomerular diseases in 46 patients (41.4%), hypertension in 21 (18.9%), diabetes in 19 (17.1%), and other etiologies in 25 patients (22.5%). The distribution of characteristics of patients by stage of CKD is shown in Table 1. There was a significant increase in the linear trend of age, systolic blood pressure, male gender, diabetes, hypertension, and use of aspirin, statins, and calcium channel blockers from stage 1 to stage 5 of CKD (p<0.05). Table 2 compares the characteristics of the patients by presence of CAD. Participants with CAD on average were older, had higher systolic blood pressure but lower eGFR, were more likely to have hypertension and heart failure, and had a higher percentage use of aspirin, statins, and calcium channel blockers (p<0.05).
Table 1.
Distribution of patients’ characteristics by stage of chronic kidney disease at the time of sampling
| GFR≥90 | GFR 60–89 | GFR 30–59 | GFR 15–29 | GFR<15 | |
|---|---|---|---|---|---|
| N | 29 | 20 | 24 | 22 | 16 |
| Age*** | 35 ± 13 | 50 ± 17 | 63 ± 13 | 61 ± 14 | 63 ± 18 |
| Male sex (%)* | 6 (20.7) | 10 (50.0) | 9 (37.5) | 11 (50.0) | 8 (50.0) |
| White race (%) | 20 (69.0) | 13 (65.0) | 17 (70.8) | 15 (68.2) | 10 (62.5) |
| SBP (mmHg)** | 125.8 ± 18.6 | 137.3 ± 24.0 | 125.4 ± 21.6 | 144.4 ± 21.6 | 143.3 ± 24.2 |
| DBP (mmHg) | 75.2 ± 12 | 78.9 ± 10.3 | 70.8 ± 11.0 | 81.4 ± 15.7 | 76.4 ± 12.9 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Weight (kg) | 82 ± 27 | 85 ± 22 | 91 ± 25 | 88 ± 21 | 87 ± 13 |
| BMI (kg/m2) | 29 ± 8 | 29 ± 8 | 34 ± 10 | 32 ± 7 | 30 ± 4 |
| Comorbidities | |||||
| Hypertension (%)*** | 16 (55.2) | 15 (75.0) | 18 (75.0) | 20 (90.9) | 16 (100) |
| Diabetes (%)** | 6 (20.7) | 5 (25.0) | 9 (37.5) | 11 (50.0) | 8 (50.0) |
| CAD (%) | 2 (6.9) | 4 (20.0) | 6 (25.0) | 8 (36.4) | 3 (18.8) |
| Heart Failure (%) | 1 (3.4) | 1 (5.0) | 3 (12.5) | 3 (1.63) | 2 (12.5) |
| PAD (%) | 1 (3.4) | 1 (5.0) | 1 (4.2) | 4 (18.2) | 2 (12.5) |
| Medications | |||||
| Aspirin (%)*** | 4 (13.8) | 6 (30.0) | 7 (29.2) | 13 (59.1) | 8 (50.0) |
| Statins (%)* | 10 (34.5) | 7 (35.0) | 13 (54.2) | 15 (68.2) | 9 (56.3) |
| ACEI/ARB (%) | 21 (72.4) | 14 (70.0) | 16 (66.7) | 16 (72.7) | 7 (43.8) |
| Ca-Blocker (%)*** | 3 (10.3) | 5 (25.0) | 10 (41.7) | 15 (68.2) | 9 (56.3) |
| Albumin (g/dL) | 3.6 ± 0.7 | 4.2 ± 0.5 | 4.0 ± 0.4 | 4.1 ± 0.3 | 3.8 ± 0.4 |
| Cholesterol (mg/dL) | 177 ± 41 | 159 ± 44 | 169 ± 53 | 162 ± 53 | 146 ± 34 |
| LDL (mg/dL) | 97 ± 36 | 80 ± 37 | 86 ± 42 | 80 ± 37 | 72 ± 23 |
| HDL (mg/dL) | 41 ± 15 | 38 ± 18 | 43 ± 19 | 35 ± 19 | 39 ± 20 |
| Triglycerides (mg/dL) | 170 ± 80 | 129 ± 68 | 125 ± 60 | 158 ± 72 | 140 ± 121 |
| UPCR† | 1.6 [0.7–1.9] | 1.2 [0.4–1.3] | 0.8 [0.1–1.4] | 1.4[0.2–1.5] | 2.3[1.2–3.3] |
| WBC (1000/μL) | 7.7 ± 4.0 | 5.9 ± 1.5 | 6.8 ± 2.2 | 8.0 ± 4.8 | 6.8 ± 1.9 |
| Creatinine (mg/dL)*** | 0.7 ± 0.1 | 1.2 ± 0.3 | 1.7± 0.5 | 2.7 ± 0.4 | 5.1 ± 1.8 |
| eGFR (mL/min)*** | 113 ± 12 | 75 ± 7 | 42 ± 9 | 23 ± 3 | 11 ± 3 |
P value of linear trend:
<0.05,
<0.01,
≤0.001.
values are median and interquartile range
Table 2.
Distribution of patients’ characteristics by stage of coronary artery disease at the time of sampling
| Without CAD | With CAD | |
|---|---|---|
| N | 88 | 23 |
| Age*** | 49 ± 18 | 67 ± 13 |
| Male sex (%) | 33 (37.5%) | 11 (47.8%) |
| White race (%) | 59 (67.0%) | 16 (69.6%) |
| SBP (mmHg)* | 131.2 ± 22.1 | 143.9 ± 23.1 |
| DBP (mmHg) | 76.2 ± 12.7 | 76.7 ± 13.3 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Weight (kg) | 87 ± 24 | 83 ± 19 |
| BMI (kg/m2) | 31 ± 8 | 29 ± 6 |
| Comorbidities | ||
| Hypertension (%)* | 63 (71.6) | 22 (95.7) |
| Diabetes (%) | 31 (35.2) | 8 (34.8) |
| Heart Failure (%)** | 4 (4.5) | 6 (26.1) |
| PAD (%) | 5 (5.7) | 4 (17.4) |
| Medications | ||
| Aspirin (%)*** | 23 (26.1) | 15 (65.2) |
| Statins (%)*** | 35 (39.8) | 19 (82.6) |
| ACEI/ARB (%) | 56 (63.6) | 18 (78.3) |
| Ca-Blocker (%)* | 29 (33.0) | 13 (56.5) |
| Serum albumin (g/dL) | 3.9 ± 0.5 | 4.0 ± 0.5 |
| Plasma total cholesterol (mg/dL) | 168 ± 46 | 152 ± 46 |
| Plasma LDL (mg/dL) | 87 ± 38 | 73 ± 31 |
| Plasma HDL (mg/dL) | 40 ± 18 | 39 ± 17 |
| Plasma Triglycerides (mg/dL) | 150 ± 82 | 131 ± 75 |
| UPCR† | 1.4 [0.3–2.3] | 1.3 [0.4–1.5] |
| WBC (1000/μL) | 7.0 ± 3.3 | 7.8 ± 3.2 |
| Serum creatinine (mg/dL) | 2.0 ± 1.7 | 2.2 ± 1.2 |
| eGFR (mL/min)** | 63 ± 40 | 42 ± 30 |
P value of linear trend:
<0.05,
<0.01,
≤0.001.
values are median and interquartile range
MPO levels by CKD and CAD
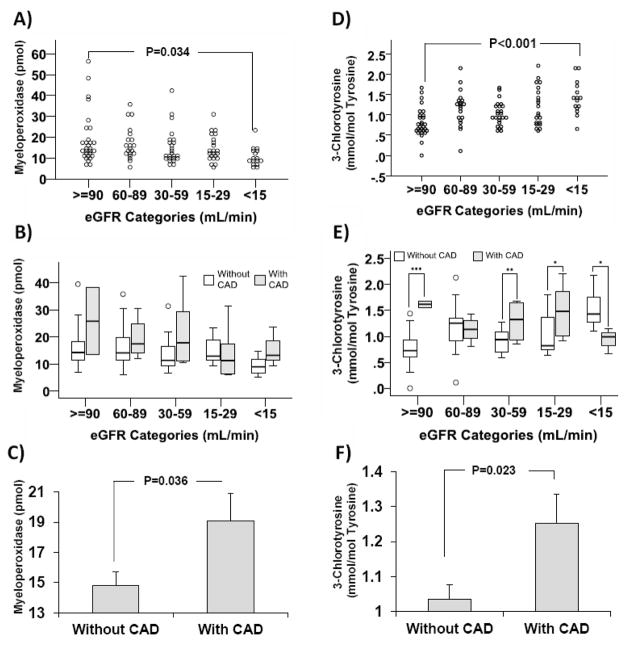
Figure 1A shows the unadjusted distribution of myeloperoxidase levels measured by ELISA according to CKD stage. Surprisingly, there is a significant decline in linear trend of myeloperoxidase from CKD stage 1 to stage 5 (p=0.003). The unadjusted mean±SD of myeloperoxidase decreased from 18.8±12.3 pmol in stage 1 to 10.5±4.7 pmol in stage 5 (p=0.034). After adjusting for the differentially distributed covariates at baseline (Table 1), the adjusted values remained similar to unadjusted values with the mean±SD of myeloperoxidase of 18.1±12.3 pmol at stage 1 and 10.9±4.7 pmol at stage 5, (p=0.011). Figure 1B shows that within each stage of CKD, participants with CAD had a trend toward higher myeloperoxidase levels, although the difference did not reach statistical significance within each CKD stage. However, Figure 1C shows that overall the CKD adjusted mean±SD of myeloperoxidase in patients with CAD was 19.1±10.1 which was significantly higher as compared to 14.8±8.7 pmol in patients without CAD (p=0.036). Logistic regression model revealed that each 10 pmol increase in myeloperoxidase was associated with 1.91 fold (95% CI: 1.09–3.36, p=0.025) higher odds of association with CAD, after adjusting for eGFR, diabetes, hypertension, gender, use of aspirin, calcium channel blockers, and statins. No difference was observed in myeloperoxidase level by diabetes status.
Figure 1.
Panel A to C show comparison of myeloperoxidase (MPO) concentration determined by enzyme linked immune assay (ELISA) by CKD stage and CAD status: A) Unadjusted MPO by CKD stage, B) Adjusted MPO distribution by presence of CAD across CKD stages, C) CKD adjusted mean myeloperoxidase by status of CAD. Panel D to F show comparison of levels of plasma protein-bound 3-chlorotyrosine (ClY) determined by LC/MS by CKD stage and CAD status: D) Unadjusted ClY by CKD stage, E) Adjusted ClY distribution by presence of CAD across CKD stage, F) CKD adjusted mean ClY by status of CAD. Analysis of Panels A and D applied Analysis of Variance and analysis of panels B, C, E, and F applied analysis of covariance adjusting for the corresponding covariate. The box plots represent median and interquartile ranges, the whiskers represent range and the dots above and below the whiskers show the outliers located 1.5 times above the third quartile or below the first quartile, respectively. Bars are mean plus standard error. *p<0.05, **p=0.008, ***p<0.001
ClY by CKD and CAD
In contrast to myeloperoxidase levels, there was a significant increase in linear trend of ClY from stage 1 to stage 5 CKD (p<0.001; Figure 1D). The adjusted mean±SD of ClY increased from 0.81±0.36 mmol/mol tyrosine in stage 1 to 1.41±0.41 mmol/mol tyrosine in stage 5 (p<0.001). Figure 1E shows that ClY level in patients with CAD was higher than those without CAD in CKD stage 1, 3 and 4 (p≤0.031). Similarly, Figure 1F shows that after multivariable adjustment (including CKD stages), the overall ClY level in patients with and without CAD was 1.25±0.44 and 1.04±0.42 mmol/mol tyrosine (p=0.023), respectively. Logistic regression model revealed that increase of each 1 mmol of ClY/mol tyrosine was associated with 3.83 fold (95% CI: 1.09–13.47, p=0.026) higher odds of association with CAD, after adjusting for eGFR, diabetes, hypertension, gender, use of aspirin, calcium channel blockers, and statins. No difference was observed in ClY level by diabetes status.
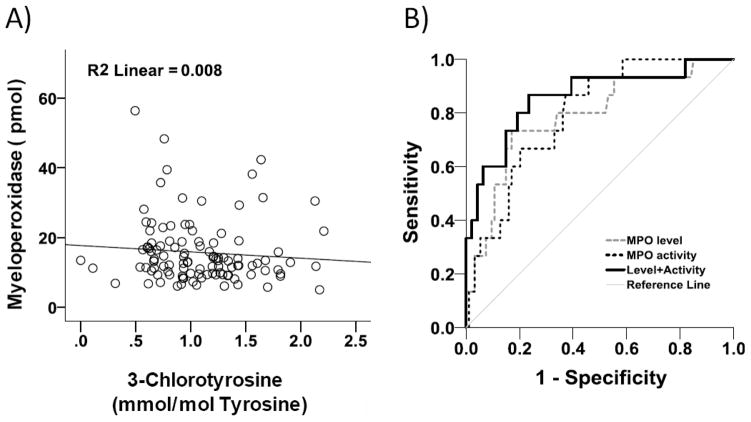
Relation between myeloperoxidase level and activity
Figure 2A shows that there was no significant relationship between myeloperoxidase and ClY level in CKD. Figure 2B shows that the c-statistic (SE) of myeloperoxidase level to predict CKD stage 5 was 0.788 (0.07), for ClY level this measure was 0.796 (0.05), and for their combination when added together it was 0.860 (0.06), showing a significantly higher level for their combination as compared to each individual measure (p=0.0097). The c-statistic (SE) of myeloperoxidase level to predict CAD was 0.687 (0.06), for ClY level this measure was 0.727 (0.05), and for their combination it was 0.727 (0.05) (not statistically different).
Figure 2.
A) Correlation of myeloperoxidase (MPO) and 3-chlorotyrosine (ClY) level, B) Comparing the area under receiver operating characteristic curves reveals a significantly higher c-statistic for myeloperoxidase activity and level combined as compared to each individual measure to predict CKD stage 5 (p=0.0097).
Discussion
In this study we observed a graded decrease in crude and adjusted myeloperoxidase level by stage of CKD. In contrast ClY level increased with increasing severity of CKD suggesting a discrepancy between the two measures in CKD subjects. Myeloperoxidase and ClY levels were higher in patients with a prior history of CAD.
Increased risk of mortality and CV outcomes in end stage kidney disease (ESKD) patients is shown in previous studies [34,35]. In an observation of 356 hemodialysis patients Kalantar-Zadeh et al showed that the highest tertile of myeloperoxidase was associated with 82% higher risk of mortality as compared to the middle tertile [34]. In another study in ESKD patients on peritoneal dialysis, Wang et al showed that doubling of myeloperoxidase was associated with 46% and 60% higher adjusted risk of mortality and cardiovascular events, respectively [35]. On the other hand, controversial reports on myeloperoxidase level at milder stages of CKD have raised question about its prognostic value at milder stages of CKD. In an observation of 41 non-dialysis CKD patients, Caimi et al did not see any significant association between myeloperoxidase circulating levels and serum creatinine or GFR [37]. In another observation of 100 patients at different stages of CKD, Madhusudhana et al reported even a graded decrease in plasma myeloperoxidase from stage 1 to stage 5 [36]. This is in contrast with the report of Capeillere-Blandin et al who showed a significantly higher level of myeloperoxidase in ESKD patients on hemodialysis when compared to non-dialysis CKD patients [40]. Increase in myeloperoxidase after hemodialysis [41], its differential regulation by hemodialysis membrane type [42,43], duration of dialysis, and variation in background variables such as anthropometric measures [44], comorbidities and medication use suggest their confounding rules in myeloperoxidase level [45]. The change in 3-ClY is less frequently studied in CKD. In a case-control observation, Himmelfarb et al matched five hemodialysis patients by age and sex with five healthy controls [46]. While the oxidized amino acid was detected in 4 out of 5 dialysis patients, the level was undetectable in healthy controls [46]. Our findings can align with this conflicting literature in that while the myeloperoxidase level was lower in our patients with more advanced CKD compatible with Madhusudhana’s report, the ClY level increased by worsening of CKD stage and was independently associated with CAD.
In our study, myeloperoxidase and ClY levels were discordant raising concern of measuring myeloperoxidase levels alone in isolation. This discrepancy might indicate that the plasma myeloperoxidase levels may not fully reflect myeloperoxidase burden. Lack of significant inverse correlation between the two measures indicates that the increased ClY level is not simply from shift of myeloperoxidase utilization. This raises potential additional mechanisms. Myeloperoxidase deficiency is categorized in to two forms including primary or congenital, and secondary or acquired [47]. Among the acquired etiologies of myeloperoxidase deficiency, diabetes mellitus, iron deficiency, thrombotic diseases, kidney transplantation, severe infectious diseases, neuronal lipofuscinosis, lead intoxication, hematological malignancies, metastatic cancers, medications (including cytotoxic agents and anti-inflammatory compounds), and pregnancy are reported [47]. CKD is characterized by a constellation of the several of the above mentioned comorbidities and complications and therefore may lead to lower MPO plasma levels. However, the mechanism of reduction of circulating MPO remains unclear in these secondary states. ClY level, on the other hand may reflect overall myeloperoxidase burden more accurately. Extravascular myeloperoxidase (derived from macrophages and inflammatory cells in sites of inflammation including vasculature) may participate in oxidation reactions but may not contribute to plasma myeloperoxidase. We previously reported that high density lipoprotein (HDL) 3-ClY content from atheroma was substantially higher than those in plasma-derived HDL in subjects with CVD [29,30]. In recent studies, we also showed that myeloperoxidase levels and activity in plasma might be discordant in subjects with rheumatoid arthritis and lupus suggesting myeloperoxidase may be modifying plasma proteins in plasma and in extravascular sites such as inflamed tissue or vasculature [25,26]. Furthermore, in this study we show that myeloperoxidase and ClY together predict the severity of CKD better than each of the individual measures alone. Although the c-statistic of myeloperoxidase activity or its combination with level to predict CAD was higher than the c-statistic of myeloperoxidase alone the difference did not reach statistical significance. Altogether, these observations support the hypothesis that myeloperoxidase oxidation markers of circulating plasma proteins may reflect added myeloperoxidase burden. Thus a combined measurement of both myeloperoxidase and ClY levels might serve as mechanistic markers of CKD severity and CAD risk in this high risk population.
This study has several strengths. We took the advantage of existing well-characterized CKD patients in CPROBE cohort which allowed correlating the biomarkers with the phenotypes. This is also one of the largest studies in CKD patients investigating not only myeloperoxidase level but also its stable end-product ClY. In fact, this approach has revealed the association of ClY with CAD despite decrease of myeloperoxidase level by CKD stage which was viewed as a controversial matter. This study is adequately powered to test the hypothesis. This study also has limitations. The utility of myeloperoxidase and ClY as a diagnostic or predictive biomarker in clinical practice is not ascertained by this work, as we have not compared classification power of MPO or ClY with well-established traditional biomarkers in clinical practice to diagnose CKD or predict its progression or CVD outcomes. The significance of our findings is in providing further insight on the inflammatory mechanism of CKD and highlighting a potential target for future studies. By design, this is a cross-sectional observation which does not establish causality. Although, it is possible that there may be unmeasured residual confounders in the cohort, it is our assessment that the strongest indicator of health status in the cohort is CKD stage which is already used for adjustment, and therefore potential for further residual confounders to substantially alter the observed relationships is low. As this was not a longitudinal study the predictive power of myeloperoxidase and ClY to estimate mortality outcome was not achieved. We would also like to highlight that the samples used for this study are 7 years old, which in our assessment is within the time frame of preserved stability given the minimum number of freeze-thaw cycles. In addition, measurement of protein-bound ClY reflects an indirect measurement of myeloperoxidase activity. Although there are very few human enzymes able to produce the oxidant HOCl, we recognize that this study doesn’t provide a directly measurement of myeloperoxidase activation. Another limitation is that CAD was based on known cases and there may be some undiagnosed subclinical disease. Finally, the conclusion drawn in this study are specific to the patients included in this cohort and we are conscious that patient with different comorbidities and/or causes of CKD could have a different myeloperoxidase profile. Whether or not myeloperoxidase inhibitors may alter the CVD outcome and impact survival requires would be in the scope of further future studies.
In conclusion, this study provides evidence for increase of ClY level by worsening stage of CKD, despite decrease in level of myeloperoxidase at advanced CKD stage, showing its added value beyond myeloperoxidase level to predict severity of CKD. It also provides evidence for independent association of myeloperoxidase and ClY levels with CAD in CKD patients. Our results confirm the hypothesis that ClY levels correlate with CKD stage and known CAD in this vulnerable population and raise the possibility that measurement of ClY, rather than myeloperoxidase levels might serve as a mechanistic marker for CKD progression and CAD risk.
Acknowledgments
Support: Supported in part by AstraZeneca. Supported by the NIH grants DK106523 (FA), DK089503, DK082841, DK081943, P30DK020572, and DK097153 (SP)
Footnotes
Conflict of Interest: Authors declare that they do not have any conflict of interest. Matthias Kretzler and Subramaniam Pennathur have received a research grant from Astra-Zeneca in partial support of the study presented.
References
- 1.Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. American journal of nephrology. 2008;28:354–360. doi: 10.1159/000111829. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Kasiske B, Herzog C, Chen SC, Everson S, Constantini E, Grimm R, McBean M, Xue J, Chavers B, Matas A, Manning W, Louis T, Pan W, Liu J, Li S, Roberts T, Dalleska F, Snyder J, Ebben J, Frazier E, Sheets D, Johnson R, Li S, Dunning S, Berrini D, Guo H, Solid C, Arko C, Daniels F, Wang X, Forrest B, Gilbertson D, St Peter W, Frederick P, Eggers P, Agodoa L. Excerpts from the United States Renal Data System 2003 Annual Data Report: atlas of end-stage renal disease in the United States. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2003;42:A5–7. S1–230. [PubMed] [Google Scholar]
- 3.Das M, Aronow WS, McClung JA, Belkin RN. Increased prevalence of coronary artery disease, silent myocardial ischemia, complex ventricular arrhythmias, atrial fibrillation, left ventricular hypertrophy, mitral annular calcium, and aortic valve calcium in patients with chronic renal insufficiency. Cardiology in review. 2006;14:14–17. doi: 10.1097/01.crd.0000148162.88296.9f. [DOI] [PubMed] [Google Scholar]
- 4.Di Lullo L, Rivera R, Barbera V, Bellasi A, Cozzolino M, Russo D, De Pascalis A, Banerjee D, Floccari F, Ronco C. Sudden cardiac death and chronic kidney disease: From pathophysiology to treatment strategies. International journal of cardiology. 2016;217:16–27. doi: 10.1016/j.ijcard.2016.04.170. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, Singer DE Investigators AS. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119:1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meisinger C, Doring A, Lowel H, Group KS. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. European heart journal. 2006;27:1245–1250. doi: 10.1093/eurheartj/ehi880. [DOI] [PubMed] [Google Scholar]
- 7.Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, Tsuruya K, Iida M, Kiyohara Y, Sueishi K. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2010;55:21–30. doi: 10.1053/j.ajkd.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Seliger SL, Gillen DL, Longstreth WT, Jr, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney international. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney international. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. Jama. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 13.Baldus S, Rudolph V, Roiss M, Ito WD, Rudolph TK, Eiserich JP, Sydow K, Lau D, Szocs K, Klinke A, Kubala L, Berglund L, Schrepfer S, Deuse T, Haddad M, Risius T, Klemm H, Reichenspurner HC, Meinertz T, Heitzer T. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113:1871–1878. doi: 10.1161/CIRCULATIONAHA.105.590083. [DOI] [PubMed] [Google Scholar]
- 14.Kubala L, Lu G, Baldus S, Berglund L, Eiserich JP. Plasma levels of myeloperoxidase are not elevated in patients with stable coronary artery disease. Clin Chim Acta. 2008;394:59–62. doi: 10.1016/j.cca.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 18.Ray RS, Katyal A. Myeloperoxidase: Bridging the gap in neurodegeneration. Neuroscience and biobehavioral reviews. 2016;68:611–620. doi: 10.1016/j.neubiorev.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff SJ. Myeloperoxidase: friend and foe. Journal of leukocyte biology. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 20.Hansson M, Olsson I, Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Archives of biochemistry and biophysics. 2006;445:214–224. doi: 10.1016/j.abb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. Journal of Clinical Investigation. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domigan NM, Charlton TS, Duncan MW, Winterbourn CC, Kettle AJ. Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. Journal of Biological Chemistry. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Soud HM, Maitra D, Shaeib F, Khan SN, Byun J, Abdulhamid I, Yang Z, Saed GM, Diamond MP, Andreana PR, Pennathur S. Disruption of heme-peptide covalent cross-linking in mammalian peroxidases by hypochlorous acid. J Inorg Biochem. 2014;140:245–254. doi: 10.1016/j.jinorgbio.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennathur S, Vivekanandan-Giri A, Locy ML, Kulkarni T, Zhi D, Zeng L, Byun J, de Andrade JA, Thannickal VJ. Oxidative Modifications of Protein Tyrosyl Residues Are Increased in Plasma of Human Subjects with Interstitial Lung Disease. Am J Respir Crit Care Med. 2016;193:861–868. doi: 10.1164/rccm.201505-0992OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, Gillespie BW, Carmona-Rivera C, Liu X, Subramanian V, Hasni S, Thompson PR, Heinecke JW, Saran R, Pennathur S, Kaplan MJ. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivekanandan-Giri A, Slocum JL, Byun J, Tang C, Sands RL, Gillespie BW, Heinecke JW, Saran R, Kaplan MJ, Pennathur S. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Annals of the rheumatic diseases. 2013;72:1725–1731. doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivekanadan-Giri A, Wang JH, Byun J, Pennathur S. Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Rev Endocr Metab Disord. 2008;9:275–287. doi: 10.1007/s11154-008-9093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O’Brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 31.Heslop CL, Frohlich JJ, Hill JS. Myeloperoxidase and C-reactive protein have combined utility for long-term prediction of cardiovascular mortality after coronary angiography. Journal of the American College of Cardiology. 2010;55:1102–1109. doi: 10.1016/j.jacc.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 32.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. Journal of the American College of Cardiology. 2007;50:159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Go AS. Cardiovascular Disease Consequences of CKD. Seminars in nephrology. 2016;36:293–304. doi: 10.1016/j.semnephrol.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 35.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE. Prognostic value of plasma myeloperoxidase in ESRD patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2010;56:937–946. doi: 10.1053/j.ajkd.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Madhusudhana Rao A, Anand U, Anand CV. Myeloperoxidase in chronic kidney disease. Indian journal of clinical biochemistry: IJCB. 2011;26:28–31. doi: 10.1007/s12291-010-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caimi G, Carollo C, Montana M, Vaccaro F, Lo Presti R. Elastase, myeloperoxidase, nitric oxide metabolites and oxidative status in subjects with clinical stable chronic renal failure on conservative treatment. Clinical hemorheology and microcirculation. 2009;43:253–258. doi: 10.3233/CH-2009-1237. [DOI] [PubMed] [Google Scholar]
- 38.Vivekanandan-Giri A, Slocum JL, Byun J, Tang C, Sands RL, Gillespie BW, Heinecke JW, Saran R, Kaplan MJ, Pennathur S. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivekanandan-Giri A, Byun J, Pennathur S. Quantitative analysis of amino Acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. doi: 10.1016/B978-0-12-385928-0.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capeillere-Blandin C, Gausson V, Nguyen AT, Descamps-Latscha B, Drueke T, Witko-Sarsat V. Respective role of uraemic toxins and myeloperoxidase in the uraemic state. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:1555–1563. doi: 10.1093/ndt/gfl007. [DOI] [PubMed] [Google Scholar]
- 41.Rao AM, Apoorva R, Anand U, Anand CV, Venu G. Effect of Hemodialysis on Plasma Myeloperoxidase Activity in End Stage Renal Disease Patients. Indian journal of clinical biochemistry: IJCB. 2012;27:253–258. doi: 10.1007/s12291-012-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono K, Ueki K, Inose K, Tsuchida A, Yano S, Nojima Y. Plasma levels of myeloperoxidase and elastase are differentially regulated by hemodialysis membranes and anticoagulants. Research communications in molecular pathology and pharmacology. 2000;108:341–349. [PubMed] [Google Scholar]
- 43.Wu CC, Chen JS, Wu WM, Liao TN, Chu P, Lin SH, Chuang CH, Lin YF. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20:1134–1139. doi: 10.1093/ndt/gfh764. [DOI] [PubMed] [Google Scholar]
- 44.Tsai MS, Shaw HM, Li YJ, Lin MT, Lee WT, Chan KS. Myeloperoxidase in chronic kidney disease: role of visceral fat. Nephrology. 2014;19:136–142. doi: 10.1111/nep.12187. [DOI] [PubMed] [Google Scholar]
- 45.Kisic B, Miric D, Dragojevic I, Rasic J, Popovic L. Role of Myeloperoxidase in Patients with Chronic Kidney Disease. Oxidative medicine and cellular longevity. 2016;2016:1069743. doi: 10.1155/2016/1069743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Himmelfarb J, McMenamin ME, Loseto G, Heinecke JW. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free radical biology & medicine. 2001;31:1163–1169. doi: 10.1016/s0891-5849(01)00697-9. [DOI] [PubMed] [Google Scholar]
- 47.Lanza F. Clinical manifestation of myeloperoxidase deficiency. J Mol Med (Berl) 1998;76:676–681. doi: 10.1007/s001090050267. [DOI] [PubMed] [Google Scholar]