Abstract
Mass administration of endectocides, drugs that kill blood-feeding arthropods, has been proposed as a complementary strategy to reduce malaria transmission. Ivermectin is one of the leading candidates given its excellent safety profile. Here we provide proof that the effect of ivermectin can be boosted at two different levels by drugs inhibiting the cytochrome or ABC transporter in the mammal host and the target mosquitoes. Using a mini-pig model, we show that drug-mediated cytochrome P450/ABC transporter inhibition results in a 3-fold increase in the time ivermectin remains above mosquito-killing concentrations. In contrast, P450/ABC transporter induction with rifampicin markedly impaired ivermectin absorption. The same ketoconazole-mediated cytochrome/ABC transporter inhibition also occurs outside the mammal host and enhances the mortality of Anopheles gambiae. This was proven by using the samples from the mini-pig experiments to conduct an ex-vivo synergistic bioassay by membrane-feeding Anopheles mosquitoes. Inhibiting the same cytochrome/xenobiotic pump complex in two different organisms to simultaneously boost the pharmacokinetic and pharmacodynamic activity of a drug is a novel concept that could be applied to other systems. Although the lack of a dose-response effect in the synergistic bioassay warrants further exploration, our study may have broad implications for the control of parasitic and vector-borne diseases.
Introduction
In spite of remarkable advances since the turn of the century, malaria continues to be a major public health problem in most tropical countries1. Most of these recent advances can be attributed to the scale-up of vector control interventions such as long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)2. Malaria is, however, a moving target and mosquito vectors do not cease to evolve and adapt in response to the pressure exerted by our control measures. The spread and intensity of insecticide resistance3 and behavioural adaptations that allow avoidance of insecticides and other home-centred control measures4, 5 are two of the major challenges faced by the malaria community today.
In this context, the mass use of drugs that can kill mosquitoes feeding on treated subjects has potential to become a new paradigm for vector control. These drugs, known as endectocides, could allow targeting of mosquitoes that avoid or are resistant to currently used insecticides and thus, could be a complementary intervention for malaria elimination6, 7. When modelling this potential intervention, the duration of the mosquito-killing effect is the parameter with the greatest impact on malaria transmission. The longer the drug is present in the blood, the larger the magnitude of effect will be8, 9.
Ivermectin is one of the most broadly studied endectocides. It effectively kills malaria vectors in the insectary10 and in the field11, 12. It has also been distributed to more than 2.5 billion people in the last 30 years for the control of onchocerciasis and other neglected tropical diseases (NTDs)13. For that use, it has an excellent safety profile14. Ivermectin, however, has a relatively short half-life of 18 hours15, which would limit the duration of the mosquito-killing effect. Concentrations that kill 50% of Anopheles gambiae in 10 days can only be sustained for around 72 hours after the single dose of 200 mcg/kg commonly used for NTDs; for other less susceptible species like Anopheles aquasalis 16, this mosquito-killing window can be shorter.
Several strategies have been proposed to overcome the relative short half-life of ivermectin and increase its potential impact on malaria transmission. These include using higher doses than approved for NTDs17, using repeated doses at regular intervals18 or developing slow-release formulations8, 19. The comparative advantages and disadvantages of each strategy has been described elsewhere20.
One additional strategy is to use a second drug to intentionally slow down ivermectin´s metabolism and elimination and boost plasma levels, sustaining them for longer periods of time. This is known as pharmacoenhancement and is commonly used in HIV treatment with protease inhibitors21.
Protease inhibitors, like many drugs including ivermectin22 are metabolised by the cytochrome P450 (CYP) 3A enzymes. The pharmacokinetic (PK) profile of the target drug is enhanced by adding either Ritonavir, a broad CYP inhibitor at doses below antiretroviral efficacy, or by using Cobicistat, a more specific CYP 3A4 inhibitor recently licensed for this purpose21. This approach reduces pill burden, improves adherence to treatment and spares active pharmaceutical ingredient of the boosted protease inhibitor21.
Modulation of the ABC transporter P-glycoprotein (P-gp) can also be used to improve the pharmacokinetic profile of certain medicines. The P-glycoprotein is an active efflux transporter; its action is normally protective by pumping out xenobiotics23. In humans, it is present primarily in endothelial cells with transport or barrier roles, such as intestinal mucosa or the capillaries in the blood-brain barrier24. In recent years, attempts have been made at reducing P-gp activity with the goal of increasing bioavailability and therapeutic benefit of certain drugs in humans, e.g. by better access to central nervous system targets or overcoming acquired P-gp-mediated resistance to chemotherapeutics25–28. Ivermectin is a substrate and an inhibitor of P-gp29.
One important challenge for using pharmacoenhancement strategies with ivermectin is the role of P-gp at the blood-brain barrier. Mice and dogs with a dysfunctional P-gp show increased susceptibility to ivermectin due to abnormal accumulation of the drug in the brain30, 31. There is a theoretical concern for this happening in humans, although supported by very little data32, 33. This potential for added toxicity has been evaluated in HIV and cancer therapy with encouraging safety results34.
We conducted a drug-drug-interaction study of ivermectin in a mini-pig model using ketoconazole, a broad CYP3A4 inhibitor. This drug was used for a first proof-of-concept step, given its capacity to inhibit both the CYP and the P-gp. Although it had been described in animal models that ketoconazole enhances systemic exposure to ivermectin35, 36, it is not completely clear whether this is done by reducing metabolism (directly related to CYP) or by reducing excretion (more related to P-gp inhibition).
Additionally, in the mosquito, metabolic resistance mechanisms drive an important proportion of insecticide resistance in Africa37, 38. There is no available data on the role of mosquito P450 in ivermectin detoxification; however, permethrin-resistant Aedes aegypti adults have a significantly increased ivermectin 5-day LC50 when compared with permethrin-sensitive counterparts39. Both compounds have different targets; this suggests a role of metabolic pathways involving P450s or xenobiotic pumps such as the P-gp.
If ivermectin is scaled up for vector control, this will exert selective pressure on mosquitoes, a process that can eventually lead to ivermectin resistance. In the face of the challenge posed by resistance to public health insecticides, a thorough understanding of the mosquito metabolic pathways and potential defence mechanisms from ivermectin can be pivotal if this novel strategy were to be used in the field. To date, ivermectin drug-class resistance in arthropods has been associated with a wide range of mechanisms: reduced cuticular penetration40, mutation of the glutamate-gated chlorine channel41 and metabolic resistance due to overexpression of xenobiotic pumps from the ABC family, like the P-gp42–44 and cytochrome P450 isoenzymes44, 45.
Since ketoconazole is a broad inhibitor of CYP and P-gp, our drug-drug interaction study provided a unique opportunity to test the concept of whether vector mortality is also enhanced by inhibition of both mechanisms in the mosquito as well. Using the original blood samples, we conducted a synergistic bioassay46 to evaluate the potential involvement of these mechanisms in the metabolism/detoxification of ivermectin in Anopheles gambiae and whether this information could be harnessed to enhance the effect of the drug on the vector.
Our main aims were: (a) to assess the PK modifications induced by a dual CYP/P-gp inhibitor, (b) to assess the safety of ivermectin in the presence of P-gp inhibition in a mini-pig model, (c) to determine the effect of ketoconazole alone on mosquito mortality and (d) to determine whether ketoconazole by means of CYP/P-gp inhibition increases the ivermectin-driven mosquito mortality.
Results
Design of drug-drug interaction study
The experiments were conducted following a randomised crossover design (Fig. 1). In phase I all mini-pigs received a single 800 mcg/kg dose of oral ivermectin and sampling was done as described below. After a washout period of 14 days, the animals were randomly assigned to pre-treatment with either ketoconazole (200 mg daily, orally, for 14 days), rifampicin (10 mg/kg daily, orally, for 14 days) or nothing in a 1:1:1 ratio and ivermectin dosage and sampling was repeated. The total wash-out period between ivermectin doses was 30 days. Ketoconazole is a dual inhibitor of the CYP and the P-glycoprotein (P-gp), which plays a key role as xenobiotic pump in the blood-brain barrier and other epithelial barriers23, 24. Inhibiting the P-gp could theoretically lead to toxicity due to abnormal accumulation of the drug in the brain30, 31. The dose of 200 mg/day was calculated to maximise the time ketoconazole remained above the 50% inhibitory concentration (IC50) for the CYP 3A4 (estimated in 0.022–0.025 µM [1.6–13.2 ng/ml]47) but remaining below the much higher IC50 for the P-gp (estimated in 5.6 µM [2975 ng/ml]48). Irrespectively of this, drug quantification in cerebrospinal fluid (CSF) was added as an additional safety point. Rifampicin a CYP/P-gp inducer49 was included as we expected to see reduced bioavailability and penetration into CSF.
Figure 1.
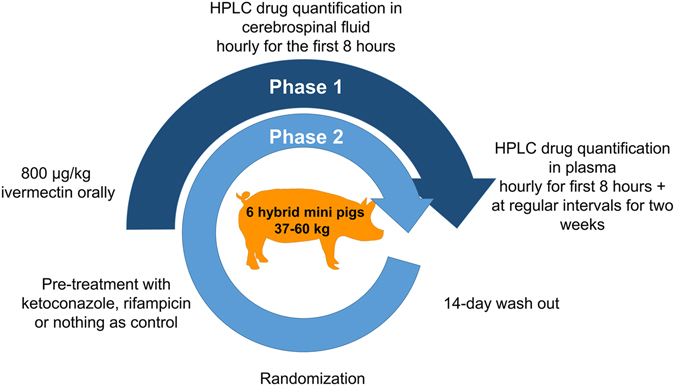
Illustration of the randomized-crossover design used in the study. In phase I all six pigs received a single 800 mcg/kg dose of oral ivermectin. After a washout period of 14 days, the animals were randomly assigned to pre-treatment with ketoconazole, rifampicin or nothing in a 1:1:1 ratio and ivermectin dosage and sampling was repeated. The time between ivermectin doses was 30 days. Figure by Juliane Chaccour.
The main outcome measure was time above the ivermectin concentration that kills 50% Anopheles gambiae in 10 days [10-day lethal concentration 50 (LC50)], which has been estimated at 6 ng/ml50. Secondary outcome measures were the maximum concentration reached (Cmax), the total area under the curve (AUC) and the area under the curve above the LC50 (AUC > LC50).
Design and sample size of mosquito bioassay
Mosquitoes were membrane fed with reconstituted blood as described by Bousema et al.51. Feedings were done with samples drawn directly from the pigs at different intervals and pair-matched according to ivermectin concentration, thus the only different between the samples in a pair was the presence or absence of ketoconazole.
Since mosquitoes grouped in cups for feeding, we used a cluster design in which the unit of intervention was mosquito cups. Our primary outcome was mean 10-day mosquito mortality after membrane feeding. Sample size calculations were conducted according to the method described by Gangnon and Kosorok52. The minimum expected 10-day mortality in mosquitoes imbibing blood containing 9.9 ng/ml of ivermectin is 66%; this is based on the predictive function described by Ouedraogo et al.50. Based on previous observations and with all mosquitoes having the same colony origin and age range, we chose a conservative intracluster correlation of 0.15. The frailty variance of clusters was resembled by a gamma of 1. All experiments were performed in triplicate. With these parameters, at least 4 clusters of 44 mosquitoes were needed per arm to achieve 80% power at 5% significance level accounting for 10% non-feeders.
After determining of the ivermectin levels in all samples in the drug-drug interaction study, we selected 4 paired samples with matching ivermectin concentrations (+/−1 ng/ml) from naïve and ketoconazole pre-treated groups. Baseline serum samples collected before ivermectin treatment in ketoconazole and naïve pigs were used as controls. These samples were shipped frozen to the insectary facilities of the Ifakara Health Institute in Bagamoyo, Tanzania for membrane feeding assays. It was hypothesised that induction of the mosquito CYP/P-gp by rifampicin could improve survival in presence of ivermectin, but this could not be tested due to a reduced absorption of ivermectin in the mini-pigs pre-treated with rifampicin (see below).
Pharmacoenhancement
Ivermectin in both phases and pre-treatments in phase II were administered uneventfully to all subjects. Blood samples were obtained according to the planned schedule. Lumbar punctures were performed with mixed success. It was possible to insert a tunnelled intrathecal catheter and reservoir in 5 out of 6 pigs. Two reservoirs were removed within 48 hours due to motor impairment with full recovery after removal. One pig (#72) was euthanised 72 hours post procedure due to non-reversible motor impairment and was replaced by a seventh animal (#75). At least one CSF sampling point was obtained from 5 out of 6 pigs in phase I. The success rate was considerably higher during phase II with CSF samples obtained from 6 out of 6 pigs.
Ketoconazole enhances ivermectin’s PK
The main plasma PK parameters in all groups are presented in Table 1. The plasma PK parameters of ivermectin-naïve pigs were compatible with previously published data53, 54.
Table 1.
Relevant PK parameters by treatment group.
| Treatment | Subject | Cmax [ng/mL] | AUC inf [h·ng/mL] | Time > LD50 [h] | AUC > LD50 [h·g/mL] |
|---|---|---|---|---|---|
| Ivermectin 1 | 69 | 14.3 | 1916.2 | 46.7 | 509.4 |
| 70 | 24.1 | 694.9 | 27.2 | 198.9 | |
| 71 | 3.0 | 16.9 | 0.0 | 0.0 | |
| 72 | 1.6 | — | 0.0 | — | |
| 73 | 27.6 | 244.3 | 9.3 | 47.3 | |
| 74 | 2.9 | 99.4 | 0.0 | 0.0 | |
| Median (Range) | 8.7 (26.0) | 244 (1899) | 4.6 (46.7) | 23.7 (509.4) | |
| Ivermectin 2 | 73.2 | 15.2 | 1380.0 | 54.5 | 226.3 |
| 75 | 20.9 | 1030.5 | 45.4 | 368.9 | |
| Median (Range) | 18 (5.6) | 1205.3 (349) | 49.9 (9.1) | 297.6 (142.6) | |
| Ivermectin(combined) | 69 | 14.3 | 1916.2 | 46.7 | 509.4 |
| 70 | 24.1 | 694.9 | 27.2 | 198.9 | |
| 71 | 3.0 | 16.9 | 0.0 | 0.0 | |
| 73.2 | 15.2 | 1380.0 | 54.5 | 226.3 | |
| 74 | 2.9 | 99.4 | 0.0 | 0.0 | |
| 75 | 20.9 | 1030.5 | 45.4 | 368.9 | |
| Median (Range) | 14.8* (21) | 862.7 (1899) | 36.3* (54) | 212.6* (509) | |
| Ivermectin + ketoconazole | 69 | 27.7 | 2320.7 | 117.1 | 703.9 |
| 72 | 28.4 | 1863.3 | 104.6 | 718.5 | |
| Median (Range) | 28.0* (0.7) | 2092 (457.4) | 110.8* (12.4) | 711.2* (14.6) | |
| Ivermectin + rifampin | 71 | 0.3 | — | 0.0 | 0.0 |
| 74 | 6.8 | — | 4.7 | — | |
| Median (Range) | 3.6 (6.5) | — | 2.4 (4.7) | — |
Footnote: Cmax: peak plasma concentration, AUC inf: area under the PK curve to infinity, LD50: the 10-day lethal concentration 50 of ivermectin for Anopheles gambiae (6 ng/ml). Pig 72 died and was replaced by pig 75. Pig 73 received ivermectin in phase 1 and was randomised to ivermectin alone in phase 2, this is denoted as 73/73.2, these observations were not combined in order to sustain sample independence. *Denotes a statistically significant difference.
Firstly, we compared the PK parameters between ivermectin-naïve pigs and those who received it in phase II without pre-treatment by means of a Mann-Whitney test and found no difference in time above LC50, Cmax, AUC or AUC > LC50 (P values of 0.12, 0.61, 0.16 and 0.32 respectively). Given these results, the small sample size and relative large variations between subjects (as expected in a mini-pig model), these two groups (excluding one animal for which lamda could not be calculated) were combined for comparisons between ivermectin alone and ketoconazole/rifampicin pre-treated pigs. In order to respect sample independence, only the values of the second treatment of mini-pig 73 were used, this penalized the main outcome by increasing the time above LC50 of the combined ivermectin.
The PK curves of all three groups (ivermectin alone, ketoconazole + ivermectin and rifampicin + ivermectin) are shown in Fig. 2.
Figure 2.
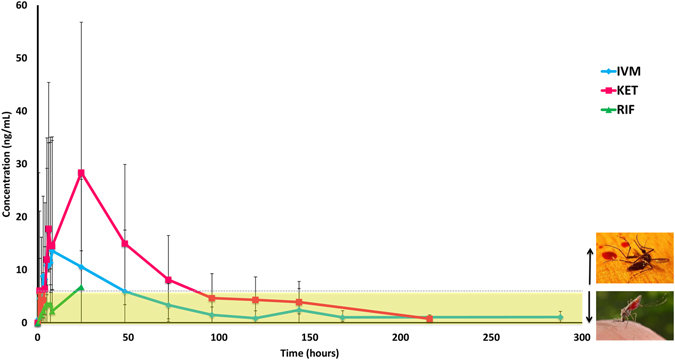
Comparison of median (±range) plasma concentrations across different treatment groups. The dashed line indicates the target 10-day lethal concentration (LC50) for Anopheles gambiae. Alive Anopheles image in public domain from CDC Public Health Image Library, photo credit: James Gathany. Dead mosquito image CC-by-sa PlaneMad/Wikimedia available at https://commons.wikimedia.org/wiki/File:Dead_mosquito.jpg. CDC: Centers for Disease Control and Prevention, IVM: ivermectin, KET: ketoconazole, RIF: rifampicin.
Pre-treatment with ketoconazole significantly increased the time above LC50 3-fold from a median of 36 to 111 hours (P = 0.033). The Cmax was also increased 2-fold (median 14 vs 28 ng/ml, P = 0.033), along with a more than 3-fold increase in the AUC > LC50 (median 212 vs 711, P = 0.033). The total AUC was also increased more than 2-fold (862 vs 2092 h*ng/ml) but this difference was not statistically significant (P = 0.067).
Pre-treatment with rifampicin seriously hampered ivermectin absorption making the AUC and AUC > LC50 calculations uninterpretable. Only one pig reached ivermectin plasma concentration above 6 ng/ml, which was maintained for less than 5 hours in total (Fig. 2).
Pre-treatment with ketoconazole did not increase ivermectin in the CSF
No clinical adverse events were observed. Although detectable in some, ivermectin was below quantification level (0.5 ng/ml) in all CSF samples obtained (Table 2). Of 24 CSF samples available from phase I, ivermectin was detectable in 4. Of 37 CSF samples available from phase II, ivermectin was detectable in 4 samples (2 in the ketoconazole group and 2 from rifampicin group).
Table 2.
Number of CSF samples with detectable and quantifiable ivermectin divided by study phase and treatment group.
| Concentration | Phase I | Phase II | ||
|---|---|---|---|---|
| Ivm | Ivm | Ket | Rif | |
| < 0.1 ng/ml | 20 | 10 | 10 | 13 |
| > 0.1 < 0.5 ng/ml | 4 | 0 | 2 | 2 |
| Total | 24 | 10 | 12 | 15 |
Footnote: Ivm: ivermectin only group, Ket: ketoconazole + ivermectin group, Rif: rifampicin + ivermectin group.
Mosquito bioassay
Out of a total of 169 samples available we selected four pairs with matching ivermectin concentrations (+/−1 ng/ml) that only differed in the presence of ketoconazole, plus two controls; one from a ketoconazole-only and one from a fully untreated pig (Table 3). In the rifampicin group, there was only one sample with matching ivermectin concentration in naïve pigs.
Table 3.
Mean survival and time to median mortality of mosquitoes feeding on blood samples with matching concentrations (+/−1 ng/ml) of ivermectin with (K) or without ketoconazole at CYP inhibition concentrations.
| Pair | Drug concentrations | Mean survival (days) | Time to median mortality (days) |
|---|---|---|---|
| 1 | IVM 9.98 | 6.73 (6.29–7.17) | 7 |
| KET 8.96 | 5.39 (4.90–5.89) | 5 | |
| 2 | IVM 16.5 | 5.11 (4.63–5.86) | 5 |
| KET 17.23 | 3.79 (3.49–4.09) | 4 | |
| 3 | IVM 20.46 | 7.43 (7.11–7.75) | 7 |
| KET 20.39 | 5.10 (4.51–5.69) | 4 | |
| 4 | IVM 27.58 | 5.28 (4.54–6.02) | 5 |
| KET 27.68 | 4.23 (3.80–4.65) | 4 |
Footnote: IVM: ivermectin alone group, KET: ketoconazole + ivermectin group.
Ketoconazole is not lethal to mosquitoes at the dose used
Firstly we assessed whether the ketoconazole concentration present in the samples could have a lethal effect per se. For this we compared the 10-day mortality of mosquitoes fed on samples containing only ketoconazole (baseline samples from ketoconazole pre-treated pigs) with the mortality of mosquitoes feeding on fully drug-free samples (baseline of control pigs). Given that all pigs received ivermectin in the first phase, this experiment served as well to assess whether there could be active metabolites increasing mortality in spite of a washing period of 30 days.
Ketoconazole alone did not increase the mortality of Anopheles gambiae, in fact it provided a slight, protective effect (Long-rank P = 0.048, n = 222), as previously seen with other antimicrobials55 (Fig. 3).
Figure 3.
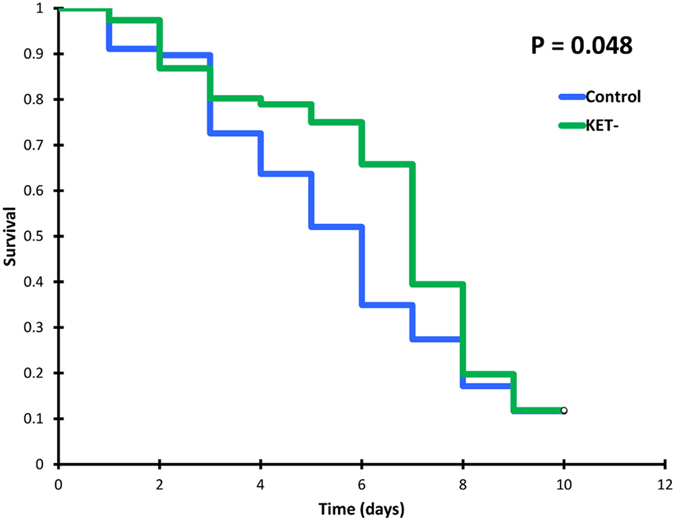
Survival curves of mosquitoes feeding on blood from one fully untreated pig (control) vs mosquitoes feeding on a ketoconazole-only treated pig (Ket-). Triplicate experiments, n = 222.
Ketoconazole synergises the mosquito mortality caused by ivermectin
Ketoconazole significantly increased the mortality of Anopheles gambiae fed in all samples containing ivermectin irrespectively of the ivermectin concentration (Fig. 4). The effect was predominantly observed at the expense of early mortality as seen by a reduction in the mean survival and the time to median mortality in all ketoconazole groups (Table 3).
Figure 4.
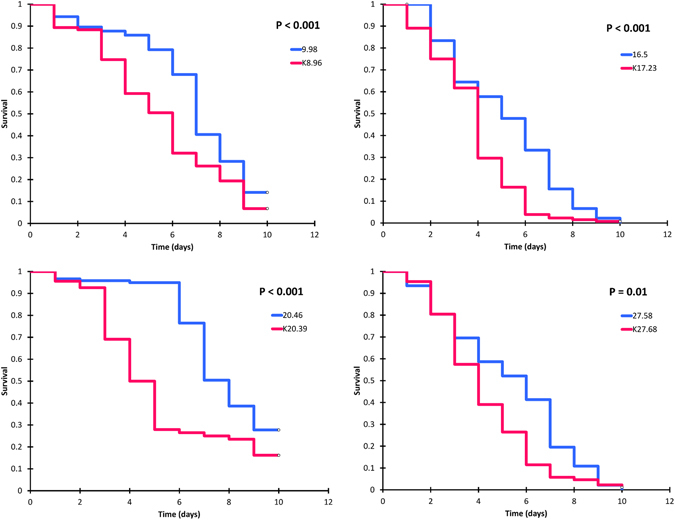
Survival curves of mosquitoes feeding on blood with matching ivermectin concentrations (+/−1 ng/ml) with (blue) or without (pink) the ketoconazole at CYP inhibition concentrations. Triplicate experiments, n = 133–220.
The mortality of mosquitoes fed on the only paired sample with rifampicin-ivermectin did not differ from that of ivermectin or rifampicin alone groups (data not shown).
Discussion
Our study shows that inhibition of the CYP 3A4 could safely increase ivermectin’s time above target insecticidal concentration in the mammal host, increasing mosquito exposure. This finding could be harnessed to simplify the dosing regime and spare active pharmaceutical ingredient. Additionally, once outside the mammal host, pharmacological CYP inhibition increases the mortality of exposed Anopheles gambiae mosquitoes, which could further improve efficacy. A natural next step would be to assess the potential implications of these findings in the field by means of modelling studies.
CYP inhibitors that also target the P-gp could theoretically increase penetration of the drug into the CNS and facilitate interaction with the GABA receptors, to which ivermectin has weak affinity. Thus, our main concern was that ketoconazole mediated CYP inhibition might reduce ivermectin’s excellent safety profile. Our results suggest that the large therapeutic window of ivermectin and the difference in IC50 for CYP and P-gp could safely allow CYP inhibition without a measurable increase of the drug in the CSF or clinically observed adverse events. Currently there are some “selective” CYP3A4 or P-gp inhibitors on the market but most drugs that affect one system typically have at least a partial effect on the other. Emerging more selective molecules56 or even combination strategies34 could overcome this issue.
Ivermectin resistance resulting from scale up of its veterinary use is well known. It was observed in nematodes as early as 198557, four years after licensure of the drug. It was soon proven that resistance could be selected in the lab after only 8 generations of Haemonchus contortus exposed to sub-optimal dosing58. In arthropods highly ivermectin-resistant Musca domestica could be selected in the lab after only seven generations in the early 1990s59. Field reports in economically or public health relevant arthropods followed shortly60, 61. The relative delay in global resistance reports was the result of ivermectin’s broad spectrum. Most intestinal parasites in this spectrum could be removed with doses as low as 20 mcg/kg, but the 200 mcg/kg dose was selected based on the less susceptible organisms, the dose-defining species. The result is that any resistance arising in the more susceptible parasites could not be detected until their susceptibility was 10-fold higher62. This phenomenon has been called the window of escalation62 and could be an important concept it if ivermectin is scaled up for vector control given that malaria vectors also differ in their ivermectin susceptibility16, 63, 64. While Anopheles gambiae 50 and Anopheles minimus seems to be remarkably susceptible based on their low LC50, others like Anopheles aquasalis or Anopheles dirus promise to become the dose-defining mosquito species16, 63, 64 for the novel vector control use of the drug. Taking advantage of a synergist could potentially delay the emergence of ivermectin resistance in malaria vectors and extend the spectrum of ivermectin towards less susceptible vectors.
The findings from our study suggest that although metabolic pathways play a role in the mosquito defence from ivermectin, the activity of the detoxification mechanisms or efflux pumps could be modulated to synergise ivermectin and boost its efficacy. These findings warrant surveillance of early signs of metabolic resistance if ivermectin is used to reduce malaria transmission. At the same time, these results offer a simple potential tool to address metabolic resistance before it challenges efficacy.
Additionally, emerging data indicates that active ivermectin metabolites could cause mosquito mortality even when the mother drug is no longer detectable in plasma65, 66. Increasing the time above mosquitocidal levels of ivermectin at the expense of reducing active metabolites could leave overall mosquito mortality unchanged20. Previous animal studies suggest dual CYP3A4/P-gp inhibitors do not significantly modify the PK of one of the major ivermectin metabolites, 3 O-desmethyl ivermectin35, 36 potentially suggesting an increase in systemic exposure mostly due to reduced excretion rather than reduced metabolism. Nonetheless, the evaluation of ivermectin metabolites is a complex process22, 67 and their potential role in the insecticidal effect of the drug would require additional investments.
Ketoconazole was used purely to test the concept of dual CYP/P-gp inhibition enhancing the PK and the ivermectin-related mosquito mortality, but it is in no way a molecule suitable for combination in MDA campaigns. Some additional limitations of this study include: a small number of mini-pigs was used for the pharmacoenhancement experiment, which explains the relative large concentration ranges; this pharmacokinetic effect, however, has previously been seen in other animal models35, 36. We included rifampicin, a dual CYP3A4/P-gp inducer49 to validate the results, evaluate changes in the CSF concentration and better understand the potential routes of manipulation of detoxification enzymes/transporters in the mosquito. Yet, Rifampicin mediated CYP 3A4/P-gp induction led to a marked reduction in systemic ivermectin exposure, possibly due to reduced intestinal absorption and/or pre-systemic metabolism; Although there is no evidence that ivermectin is a substrate of Organic Anion-Transporting Polypeptides (OATPs) in hepatic tissue or the intestinal epithelium, decreased bioavailability when co-administered with orange juice suggests this could occur68, 69. If this was the case, inhibition of OATPs mediated uptake by rifampicin70 could have also contributed to the reduced ivermectin absorption seen in our rifampicin pre-treated mini-pigs. Rifampicin-ivermectin samples were insufficient for the mosquito experiment. Nonetheless, the role of MDR1 polymorphism on the pharmacokinetics and pharmacodynamics of ivermectin in humans requires further exploration32, 33, particularly in the African setting71, 72. Finally, we acknowledge the lack of a dose-response effect in the membrane feeding assays. These assays were performed with reconstituted blood which can lead to varying final drug concentrations given to mosquitoes caused by the manual mixture; this could explain the apparent absence of a dose-response effect in our experiments but does not affect our conclusion about a pharmacodynamic synergism. Future experiments assessing drug concentrations inside the mosquito could help clarify this potential source of bias.
When used as anthelmintics, dual pharmacokinetic and pharmacodynamic enhancement of macrocyclic lactones such as ivermectin can occur by modulation of the ABC transporter. This results in higher drug bioavailability in the host and higher drug penetration in the nematode73, 74, which is the main driver of efficacy. However, this dual in vivo enhancement by inhibiting the CYP has not been reported, even though it could have important implications for the treatment of certain helminths75–78. Particularly in the context of wider use of ivermectin mass-treatment as resistance in endo- and ectoparasites can be induced simultaneously79.
Provided an appropriate regimen of a selective molecule is defined and the appropriate cost-effectiveness evaluations are conducted, our findings could be applied to other systems and have important implications for the control of different vector-borne diseases.
Materials and Methods
Mini-pig procedures
The pre-medication drugs were administered by crushing the tablets and mixing them with canned meat products used for enrichment. For ivermectin administration and sampling during the first 8 hours, the animals were sedated with a single intramuscular dose of tiletamine-zolazepan (4 mg/kg) and maintained with inhalatory isoflurane (1.5–2%). In pigs, there is no evidence of a significant interaction (other than being a substrate) of these drug classes with the CYP3A4 or the P-gp80. Ivermectin was administered via nasogastric tube.
For blood sampling during the first eight hours, a femoral line was left in place and later removed during recovery from anaesthesia. Blood sampling thereafter was done by jugular puncture under restrain. Sampling points for blood were: pre-treatment, at 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 24, 48/72 hours and at days 6/7, 9 and 14. For the mosquito bioassay, additional serum samples were obtained at every time point, and frozen at −20 °C.
For obtaining CSF samples during the first 8 hours, an intrathecal catheter was placed by means of fluoroscopy-guided lateral lumbar puncture81 (Fig. 5). Given the challenges posed by the particular anatomy of the pig, efforts were done to tunnel the intrathecal catheter and connect it to a subcutaneous reservoir in order to avoid repeating the technically demanding lumbar puncture during phase II of the study (Fig. 5). Given the risk of damaging nerve roots when accessing the reduced thecal space, the pigs received at least 20 ml/kg of isotonic IV fluids during the first 8 hours and motor impairment lasting more than 72 hours was included as a humane endpoint criterion. Sampling points for CSF were: pre-treatment and at 0.5, 1, 2, 3, 4, 5, 6, 7 and 8 hours.
Figure 5.
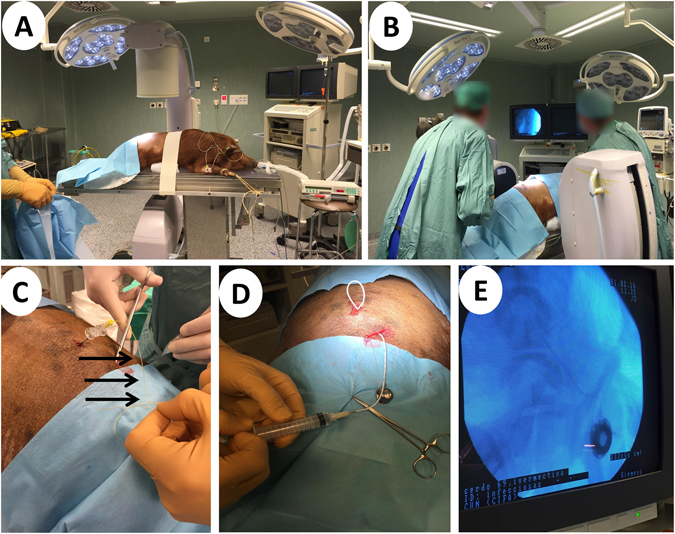
Cerebrospinal fluid sampling. (A) The pigs were sedated with tiletamine-zolazepan, intubated and maintained with inhalatory isoflurane. (B) Lateral lumbar puncture was performed in aseptic conditions by an anaesthetist and an interventional radiologist under fluoroscopic guidance. (C) After introduction of the intrathecal catheter (black arrows), it was tunnelled (D) and connected to a reservoir placed in subcutaneous tissue (E) to avoid a second round of laborious localization of the intrathecal space.
Mosquito procedures
For all experiments, we used the fully susceptible Anopheles gambiae s.s. colony maintained at the insectary in Bagamoyo. Mosquitoes were reared at 28 °C, 80% humidity and with a photoperiod of 12:12. Larvae were kept in large plastic bowls covered with netting containing approximately 2 litres of water with larval densities not exceeding one 4th instar larvae per ml. Larvae were fed Tetramine fish flakes ad libitum. Once they developed into pupae these were collected using disposable plastic Pasteur pipettes and transferred to small bowls containing approximately 200 ml of clean water. The pupae bowls were then placed inside fully screened cages (30 × 30 × 30 cm) where adult mosquitoes were allowed to emerge and given 10% glucose as a sugar source ad libitum.
Only mosquitoes of 2–5 days of age were used for the experiments. Before the experiments, mosquitoes were starved from sugar for 12–18 hours and from water for one hour. Hungry females were selected by applying a bottle with heated water (37–40 °C) to the cage. 40–50 hungry females were transferred to each paper cup using a mouth aspirator.
We performed membrane feeding assays with serum replacement as previously described51. For each experiment, the selected pig sample was thawed and 1 ml of whole blood was centrifuged at 2100 rpm for 10 minutes. The serum volume was noted and replaced with the same amount of the sample pig-serum being tested. After this, tubes were inverted 10 times. Each sample was tested in triplicate.
In order to reduce potential confounding arising from variations in bloodmeal size, partially fed mosquitoes were discarded and only fully engorged females followed up for the mortality assessment. After each feeding experiment, the cups were placed on ice for 1–2 minutes to select and transfer fully engorged females.
Cups with fully engorged mosquitoes were kept at 24–26 °C and 65–70% relative humidity inside climate controlled incubators. Mosquitoes were maintained with 10% glucose solution using impregnated cotton. Mortality was recorded daily for 9 days by a person blinded to the allocation of the cups. Dead mosquitoes were removed from the cups daily.
Analytics and PK calculations
Blood samples were drawn in 3 ml EDTA tubes and centrifuged. Plasma was separated and frozen at −20 °C until analysis. CSF samples were frozen at −20 °C.
Ivermectin levels in plasma and CSF were determined using a validated adaptation of a previously described HPLC-FLD82. The detection limit in plasma and CSF was 0.1 ng/ml, the quantification limit was 0.5 ng/ml.
We used the lethal concentration 50 of Anopheles gambiae described by Ouedraogo50 (6 ng/ml) as target concentration and particularly aimed at increasing the time ivermectin was present in plasma above this level. Pharmacokinetic analyses were performed using Phoenix WinNonlin 6.4 (Certara, Princeton, NJ, USA). Pharmacokinetic parameters were derived from non-compartmental analyses.
Animals
Six hybrid mini-pigs (2 male, 4 female) weighing between 46 and 70 kg were procured from our accredited breeding centre at the University of Navarra. They were housed individually throughout the study at the animal research facilities in the University of Navarra. From arrival onward, the animals were assessed daily for general wellbeing and after the intervention, also for specific ivermectin toxicity signs.
Drugs and reagents
Ivermectin (Noromectin® 0.08% oral solution, Norbrook) and ketoconazole (Fungiconazol 200 mg dog tablets, Fatro) were procured from our veterinary supplier. Rifampicin (Rifaldin® 300 mg tablets, Sandoz) was procured through the hospital pharmacy service.
European pharmacopeia standard ivermectin for HPLC calibration was procured from Sigma Aldrich.
Statistics
The comparison of PK parameters between ivermectin groups was done by means of a two-sided Mann-Whitney test. Comparison between combined ivermectin and ketoconazole plus ivermectin groups was done with a one sided Mann-Whitney test.
Survival Kaplan-Meier analysis was performed in Addinsoft’s XLSTAT ® Version 2016.04.32229 (New York, NY, USA). Comparisons of survival patterns were done with Log-rank test using a 5% significance level adjusted for cluster design.
Ethics
All procedures were reviewed and approved by the animal experimentation ethics committee of the Universidad de Navarra (Registry number E27-15(135-12E4)). All procedures were performed in accordance with the relevant guidelines and regulations.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Acknowledgements
This work was funded by the University of Navarra PIUNA grant scheme. Carlos Chaccour is supported by a Ramón Areces fellowship. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. We thank Juliane Chaccour for designing Figure 1 and proofreading
Author Contributions
C.Ch., J.L.D.P. and M.M. conceived and designed the study. F.H. and A.A. defined the co-administration doses. C.Ch., S.C., G.A., and J.L.D.P. performed the mini-pig experiments with the exception of lumbar punctures and reservoir placement that was done by R.M. and J.I.B. M.A., B.T. and M.M. performed the mosquito experiments. A.I.B. analysed the HPLC samples. C.Ch. and H.M.S. calculated the sample size for the mosquito trial. C.Ch., F.H. and M.A. analysed the data. C.Ch. and M.A. wrote the first draft. All authors reviewed, contributed and approved the final draft.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Felix Hammann, Marta Alustiza, Marta Maia and José Luis Del Pozo contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World Malaria report 2016. http://www.who.int/entity/malaria/publications/world-malaria-report-2016/report/en/index.html (Accessed Jan 2017). (Geneva, World Health Organization, 2016).
- 2.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranson H, Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Killeen GF. A second chance to tackle African malaria vector mosquitoes that avoid houses and don’t take drugs. Am J Trop Med Hyg. 2013;88:809–816. doi: 10.4269/ajtmh.13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killeen GFC. controlling and eliminating residual malaria transmission. Malar J. 2014;13 doi: 10.1186/1475-2875-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaccour CJ, et al. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J. 2013;12 doi: 10.1186/1475-2875-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foy BD, Kobylinski KC, da Silva IM, Rasgon JL, Sylla M. Endectocides for malaria control. Trends Parasitol. 2011;27:423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellinger AM, et al. Oral, ultra-long-lasting drug delivery: Application toward malaria elimination goals. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aag2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slater HC, Walker PG, Bousema T, Okell LC, Ghani AC. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. J Infect Dis. 2014;210:1972–1980. doi: 10.1093/infdis/jiu351. [DOI] [PubMed] [Google Scholar]
- 10.Chaccour C, Lines J, Whitty CJ. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis. 2010;202:113–116. doi: 10.1086/653208. [DOI] [PubMed] [Google Scholar]
- 11.Alout H, et al. Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malar J. 2014;13 doi: 10.1186/1475-2875-13-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sylla M, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9 doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mectizan Donation Program. Annual highlights 2015. http://www.mectizan.org/sites/www.mectizan.org/files/attachments/resources/MDP_AnnHigh2015_Design%20041516FINAL2%20%281%29.pdf (Accessed August 2016).
- 14.Ejere HO, Schwartz E, Wormald R, Evans JR. Ivermectin for onchocercal eye disease (river blindness) The Cochrane database of systematic reviews. 2012;8 doi: 10.1002/14651858.CD002219.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merck & Co. Stromectrol. FDA approved Package insert 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050742s026lbl.pdf (Accessed October, 2016).
- 16.Sampaio VS, et al. Filling gaps on ivermectin knowledge: effects on the survival and reproduction of Anopheles aquasalis, a Latin American malaria vector. Malar J. 2016;15 doi: 10.1186/s12936-016-1540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smit MR, et al. Efficacy and Safety of High-Dose Ivermectin for Reducing Malaria Transmission (IVERMAL): Protocol for a Double-Blind, Randomized, Placebo-Controlled, Dose-Finding Trial in Western Kenya. JMIR Res Protoc. 2016;5 doi: 10.2196/resprot.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foy, B. D. Results from RIMDAMAL, a pilot randomized cluster-design trial in Burkina Faso, designed to assess the safety and efficacy of repeat ivermectin mass drug administrations to control malaria and NTDs. Oral presentation during the 65th Annual Meeting of the ASTMH. Atlanta 2016.
- 19.Chaccour C, et al. Screening for an ivermectin slow-release formulation suitable for malaria vector control. Malar J. 2015;14 doi: 10.1186/s12936-015-0618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaccour C, Hammann F, Rabinovich NR. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar J. 2017;16 doi: 10.1186/s12936-017-1801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson KB, Wang K, Delille C, Otofokun I, Acosta EP. Pharmacokinetic enhancers in HIV therapeutics. Clin Pharmacokinet. 2014;53:865–872. doi: 10.1007/s40262-014-0167-9. [DOI] [PubMed] [Google Scholar]
- 22.Zeng Z, Andrew NW, Arison BH, Luffer-Atlas D, Wang RW. Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes. Xenobiotica; the fate of foreign compounds in biological systems. 1998;28:313–321. doi: 10.1080/004982598239597. [DOI] [PubMed] [Google Scholar]
- 23.Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161–178. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 24.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–473. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Stouch TR, Gudmundsson O. Progress in understanding the structure-activity relationships of P-glycoprotein. Advanced drug delivery reviews. 2002;54:315–328. doi: 10.1016/S0169-409X(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 26.Varma MV, Perumal OP, Panchagnula R. Functional role of P-glycoprotein in limiting peroral drug absorption: optimizing drug delivery. Curr Opin Chem Biol. 2006;10:367–373. doi: 10.1016/j.cbpa.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Callaghan R, Luk F, Bebawy M. Inhibition of the multidrug resistance P-glycoprotein: time for a change of strategy? Drug Metab Dispos. 2014;42:623–631. doi: 10.1124/dmd.113.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets. 2013;13:326–346. doi: 10.2174/15680096113139990076. [DOI] [PubMed] [Google Scholar]
- 29.Didier A, Loor F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anticancer Drugs. 1996;7:745–751. doi: 10.1097/00001813-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Schinkel AH, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 31.Mealey, K. L., Bentjen, S. A., Gay, J. M. & Cantor, G. H. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics11, 727–733 (2001). [DOI] [PubMed]
- 32.Bourguinat C, et al. Analysis of the mdr-1 gene in patients co-infected with Onchocerca volvulus and Loa loa who experienced a post-ivermectin serious adverse event. Am J Trop Med Hyg. 2010;83:28–32. doi: 10.4269/ajtmh.2010.09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards, G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J2 Suppl 1, S8, doi:10.1186/1475-2883-2-S1-S8 (2003). [DOI] [PMC free article] [PubMed]
- 34.Hendrikx JJ, et al. Oral co-administration of elacridar and ritonavir enhances plasma levels of oral paclitaxel and docetaxel without affecting relative brain accumulation. Br J Cancer. 2014;110:2669–2676. doi: 10.1038/bjc.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hugnet C, Lespine A, Alvinerie M. Multiple oral dosing of ketoconazole increases dog exposure to ivermectin. Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2007;10:311–318. [PubMed] [Google Scholar]
- 36.Alvinerie M, Dupuy J, Kiki-Mvouaka S, Sutra JF, Lespine A. Ketoconazole increases the plasma levels of ivermectin in sheep. Veterinary parasitology. 2008;157:117–122. doi: 10.1016/j.vetpar.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Djouaka R, Irving H, Tukur Z, Wondji CS. Exploring mechanisms of multiple insecticide resistance in a population of the malaria vector Anopheles funestus in Benin. PloS one. 2011;6 doi: 10.1371/journal.pone.0027760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulamba C, et al. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PloS one. 2014;9 doi: 10.1371/journal.pone.0110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deus, K. M., Saavedra-Rodriguez, K., Butters, M. P., Black, W. C. t. & Foy, B. D. The effect of ivermectin in seven strains of Aedes aegypti (Diptera: Culicidae) including a genetically diverse laboratory strain and three permethrin resistant strains. J Med Entomol49, 356–363 (2012). [DOI] [PMC free article] [PubMed]
- 40.Chen LP, Wang P, Sun YJ, Wu YJ. Direct interaction of avermectin with epidermal growth factor receptor mediates the penetration resistance in Drosophila larvae. Open Biol. 2016;6 doi: 10.1098/rsob.150231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, et al. A point mutation in the glutamate-gated chloride channel of Plutella xylostella is associated with resistance to abamectin. Insect Mol Biol. 2016;25:116–125. doi: 10.1111/imb.12204. [DOI] [PubMed] [Google Scholar]
- 42.Luo L, Sun YJ, Wu YJ. Abamectin resistance in Drosophila is related to increased expression of P-glycoprotein via the dEGFR and dAkt pathways. Insect Biochem Mol Biol. 2013;43:627–634. doi: 10.1016/j.ibmb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Mangia C, et al. Evaluation of the in vitro expression of ATP binding-cassette (ABC) proteins in an Ixodes ricinus cell line exposed to ivermectin. Parasit Vectors. 2016;9 doi: 10.1186/s13071-016-1497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon KS, et al. Brief exposures of human body lice to sublethal amounts of ivermectin over-transcribes detoxification genes involved in tolerance. Insect Mol Biol. 2011;20:687–699. doi: 10.1111/j.1365-2583.2011.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao X, et al. Identification and Characterization of the Gene CYP340W1 from Plutella xylostella and Its Possible Involvement in Resistance to Abamectin. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chouaibou M, Zivanovic GB, Knox TB, Jamet HP, Bonfoh B. Synergist bioassays: A simple method for initial metabolic resistance investigation of field Anopheles gambiae s.l. populations. Acta Trop. 2014;130:108–111. doi: 10.1016/j.actatropica.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perloff ES, et al. Validation of cytochrome P450 time-dependent inhibition assays: a two-time point IC50 shift approach facilitates kinact assay design. Xenobiotica; the fate of foreign compounds in biological systems. 2009;39:99–112. doi: 10.1080/00498250802638155. [DOI] [PubMed] [Google Scholar]
- 48.Wang EJ, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with P glycoprotein. Antimicrobial agents and chemotherapy. 2002;46:160–165. doi: 10.1128/AAC.46.1.160-165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Raymond K. Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob. 2006;5 doi: 10.1186/1476-0711-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouedraogo AL, et al. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clin Infect Dis. 2015;60:357–365. doi: 10.1093/cid/ciu797. [DOI] [PubMed] [Google Scholar]
- 51.Bousema T, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PloS one. 2012;7 doi: 10.1371/journal.pone.0042821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gangnon RE, Kosorok MR. Sample‐size formula for clustered survival data using weighted log‐rank statistics. Biometrika. 2004;91:263–275. doi: 10.1093/biomet/91.2.263. [DOI] [Google Scholar]
- 53.Chiu, S. H. & Lu, A. Y. in W.C. Campbell (ed), Ivermectin and Abamectin 131–143 (Springer-Verlag, 1989).
- 54.Lifschitz A, et al. Bioequivalence of ivermectin formulations in pigs and cattle. J Vet Pharmacol Ther. 1999;22:27–34. doi: 10.1046/j.1365-2885.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 55.Gendrin M, et al. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nature communications. 2015;6 doi: 10.1038/ncomms6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherman EM, Worley MV, Unger NR, Gauthier TP, Schafer JJ. Cobicistat: Review of a Pharmacokinetic Enhancer for HIV Infection. Clin Ther. 2015;37:1876–1893. doi: 10.1016/j.clinthera.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 57.Carmichael I, Visser R, Schneider D, Soll M. Haemonchus contortus resistance to ivermectin. Journal of the South African Veterinary Association. 1987;58 [PubMed] [Google Scholar]
- 58.Egerton JR, Suhayda D, Eary CH. Laboratory selection of Haemonchus contortus for resistance to ivermectin. The Journal of parasitology. 1988;74:614–617. doi: 10.2307/3282179. [DOI] [PubMed] [Google Scholar]
- 59.Scott JG, Roush RT, Liu N. Selection of high-level abamectin resistance from field-collected house flies. Musca domestica. Experientia. 1991;47:288–291. doi: 10.1007/BF01958163. [DOI] [PubMed] [Google Scholar]
- 60.Martins JR, Furlong J. Avermectin resistance of the cattle tick Boophilus microplus in Brazil. Vet Rec. 2001;149 [PubMed] [Google Scholar]
- 61.Currie BJ, Harumal P, McKinnon M, Walton SF. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis. 2004;39:e8–12. doi: 10.1086/421776. [DOI] [PubMed] [Google Scholar]
- 62.Shoop WL. Ivermectin resistance. Parasitol Today. 1993;9:154–159. doi: 10.1016/0169-4758(93)90136-4. [DOI] [PubMed] [Google Scholar]
- 63.Escobedo-Vargas, K. S. et al. The effect of ivermectin on the Amazonian malaria vector Anopheles darlingi: LC50 determination. Poster session presentes at the 65thAnnual meeting of the American Society of Tropical Medicine and Hygiene; 2016 Nov 11–16; Atlanta, USA. (2016).
- 64.Kobylinski, K. et al. Assessing ivermectin susceptibility of Greater Mekong Subregion malaria vectors. Poster session presentes at: Annual meeting of the American Society of Tropical Medicine and Hygiene; 2014 Nov 2–6; New Orleans, USA.
- 65.Kobylinski, K. et al. Mosquito-lethal efficacy of ivermectin, dihydroartemisinin-piperaquine, and primaquine: Ivermectin for Malaria in Southeast Asia (IMSEA Study). Oral presentation during the 65th Annual Meeting of the ASTMH. Atlanta 2016 (2016).
- 66.Smit, M., Ochomo, E. & ter Kuile, F. Efficacy and safety of high-dose ivermectin for reducing malaria transmission: A dose finding study (IVERMAL). Oral presentation during the 65th Annual Meeting of the ASTMH. Atlanta 2016.
- 67.Chiu SH, Taub R, Sestokas E, Lu AY, Jacob TA. Comparative in vivo and in vitro metabolism of ivermectin in steers, sheep, swine, and rat. Drug Metab Rev. 1987;18:289–302. doi: 10.3109/03602538708998309. [DOI] [PubMed] [Google Scholar]
- 68.Vanapalli SR, et al. Orange juice decreases the oral bioavailability of ivermectin in healthy volunteers. Clinical Pharmacology & Therapeutics. 2003;73:P94–P94. doi: 10.1016/S0009-9236(03)90702-8. [DOI] [Google Scholar]
- 69.Glaeser H, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 70.Imaoka T, et al. Integrated approach of in vivo and in vitro evaluation of the involvement of hepatic uptake organic anion transporters in the drug disposition in rats using rifampicin as an inhibitor. Drug Metab Dispos. 2013;41:1442–1449. doi: 10.1124/dmd.113.051052. [DOI] [PubMed] [Google Scholar]
- 71.Dandara C, Swart M, Mpeta B, Wonkam A, Masimirembwa C. Cytochrome P450 pharmacogenetics in African populations: implications for public health. Expert Opin Drug Metab Toxicol. 2014;10:769–785. doi: 10.1517/17425255.2014.894020. [DOI] [PubMed] [Google Scholar]
- 72.Kudzi W, Dodoo AN, Mills JJ. Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a Ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans? BMC Med Genet. 2010;11 doi: 10.1186/1471-2350-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lespine A, Alvinerie M, Vercruysse J, Prichard RK, Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol. 2008;24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Lespine A, Menez C, Bourguinat C, Prichard RK. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: Prospects for reversing transport-dependent anthelmintic resistance. International journal for parasitology. Drugs and drug resistance. 2012;2:58–75. doi: 10.1016/j.ijpddr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devine C, et al. Inhibition of cytochrome P450-mediated metabolism enhances ex vivo susceptibility of Fasciola hepatica to triclabendazole. Parasitology. 2010;137:871–880. doi: 10.1017/S003118200999148X. [DOI] [PubMed] [Google Scholar]
- 76.Morales-Montor J, Hallal-Calleros C, Romano MC, Damian RT. Inhibition of p-450 aromatase prevents feminisation and induces protection during cysticercosis. Int J Parasitol. 2002;32:1379–1387. doi: 10.1016/S0020-7519(02)00130-3. [DOI] [PubMed] [Google Scholar]
- 77.Seif El-Din SH, Abdel-Aal Sabra AN, Hammam OA, El-Lakkany NM. Effect of ketoconazole, a cytochrome P450 inhibitor, on the efficacy of quinine and halofantrine against Schistosoma mansoni in mice. Korean J Parasitol. 2013;51:165–175. doi: 10.3347/kjp.2013.51.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ziniel PD, et al. The Schistosoma mansoni Cytochrome P450 (CYP3050A1) Is Essential for Worm Survival and Egg Development. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alegria-Lopez MA, Rodriguez-Vivas RI, Torres-Acosta JF, Ojeda-Chi MM, Rosado-Aguilar JA. Use of Ivermectin as Endoparasiticide in Tropical Cattle Herds Generates Resistance in Gastrointestinal Nematodes and the Tick Rhipicephalus microplus (Acari: Ixodidae) J Med Entomol. 2015;52:214–221. doi: 10.1093/jme/tju025. [DOI] [PubMed] [Google Scholar]
- 80.Wong A, Bandiera SM. Induction of hepatic cytochrome P450 2B and P450 3A isozymes in rats by zolazepam, a constituent of Telazol. Biochem Pharmacol. 1998;55:201–207. doi: 10.1016/S0006-2952(97)00432-2. [DOI] [PubMed] [Google Scholar]
- 81.Pleticha J, et al. Pig lumbar spine anatomy and imaging-guided lateral lumbar puncture: a new large animal model for intrathecal drug delivery. J Neurosci Methods. 2013;216:10–15. doi: 10.1016/j.jneumeth.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott EW, McKellar QA. The distribution and some pharmacokinetic parameters of ivermectin in pigs. Veterinary research communications. 1992;16:139–146. doi: 10.1007/BF01839011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.


