Abstract
Background:
Many patients with asthma are potentially overtreated, which results in unnecessary cost and unnecessary exposure to drugs that may result in adverse events. Step down helps reduce overtreatment, may mitigate these harms, and is advocated by major guidelines. Unfortunately, data that support step down are sparse.
Objectives:
This systematic review aimed to examine the effect of stepping down from scheduled inhaled corticosteroids (ICS) to as-needed ICS in patients with stable asthma.
Methods:
Several electronic databases were systematically searched in April 2014. Articles were screened independently in duplicate. Studies were required to have at least a 12-week follow-up duration and to have compared stepping down from scheduled ICS to as-needed ICS and maintenance of scheduled ICS. Patients were required to have stable asthma as evidenced by at least 4 weeks without asthma exacerbation before intervention.
Results:
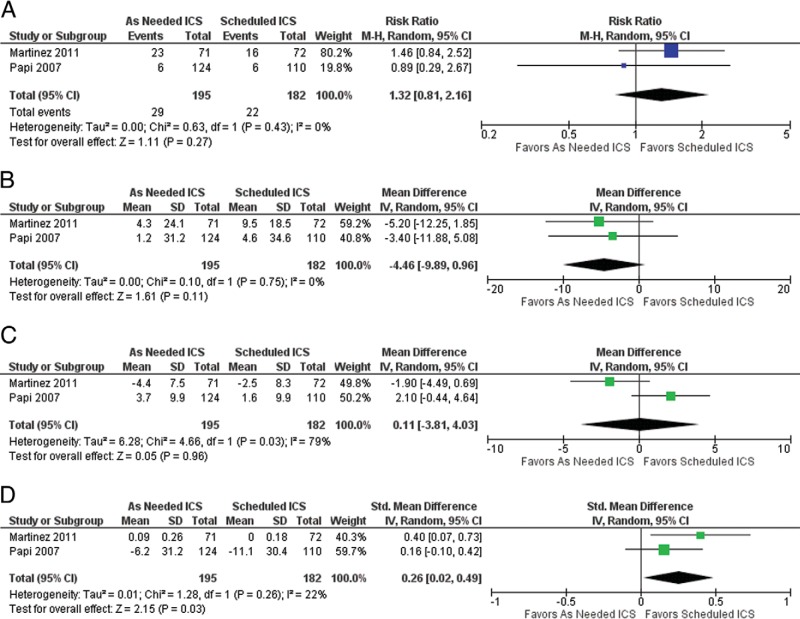
A total of 3025 abstracts were retrieved initially, 77 of which were retrieved for full-text screening. Of these, only two articles were found to be eligible for inclusion, both were randomized controlled trials. By using random effects meta-analysis, it was determined that, after a follow-up of 6–10 months, the relative risk of exacerbation of stepping down from scheduled to as-needed ICS was 1.32 (95% confidence interval [CI], 0.81–2.16; p = 0.27, I2 = 0%). Those who did not step down had more symptom-free days (standard mean difference 0.26 [95% CI, 0.02–0.49; p = 0.03; I2 = 22%]).
Conclusion:
There is currently insufficient evidence to associate stepping down from scheduled to as-needed ICS with a change in exacerbations, although it may lead to fewer symptom-free days.
Keywords: Asthma, asthma medication, step-down, inhaled corticosteroids, overtreatment, systematic review, meta-analysis
Worldwide asthma affects more than 300 million persons.1 Country-dependent estimates of asthma prevalence range from 1 to 18%. In the United States, asthma occurs in ∼8% of the population, which affects nearly 19 million adults and 7 million children.2 Asthma causes significant morbidity, mortality, and cost. Worldwide, annual asthma-related deaths total 250,000.1 In addition, worldwide, asthma results in 15 million disability adjusted life years lost each year. In the United States, asthma is responsible for more than 3000 deaths each year.2 Furthermore, in the United States, asthma is associated with nearly 480,000 hospitalizations, 2 million emergency department visits, and 9 million physician visits as well as nearly 25 million missed days of work or school each year. In all, asthma results in $56 billion in costs to the U.S. health care system each year.2
Although these figures may represent an undertreatment of asthma, many patients with asthma are potentially overtreated. Overtreatment of asthma exposes patients not only to unnecessary cost but unnecessary exposure to drugs that may result in adverse events. Long-acting β-agonists have been associated with asthma-related death3 and inhaled corticosteroids (ICS) have been associated with a variety of adverse effects, including reduced growth velocity in children, osteoporosis, hoarseness and/or dysphonia, and oral candidiasis.1 Therefore, reducing or “stepping down” the amount of asthma medication may reduce the patient's chance of experiencing an adverse event4 and reduce the financial burden of care.
Step down is advocated by major guidelines5,6 and is often successful but not frequently done.7 The current recommendations state that step down can be attempted if the patient's asthma has been stable for at least 3 months. Unfortunately, data that support this recommendation are sparse, and there is uncertainty about the optimal timing and benefit-risk balance of stepping down, which is compounded by the variety of ways that patients can step down asthma medication. Recent systematic reviews8–10 indicate that stepping down can be accomplished safely for some patients and that providers who care for patients with asthma should inform them about the risk-benefit balance of stepping down. This is especially important because many patients may step down without informing their care provider.7,11 One step-down approach for patients with stable asthma is of transitioning from a scheduled dose of ICS to an as-needed regimen. This approach reduces the amount of drug to which patients are exposed, reduces patients' risk of adverse events,4 reduces patients' out of pocket costs, and may improve adherence to the medication regimen. Therefore, the aim of this systematic review was to help inform the step-down conversation between providers and patients by examining the effect of stepping down from scheduled ICS to as-needed ICS in patients with stable asthma.
METHODS
Study Design
This systematic review and meta-analysis is consistent with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement,12 and the protocol is registered with PROSPERO13 (registration CRD42014009465.
Criteria for Considering Studies for This Review
Type of studies.
We included randomized controlled trials and observational studies that compared a treatment step down from scheduled ICS with as-needed ICS and maintenance of scheduled ICS. Trials were required to have run-in periods of at least 4 weeks to establish a diagnosis of stable asthma for participants.
Type of participants.
Participants were required to have stable asthma as defined by at least 4 weeks without an asthma exacerbation before intervention. Study eligibility was not restricted based on participant age.
Type of interventions.
Studies that evaluated the effect of stepping down from scheduled ICS to as-needed ICS were included. The follow-up duration of at least 12 weeks was required for eligibility to ensure an adequate length of follow-up for the outcomes to occur.
Type of outcome measurements.
The primary outcome measurement was asthma exacerbation as defined within studies. Secondary outcome measurements included emergency department visits, hospitalizations, asthma-related death, cost, asthma symptoms, asthma quality of life, and lung function as defined by peak expiratory flow and forced expiratory volume in the first second. Asthma exacerbation was chosen as the primary outcome because it is a patient important outcome that has significant consequences for persons with asthma.
Search Methods for the Identification of Studies
We designed and conducted a search strategy by using methods recommended by the Institute of Medicine,14 which included a search of several databases, including PubMed, Scopus (Elsevier B.V., Amsterdam, The Netherlands), Ovid MEDLINE, Ovid EMBASE, Ovid EBM Reviews CENTRAL, and Ovid PsycInfo (Ovid Technologies, New York, NY). The databases were searched from the time of their inception to April 2014, with no language restrictions. A copy of the search strategy can be found in Supplemental Appendix 1. The initial electronic search strategy was supplemented by hand searching the reference lists from the included studies, through contacting experts in the field as well as authors of included studies, and through searching our personal collections. In addition, a recent article on a similar topic was reviewed to identify any potentially eligible studies that may have been missed through other methods.15 Finally, clinical trial registries were searched to identify in-progress and completed studies.
Selection of Studies
Search results were uploaded into systematic review software (DistillerSR, Evidence Partners, Ottawa, Canada). Abstracts and full texts were assessed independently and in duplicate. Any disagreements were resolved by consensus. When consensus was not achieved between two reviewers, a third reviewer arbitrated.
Data Collection
For each study, we extracted our primary and secondary outcomes. Extraction was done independently and in duplicate, and disagreements were resolved by discussion and consensus. Unclear data were confirmed with the study author when possible.
Risk of Bias Assessment
We used the Cochrane Collaboration's risk of bias tool to evaluate the methodologic quality of included studies.16 The risk of bias in included studies was assessed in duplicate by reviewers working independently. Any disagreements were resolved by consensus.
Analysis
Review Manager version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) was used for statistical analysis. Random-effect models by DerSimonian and Laird17 were used to calculate the relative risk with 95% confidence intervals (CI) for dichotomous variables and weighted mean difference among groups for continuous variables. For outcomes assessed by using different measurements, the standardized mean difference was used. A minimum important difference was defined as 0.5 standard deviations.18 Heterogeneity was assessed by using the I2 statistic, with values greater than 75% indicative of high heterogeneity.19
RESULTS
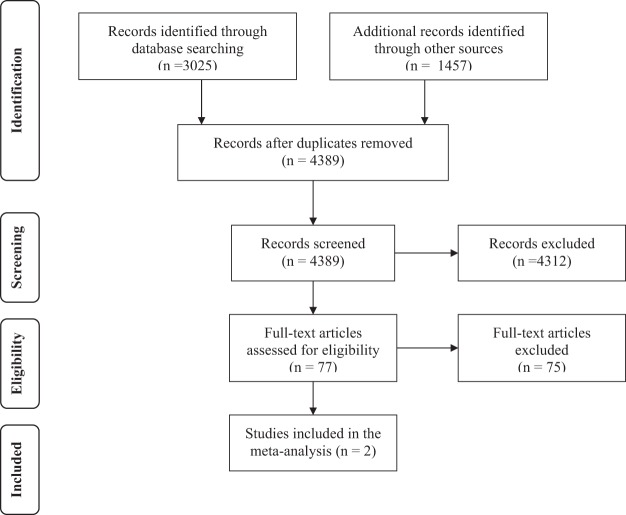
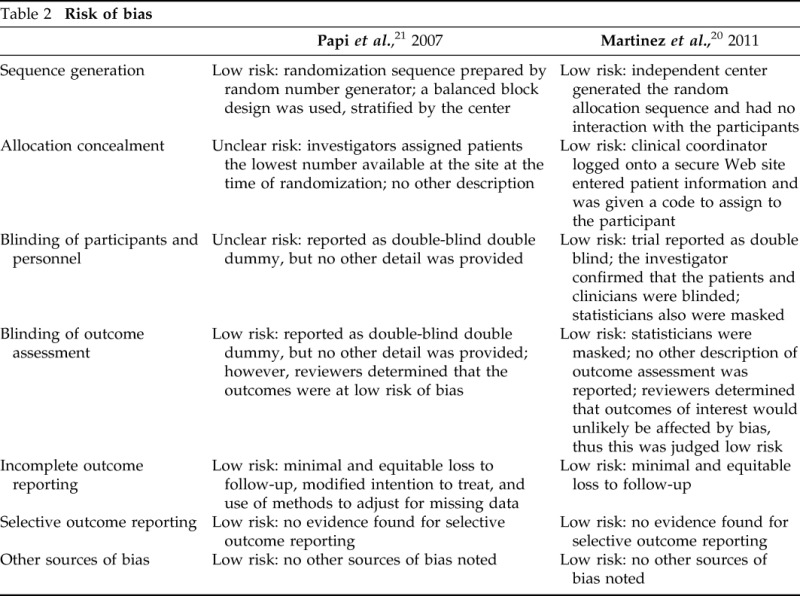
Our initial search of electronic databases identified 3025 abstracts. Of these abstracts, 77 were retrieved for full-text screening, and two were included for analysis. Both of the included studies were randomized controlled trials. Additional searches and contacting experts did not reveal any additional studies. The Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram can be seen in Fig. 1. The two identified studies were Martinez et al.20 and Papi et al.21 Characteristics regarding the studies can be found in Table 1. The risk of bias assessment of these studies can be found in Table 2. Most of the items in the assessment were judged as low risk for bias, and the overall risk of bias for both studies was judged to be low.
Figure 1.
PRISMA flow diagram describing the steps of study selection. Source: Ref. 12.
Table 1.
Characteristics of studies
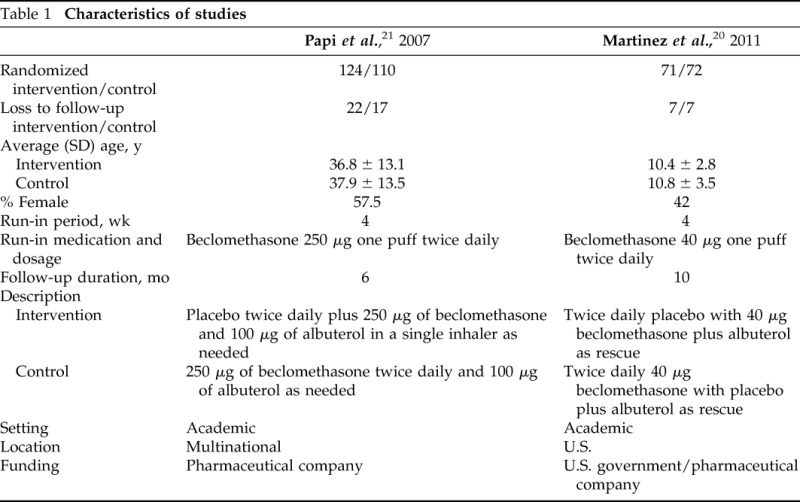
Table 2.
Risk of bias
Forest plots for the available outcomes can be found in Fig. 2. We did not find sufficient evidence to confirm a difference in exacerbations between those patients who were stepped down from scheduled ICS to as-needed ICS and those who continued scheduled ICS: relative risk 1.32 (95% CI, 0.81–2.16; p = 0.27, I2 = 0%). Similarly, for both morning peak expiratory flow and percent predicted forced expiratory volume in the first second, we did not find sufficient evidence to confirm a difference between those patients who were stepped down from scheduled ICS to as-needed ICS and those who continued on scheduled ICS, mean difference –4.46 (95% CI, –9.89 to 0.96; p = 0.11, I2 = 0%) and mean difference 0.11 (95% CI, –3.81 to 4.03; p = 0.96, I2 = 79%), respectively. However, we did find a statistically significant difference for change in symptoms and/or proportion of asthma control days for those patients who were stepped down from scheduled ICS to as-needed ICS compared with those patients who continued with scheduled ICS, with a standardized mean difference 0.26 (95% CI, 0.02–0.49; p = 0.03, I2 = 22%), which indicates that those who do not step down have more symptom-free days. Finally, we were unable to calculate a pooled effect for hospitalizations. None were reported in Martinez et al.20 Three were reported in the scheduled ICS arm of Papi et al.,21 compared with zero in the as-needed ICS arm. Due to the small number of studies, we were unable to assess the risk of publication bias.
Figure 2.
Asthma outcomes in patients who step down from scheduled to as needed ICS compared to those who remain on a scheduled dose of ICS. A) RR of asthma exacerbation in patients who step down to an as needed dosage of ICS compared to remaining on a scheduled dosage of ICS. B) Mean difference in Morning PEF in patients who step down to an as needed dosage of ICS compared to remaining on a scheduled dosage of ICS. C) Mean Difference in percent predicted FEV1 in patients who step down to an as needed dosage of ICS compared to remaining on a scheduled dosage of ICS.). Change in Symptoms/Proportion of Asthma Control Days in patients who step down to an as needed dosage of ICS compared to remaining on a scheduled dosage of ICS.
DISCUSSION
Several systematic reviews and meta-analyses have examined step down of asthma medications.8–10,15,22 Two previous systematic reviews examined the question of stepping down from scheduled to as-needed ICS15,22; however, both also examined patients with wheezing and did not explicitly require patients to have stable asthma as evidenced by 4 weeks without an asthma exacerbation.15,22 Therefore, our review is the first to examine step down from scheduled to as-needed ICS, specifically in patients with stable asthma.
Similar to other reviews, we found few trials with few events. Consequently, the accumulated evidence does not allow one to make confident conclusions regarding the safety of stepping down from scheduled to as-needed ICS, specifically regarding the risk of exacerbation or measurements of lung function. Despite the limited available data, we did find a statistically significant difference that indicates that those patients who step down from scheduled ICS to as-needed ICS have more symptoms than those who do not step down and continue with scheduled ICS. However, this difference does not meet our protocol-defined threshold of a minimally important difference, so the clinical significance of this finding is unclear.
Our findings highlight the need for additional studies in this area, a point that has been emphasized by other researchers.1,15,23 Additional studies may identify subgroups of patients, e.g., children versus adults, in whom step down may be more likely to be successful. Heterogeneity of results may sometimes indicate a subgroup effect and, although our studies did differ on patient age and although a heterogeneity was found in the outcome of the mean difference in the percent predicted forced expiratory volume in the first second, our review did not allow for a robust evaluation of heterogeneity. Additional studies may also confirm findings, e.g., the finding in Martinez et al.20 that as-needed exposure to ICS did not significantly suppress growth in children. Continuous exposure to ICS was found to suppress growth in the study by Martinez et al.20 and has been found in other studies as well.24,25
In conclusion, our systematic review and meta-analysis found that there is limited evidence available to assess whether stepping down from scheduled ICS to as-needed ICS is safe or effective. Based on data from two randomized trials, we found a statistically significant difference in symptoms, with those not stepping down having fewer symptoms, but, we could not find enough evidence to make confident conclusions about the effect step-down treatment has on exacerbations or measurements of lung function. Based on this paucity of data, we call for future studies, both randomized and nonrandomized. In the meantime, analysis of our data, and the uncertainty it represents, supports the need for patients and their clinicians to have informed conversations about the risks and benefits of stepping down asthma treatment.
ACKNOWLEDGMENTS
The authors thank Larry J. Prokop and Patricia J. Erwin, M.L.S., for their expert assistance in the development and execution of the search strategy, and Victor M. Montori, M.D., M.S., for his invaluable insight and guidance throughout this project.
Footnotes
M.R. Gionfriddo was supported by Clinical and Translational Science Award grant TL1 TR000137 from the National Center for Advancing Translational Science
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2012. Available from: http://www.ginasthma.org/
- 2. National Center for Environmental Health. Asthma's impact on the nation, 2012. http://www.cdc.gov/asthma/impacts_nation/asthmafactsheet.pdf
- 3. McMahon AW, Levenson MS, McEvoy BW, et al. Age and risks of FDA-approved long-acting adrenergic receptor agonists. Pediatrics 128:e1147–e1154, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: An evidence-based approach. Med J Aust 178:223–225, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2014. Available from: http://www.ginasthma.org/
- 6. National Heart Lung Blood Institute. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma— Full report. August 2007. http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf
- 7. Rank MA, Branda ME, McWilliams DB, et al. Outcomes of stepping down asthma medications in a guideline-based pediatric asthma management program. Ann Allergy Asthma Immunol 110:354–358.e352, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Hagan JB, Samant SA, Volcheck GW, et al. The risk of asthma exacerbation after reducing inhaled corticosteroids: A systematic review and meta-analysis of randomized controlled trials. Allergy 69:510–516, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Brozek JL, Kraft M, Krishnan JA, et al. Long-acting β2-agonist step-off in patients with controlled asthma. Arch Intern Med 172:1365–1375, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Rank MA, Hagan JB, Park MA, et al. The risk of asthma exacerbation after stopping low-dose inhaled corticosteroids: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol 131:724–729, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Rank MA, Ziegenfuss JY, Shah KM, et al. Factors associated with decisions to step down asthma medications. J Allergy Clin Immunol Pract 1:312–314, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 62:1006–1012, 2009. [DOI] [PubMed] [Google Scholar]
- 13. PROSPERO. Centre for Reviews and Dissemination, University of York, York, United Kingdom. http://www.crd.york.ac.uk/PROSPERO
- 14. Eden J., Levit L., Berg A., Morton S., eds. Finding what works in health care: Standards for systematic reviews. Institute of Medicine. National Academies Press, Washington D.C.; 2011 [PubMed] [Google Scholar]
- 15. Chauhan BF, Chartrand C, Ducharme FM. Intermittent versus daily inhaled corticosteroids for persistent asthma in children and adults. Cochrane Database Syst Rev 2:CD009611, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S. (Eds). Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2011. http://handbook.cochrane.org/ [Google Scholar]
- 17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 7:177–188, 1986. [DOI] [PubMed] [Google Scholar]
- 18. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 41:582–592, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 327:557–560, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): A randomised, double-blind, placebo-controlled trial. Lancet 377:650–657, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med 356:2040–2052, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Rodrigo GJ, Castro-Rodriguez JA. Daily vs. intermittent inhaled corticosteroids for recurrent wheezing and mild persistent asthma: A systematic review with meta-analysis. Respir Med 107:1133–1140, 2013. [DOI] [PubMed] [Google Scholar]
- 23. Rank MA, Peters SP. The risks, benefits, and uncertainties of stepping down asthma medications. J Allergy Clin Immunol Pract 2:503–509, quiz 510, 2014. [DOI] [PubMed] [Google Scholar]
- 24. Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: Effects on growth. Cochrane Database Syst Rev 7:CD009471, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pruteanu AI, Chauhan BF, Zhang L, et al. Inhaled corticosteroids in children with persistent asthma: Dose-response effects on growth. Cochrane Database Syst Rev 7:CD009878, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]