Abstract
Prediction of recurrence is a challenge for the development of adjuvant treatments in clear-cell renal cell carcinoma (ccRCC). In these tumors, expression of long non-coding RNAs (lncRNAs) are deregulated and closely associated with prognosis. Thus, we aimed to predict ccRCC recurrence risk using lncRNA expression. We identified prognostic lncRNAs in a training set of 351 localized ccRCCs from The Cancer Genome Atlas and validated lncRNA-based recurrence classification in an independent cohort of 167 localized ccRCCs. We identified lncRNA MFI2-AS1 as best candidate in the training set. In the validation cohort, MFI2-AS1 expression was independently associated with shorter disease-free survival (Hazard Ratio (HR) for relapse 3.5, p = 0.0001). Combined with Leibovich classification, MFI2-AS1 status improved prediction of recurrence (C-index 0.70) compared to MFI2-AS1 alone (0.67) and Leibovich classification alone (0.66). In patients with aggressive tumors (Leibovich ≥5), MFI2-AS1 expression was associated with dramatically increased risk of relapse (HR 12.16, p < 0.0001) compared to patients with undetectable MFI2-AS1 who had favorable outcomes. Compared to normal samples, MFI2-AS1 was upregulated in tumor tissue, and higher expression was associated with metastatic dissemination. Overall, MFI2-AS1 status improves patient stratification in localized ccRCC, which supports further integration of lncRNAs in molecular cancer classifications.
Introduction
Almost half of the 300,000 people diagnosed with kidney cancer each year die from cancer progression1. Clear cell renal cell carcinoma (ccRCC), more than 75% of renal cell carcinomas2, is diagnosed as localized disease in 70% of cases. Partial or radical nephrectomy is standard therapy for stage I–III ccRCC, one third of which will recur.
Improving prognostic classifications is a major challenge. Indeed, adjuvant clinical trials use clinicopathological criteria associated with poor prognosis, such as elevated T stage, high pathological grade or nodal involvement3–5. Thus, most patients in these trials belong to intermediate or high-risk groups according to the historical Leibovich classification6. However, 70% of patients in the intermediate group and 30% of patients in the high-risk group will not have ccRCC recurrence at 5 years. Thus, many adjuvant trials report negative results for improved recurrence-free survival, and many patients are treated needlessly. New insights in tumor biology are needed to improve prognostic classifications.
Historically, ccRCC is known for alterations of the VHL protein, which leads to the activation of the hypoxia inducible factor pathway that promotes angiogenesis7. Recent studies have found alterations of chromatin remodeling genes thought to be crucial to carcinogenesis, such as BAP1, PBRM1 and SETD2, in up to 40% of ccRCCs8, which might indicate that epigenetic regulation is important for ccRCC progression.
Long non-coding RNAs (lncRNAs), ribonucleic acids with more than 200 bases that act as genome-wide epigenetic regulators, are new players that might be involved in ccRCC ontogeny. Indeed, multiple lncRNAs are reported to be deregulated in ccRCC9, 10. Importantly, study of lncRNA signatures might help discriminate malignant and benign tumors11 and improve cancer subtype classifications12. We recently attempted to study the expression profile of lncRNAs in ccRCC through unsupervised clustering and found 4 different clusters, including a subgroup C2 that was associated with dismal prognosis and aggressive tumor features13. Thus, lncRNAs appear to be putative potent predictors of survival in ccRCC.
Despite attempts to classify recurrence risk according to molecular features of ccRCC14, strong markers of recurrence easily implemented in clinic are lacking. Thus, we sought to identify lncRNAs strongly associated with prognosis and improve patient stratification with a recurrence risk assessment based on lncRNA expression in patients with localized ccRCC.
Material and Methods
Selection of the reference recurrence model
Two scores are validated to evaluate the risk of relapse in localized ccRCC after radical nephrectomy, the UCLA staging system (UISS) and the Leibovich score. The UISS classification includes T and N status, pathological grade and clinical performance status, while the Leibovich score includes T and N status, tumor size, pathological grade and necrosis.
Several considerations made us choose the Leibovich score over the UISS classification. Published literature suggests that the Leibovich scoring system is more accurate than the UISS classification regarding the prediction of outcomes in localized ccRCC after radical nephrectomy15. In addition, the Leibovich score was the standard chosen for the recent evaluation of molecular classifications for recurrence in localized ccRCC14. Finally, the recent major adjuvant clinical trials in clear-cell renal cell carcinoma (ccRCC) selected patients based on modified UISS risk groups that were not validated16–18. These considerations made the choice of the Leibovich score the most relevant validated classification for the evaluation of recurrence risk in localized ccRCC.
Leibovich classification stratifies patients with low (0–2), intermediate (3–5) or high (>5) recurrence risk. Current adjuvant trials select patients on the basis of aggressive pathological features that belong to both intermediate and high-risk groups17. Thus, we chose to discriminate between patients with Leibovich scores <5 and those with scores ≥5 to account for intermediate-risk patients with aggressive features and comply with current adjuvant trials.
Patients
We evaluated two independent retrospective cohorts: a discovery set from the TCGA database19 and a validation set from Pitié-Salpêtrière Hospital.
The discovery set included patients with stages I, II and III ccRCC who underwent partial or radical nephrectomy between August 2005 and January 2016, with available RNA-sequencing data and clinical annotations. Clinical data included disease-free survival (DFS), overall survival (OS) and follow-up (FU).
The validation cohort included patients with available fresh frozen tumor samples from stages I, II and III ccRCC. The samples underwent central review by an expert pathologist according to the guidelines of the International Society of Urological Pathology20. Patients with TFE3 or TFEB translocations and hereditary cancers were excluded because the natural history and oncogenic alterations might differ from those associated with sporadic ccRCC. Other exclusion criteria were insufficient RNA yield (<2,000 ng) and FU less than 3 months. Clinicopathological data included age, gender, tumor size according to TNM staging, pathological grade, sarcomatoid or rhabdoid features, necrosis, renal vein thrombosis, Leibovich classification, tumor recurrence (local or distant) and DFS. Up to 4 samples from the primary tumor were collected in six patients to assess tumor heterogeneity for lncRNA expression. For exploratory analyses, matched normal tissue samples from 21 patients were included, as well as additional primary tumors from 21 patients who had metastases at diagnosis. Each tissue sample was collected through surgical resection.
All patients had previously provided written informed consent for tumor collection and analysis. The study was approved by the ethical committee of Pitié-Salpêtrière Hospital (IDF-6, Ile de France). The collection and use of tissues followed ethical procedures formulated in the Helsinki Declaration.
Procedures
In the discovery set, RNA expression had been assessed by RNA sequencing from fresh frozen primary tumor samples. Total RNA from each sample was converted into cDNA with the Illumina TruSeq RNA sample preparation kit, and cDNA was sequenced with the Illumina HiSeq. 2000 platform according to the manufacturer’s instructions.
For the validation set, expression of target lncRNA MFI2-AS1 was assessed with real-time quantitative PCR from fresh frozen primary tumor samples. Total RNA was extracted from tumor samples using the ThermoFisher Scientific PureLink® RNA mini kit. Total RNA quantitation was performed with ThermoFisher Scientific Multiskan GO® microplate spectrophotometer. Supplementary extractions were performed if the total RNA yield was less than 2000ng of total RNA. After extraction, total RNA was converted into cDNA using the ThermoFisher Scientific high capacity RNA to cDNA kit. Real-time quantitative PCR was performed using the ThermoFisher Scientific TaqMan® master mix and predesigned primer and probe sets for MFI2-AS1 (reference: Hs04274310_g1). Each experiment was conducted in duplicate, with 40ng of RNA in each well. The experiment was performed on a Roche LightCycler® 480 platform up to 40 PCR cycles, according to the manufacturer’s instructions. Relative quantification of gene expression was performed using the Delta Cp method21. Detectable expression was defined by a crossing point (Cp) <40, with similar expression in the duplicate experiment (differential Cp <1) and adequate curve aspect (Supplementary Figure 1). We chose PPIA as the reference gene for relative quantitation of gene expression, as it has been reported as a top-ranked reference gene for gene expression analysis in ccRCC22–24. To validate this approach, we internally evaluated multiple reference genes in addition to PPIA, including GUSB, reported as a stable marker in ccRCC25, as well as 18 S and GAPDH. We assessed the expression of a known target lncRNA, HOTAIRM1, in five reference ccRCC samples, using RNA-sequencing and qRT-PCR with each reference gene. PPIA was the most accurate reference gene, allowing the highest correlation between qRT-PCR and RNA-sequencing data (Spearman r = 0,800).
Endpoints and statistical analysis
The primary endpoint was the impact of lncRNA expression to predict disease recurrence in localized ccRCC. The primary outcome for both cohorts was DFS, defined as the time from initial surgery to first relapse, identified by physical examination, biopsy or imaging. DFS was censored at the last FU or death in patients without documented recurrence.
The secondary endpoint in the training set was to identify associations between MFI2-AS1 expression and genome-wide expression changes. In the validation cohort, secondary endpoints were DFS according to clinicopathological subgroups, DFS according to lncRNA expression and Leibovich classification, and differential lncRNA expression between normal tissue, localized tumors, and metastatic tumors in the validation cohort.
To identify candidate prognostic lncRNAs, we studied 1934 lncRNAs previously identified13 as significantly expressed in the TCGA ccRCC cohort, with reads per kilobase per million mapped reads (RPKM) ≥1 in at least 10% of ccRCC samples. The choice of candidate lncRNAs was based on their association with DFS and OS, expression relative to tumor stage, oncogenic potential, and availability for standard gene expression assessment. A multiple Cox regression model was used to identify lncRNAs associated with DFS (false discovery rate (FDR) <0.01) and OS (FDR <0.05). T-tests were used to compare lncRNA expression between primary tumors from metastatic patients, localized tumors, and normal tissue.
We sought to identify pathways and mechanisms that could be altered by the dysregulation of MFI2-AS1 expression. In TCGA dataset, we studied differential gene expression in the top 10% of tumors expressing MFI2-AS1 compared to the bottom 10%. We defined upregulated genes by a twofold increased expression FDR < 0.05 and p < 0.05. We performed functional annotations of upregulated genes with Gene Ontology (GO) terms using DAVID functional annotation tool.
In the validation set, the correlation between lncRNA expression and DFS was estimated by the Kaplan-Meier model. Comparison of survival curves in univariate analysis was performed with the log-rank test. A multivariate Cox regression model was used in the analysis. In the survival models, the p-value was calculated under the null hypothesis of a hazard ratio (HR) of 1. C-statistics were determined to assess model adequacy and were compared to the Leibovich classification accuracy. Associations between lncRNA expression and clinicopathological data were evaluated with the Fisher exact test or Wilcoxon test, depending on the variables.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
LncRNA MFI2-AS1 as top-rank marker of ccRCC recurrence
We analyzed 1934 lncRNAs expressed in tumors from the TCGA ccRCC cohort19 with appropriate annotations and RNA-sequencing data (N = 423). In 351 patients with localized disease (Table 1), we looked for lncRNAs associated with shorter DFS and identified 47 statistically significant lncRNAs (adjusted p-value < 0.01). Of those, 40 lncRNAs were also associated with shorter OS (adjusted p-value < 0.05) (Fig. 1 and Supplementary dataset 1).
Table 1.
Clinicopathologic characteristics of the patients in the training and validation sets.
| Variable | Training set population with localized ccRCC | Validation set population with localized ccRCC | |||
|---|---|---|---|---|---|
| Population | N = 351 | % | N = 167 | % | |
| Gender | Male | 224 | 64 | 111 | 66 |
| Female | 127 | 36 | 56 | 34 | |
| T stage | 1/2 | 244 | 70 | 144 | 86 |
| 3/4 | 107 | 30 | 23 | 14 | |
| Grade | 1/2 | 173 | 49 | 110 | 66 |
| 3/4 | 178 | 51 | 57 | 34 | |
| Sarcomatoid | Yes | — | — | 14 | 8 |
| No | — | — | 153 | 92 | |
| Rhabdoid | Yes | — | — | 6 | 4 |
| No | — | — | 161 | 96 | |
| Necrosis | Yes | — | — | 12 | 7 |
| No | — | — | 155 | 93 | |
| Thrombosis | Yes | — | — | 7 | 4 |
| No | — | — | 160 | 96 | |
| Leibovich score | Low | — | — | 107 | 64 |
| Intermediate | — | — | 40 | 24 | |
| High | — | — | 20 | 12 | |
| Leibovich score | <5 | — | — | 135 | 81 |
| ≥5 | — | — | 32 | 19 | |
| Age (median, years) | 61 (26–90) | 63 (31–90) | |||
| Follow-up (median, months) | 38 (0–122) | 41 (3–122) | |||
Figure 1.
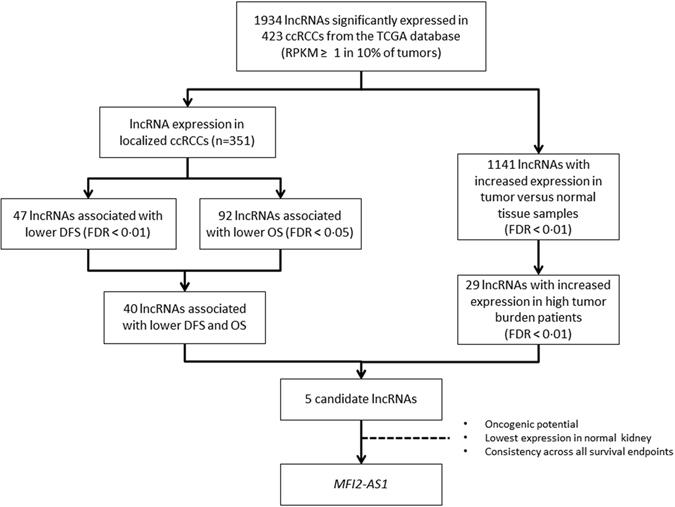
Selection of prognostic long non-coding RNA in the discovery set from The Cancer Genome Atlas.
To determine which lncRNAs might be oncogenic and important in ccRCC metastasis, we focused on lncRNAs overexpressed in cancer versus normal kidney tissue (1134 lncRNAs, p < 0.01) or overexpressed in metastatic kidney cancer compared with non-metastatic kidney cancer (71 lncRNAs, p < 0.01). We found 5 candidate lncRNAs: MFI2-AS1, SNHG15, RP4-584D14.7, RP11-465L10.7, and CTC-444N24.8. Of those, only MFI2-AS1 26 and SNHG15 27, 28 have been reported as putative oncogenes in other cancers. MFI2-AS1 had the lowest expression in normal kidney tissue and was thus chosen as the top candidate (Fig. 1 and Supplementary dataset 1).
We investigated the implication of MFI2-AS1 in regard to the lncRNA-based unsupervised clustering of ccRCC13. MFI2-AS1 was overexpressed in cluster 2 as compared to other clusters (Fold change = 3.02, p-value = 8.52 × 10−15, FDR = 5,15 × 10−13), which was associated with dismal prognosis and aggressive tumor features. Correlations between MFI-2AS1 expression and copy-number variations found a high correlation with amplification of its locus at 3q29 (correlation coefficient = 0.34, corrected p-value = 4,44 × 10−16).
Among 13656 genes, we identified 541 genes that were upregulated in tumors with high expression of MFI2-AS1. Functional analysis revealed that these genes were associated with acute phase inflammatory response (Fig. 2 and Supplementary dataset 2). These genes include haptoglobin, erythropoietin, interleukin 6, CEBPB, lipopolysaccharide binding protein, Serpin A3, and serum amyloid proteins A1, A2, A4.
Figure 2.
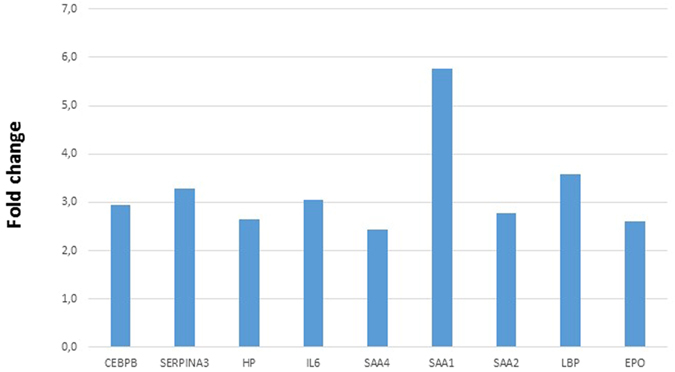
Differential expression of genes involved in acute-phase response in the top 10% of tumors expressing MFI2-AS1 compared to the bottom 10%. CEBPB: CCAAT/enhancer binding protein beta(CEBPB), EPO: erythropoietin, HP: haptoglobin, IL6: Interleukin 6, LBP: lipopolysaccharide binding protein, SERPINA3: Serpin A3, SAA: serum amyloid protein.
MFI2-AS1 is a potent and independent predictive marker for ccRCC recurrence
The validation cohort included 204 patients with localized ccRCC. Five patients were excluded for insufficient RNA yield, another five for insufficient quality of PCR data (attributed to degraded RNA), and 27 were excluded for insufficient FU. Characteristics of the analysis population are reported in Table 1. The median FU was 41 months (range, 3–122 months); median age at diagnosis was 63 years (range, 31–90 years), with a male to female ratio of 1.9 to 1. Most patients had low T stage (86% of T stage 1–2) and low-grade tumors (66% with grade 1–2). Less than 10% of patients had sarcomatoid or rhabdoid components, tumor necrosis or renal vein thrombosis in pathological review. According to Leibovich scores, 107 (64%) patients had low recurrence risk, while 40 (24%) and 20 (12%) had intermediate or high risk, respectively. Overall, 135 (81%) patients had Leibovich scores <5 and 32 (19%) patients had Leibovich scores ≥5.
Analysis of MFI2-AS1 expression by quantitative PCR revealed that two thirds of tumors (66.5%) did not express MFI2-AS1 at a detectable level. We thus plotted a receiver operating characteristic (ROC) curve for recurrence based on the relative expression of MFI2-AS1 in the validation cohort. Analysis of the ROC curves determined that the best performing stratification of patients to assess recurrence risk was based on the discrimination between the presence or absence of detectable MFI2-AS1 transcripts (Supplementary Figure 2).
MFI2-AS1 expression was associated with significantly shorter DFS in the univariate analysis, with a HR for relapse of 3.5 (95% CI [1.64–7.49], p = 0.0001) (Table 2 and Fig. 3A). Of note, the median DFS was not reached in both groups. Patients with MFI2-AS1 expression also had more aggressive tumor features, including sarcomatoid or rhabdoid contingents (p = 0.0424), tumor necrosis (p = 0.0219) or renal vein thrombosis (p = 0.0429) (Table 3). While patients with MFI2-AS1 expression more often had high Leibovich scores (p = 0.0205), this association was not reported when comparing Leibovich scores ≥5 to those <5. No association was found between MFI2-AS1 and tumor grade (p = 0.1078). Other variables associated with increased DFS in univariate analysis were renal vein thrombosis (p < 0.0001), necrosis (p = 0.0008), grade 3–4 (p = 0.0003), sarcomatoid or rhabdoid contingent (p < 0.0001), and Leibovich score ≥5 (p < 0.0001) (Table 2).
Table 2.
Hazard ratio for disease-free survival according to patient and tumor characteristics.
| Variable | Hazard ratio [95% CI] | p-value in univariate analysis |
|---|---|---|
| Age (years) >65 vs <65 | 1.52 [0.77–3.03] | 0.2238 |
| Gender Male/Female | 1.14 [0.55–2.36] | 0.7267 |
| Thrombosis Yes/No | 7.57 [0.58–99.38] | <0.0001 |
| Necrosis Yes/No | 4.02 [0.87–18.60] | 0.0008 |
| Sarcomatoid or rhabdoid Yes/No | 5.50 [1.49–20.40] | <0.0001 |
| Tumor grade 3–4/1–2 | 3.27 [1.55–6.93] | 0.0003 |
| T Stage 3–4/1–2 | 2.11 [0.72–6.19] | 0.0721 |
| Leibovich score ≥5/<5 | 4.11 [1.52–11.10] | <0.0001 |
| Leibovich score High vs. intermediate | 3.33 [1.11–9.99] | <0.0001 |
| Intermediate vs. low | 1.99 [0.80–4.93] | |
| High vs. low | 7.29 [1.63–32.58] | |
| MFI2-AS1 expression Yes/No | 3.50 [1.64–7.49] | 0.0001 |
| Hazard ratio [95% CI] | p-value in multivariate analysis | |
| Tumor grade *3–4/1–2 | 3.37 [1.68–6.76] | 0.0006 |
| MFI2-AS1 expression *Yes/No | 3.62 [1.80–7.27] | 0.0003 |
| Leibovich score ** ≥5/<5 | 5.18 [2.49–10.81] | <0.0001 |
| MFI2-AS1 expression **Yes/No | 4.24 [2.07–8.70] | 0.0001 |
*Multivariate analysis according to MFI2-AS1 expression, T stage and grade. **Multivariate analysis according to MFI2-AS1 expression and Leibovich score.
Figure 3.
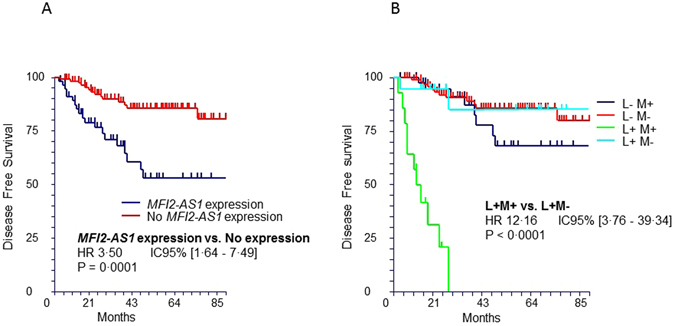
Kaplan-Meier estimates for disease-free survival (DFS) in the validation set. (A) DFS according to MFI2-AS1 expression in the entire cohort. (B) DFS according to MFI2-AS1 expression and Leibovich subgroups. L+ M+ (N = 14): Leibovich ≥5+ MFI2-AS1 expression. L+ M− (N = 18): Leibovich ≥5+ No MFI2-AS1 expression. L− M+ (N = 42): Leibovich <5+ MFI2-AS1 expression. L− M− (N = 93): Leibovich <5+ No MFI2-AS1 expression.
Table 3.
Characteristics of the patients according to MFI2-AS1 expression.
| Variable | Validation set | |||
|---|---|---|---|---|
| Population | MFI2-AS1 expression N = 56 | No MFI2-AS1 expression N = 111 | p-value | |
| Age (years) | >63 | 31 | 57 | 0.7429 |
| <63 | 25 | 54 | ||
| Gender | Male | 37 | 74 | 1.000 |
| Female | 19 | 37 | ||
| T Stage | 3/4 | 9 | 14 | 0.6352 |
| 1/2 | 47 | 97 | ||
| Tumor grade | 3/4 | 24 | 33 | 0.1078 |
| 1/2 | 32 | 78 | ||
| Sarcomatoid or rhabdoid | Yes | 11 | 9 | 0.0424 |
| No | 45 | 102 | ||
| Necrosis | Yes | 8 | 4 | 0.0219 |
| No | 48 | 107 | ||
| Thrombosis | Yes | 5 | 2 | 0.0429 |
| No | 51 | 109 | ||
| Leibovich | ≥5 | 13 | 18 | 0.2961 |
| <5 | 43 | 93 | ||
| Leibovich | Low | 30 | 77 | 0.0205 |
| Intermediate | 14 | 26 | ||
| High | 12 | 8 | ||
The prognostic impact of MFI2-AS1 expression remained highly significant in the multivariate analyses (Table 2). As the Leibovich score involves tumor grade, we used two distinct Cox regression models to avoid statistical bias. The first analysis included MFI2-AS1 expression and Leibovich score (<5 or ≥5), which remained independent from each other, with a p-value of 0.0001 for MFI2-AS1 and <0.0001 for the Leibovich score. The second analysis included MFI2-AS1 expression and tumor grade, which remained independent, with respective p-values of 0.0003 and 0.0006.
Intratumor variation of MFI2-AS1 expression
Spatial and temporal heterogeneity of clear-cell renal cell carcinoma have been recently uncovered29–31, and contribute to challenging the reproducibility of biomarker studies. Herein we studied intratumor heterogeneity of MFI2-AS1 expression in 17 samples from 6 primary tumors, with 2 to 4 distinct regions collected from each tumor.
All tumors had documented MFI2-AS1 expression. Multiregion analysis for MFI2-AS1 expression revealed that only one tumor had differential detection of MFI2-AS1 with absence of MFI2-AS1 expression reported in one region out of three studied (Supplementary Table 1). Normalized quantitation of MFI2-AS1 expression highlighted high variation between tumor samples. Two tumors had very similar MFI2-AS1 expression levels across the collected samples, with variations under 1,8 fold, while the three other tumors had variations from 7 to 91-fold.
Improved stratification of ccRCC recurrence risk using MFI2-AS1 expression and Leibovich score
To evaluate the clinical utility of MFI2-AS1 expression for DFS prediction, we stratified Leibovich subgroups according to MFI2-AS1 expression, namely Leibovich ≥5+ MFI2-AS1 expression (L+ M+), Leibovich ≥5+ no expression of MFI2-AS1 (L+ M−), Leibovich <5+ MFI2-AS1 expression (L− M+), and Leibovich <5+ no expression of MFI2-AS1 (L− M−). The L+ M− group had a spectacular improvement in DFS compared to the L+ M+ group. Indeed, in the L+ M+ group, median DFS was 10 months, which was not reached in the L+ M− group. The HR for recurrence in the L+ M+ group compared to the L+ M− group was 12, 95% CI [3.76–39.34], p < 0.0001 (Fig. 3B). Stratification of Leibovich groups according to MFI2-AS1 expression reclassified 56% of the patients with Leibovich scores ≥5 (18/32 patients), who were ultimately considered to have favourable outcomes.
The recurrence risk did not differ between the L− M+ and L− M− groups according to MFI2-AS1 expression. There was also no significant difference in DFS and recurrence risk between the L+ M− group and both the L− M+ and L− M− groups.
Overall, the classification combining the Leibovich score and MFI2-AS1 expression more accurately estimated the risk of ccRCC recurrence than each characteristic alone, with a C-statistic of 0.70 compared to 0.64 for MFI2-AS1 stratification alone and 0.66 for Leibovich stratification alone (Supplementary Figure 2).
The results observed in these survival analyses were reproduced with different cutoffs for the Leibovich score, which ultimately led to similar significant results (Supplementary Figure 3).
Increased MFI2-AS1 expression is associated with metastatic disease
In the training set from TCGA, MFI2-AS1 expression increased in primary tumors from metastatic patients (N = 72) compared to localized tumors (N = 351), and in localized tumors compared to normal tissue from the entire cohort (N = 423) (Fig. 4A). Similar results were observed in the validation set between primary tumors from metastatic patients (N = 21), localized tumors (N = 167), and normal tissue (N = 21) (Fig. 4B). In paired normal tissue and localized tumor samples, we found a significant increase in MFI2-AS1 expression (p < 0.0001). Notably, out of 21 matched localized tumor and normal tissue samples, only 3 patients had detectable expression of MFI2-AS1 in normal kidney tissue, while all 21 patients had expression of MFI2-AS1 in the primary tumor (Fig. 4C). Together, these data confirm that MFI2-AS1 is upregulated in tumor samples compared to normal tissue and that higher expression of MFI2-AS1 is associated with metastatic dissemination.
Figure 4.
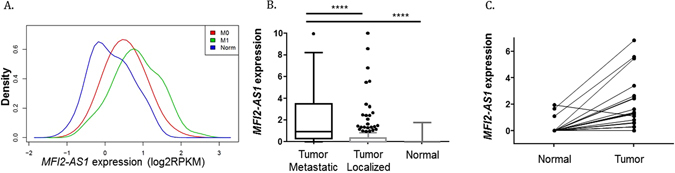
Comparison of MFI2-AS1 expression in normal tissue, localized tumors, and primary tumors from metastatic patients. (A) Development cohort. M0: localized tumors, M1: primary tumors from metastatic patients, Norm: normal tissue. (B) Validation cohort. (C) Expression of MFI2-AS1 in the validation cohort between localized ccRCCs and matched normal tissue.
Discussion
Our work supports the use of lncRNA MFI2-AS1 as a potent and independent biomarker to predict disease recurrence in localized ccRCC. Patients with MFI2-AS1 expression are nearly four times more likely to experience disease relapse after partial or radical nephrectomy. More importantly, the implementation of MFI2-AS1 status along with Leibovich score stratifies patients who have tumor features usually associated with aggressive disease into two populations with drastically different outcomes. Given the development of adjuvant trials involving immune checkpoint inhibitors and antiangiogenics in localized ccRCC32, 33, identifying patients with high recurrence risk is crucial to increase the likelihood of positive outcomes and ensure personalized care.
Indeed, multiple trials have failed to demonstrate improved DFS in the adjuvant setting16, 34. The only trial that met its primary endpoint in this setting was based on a careful selection of patients17, which corroborates our approach. Compared to classifying patients according to tumor stage and grade, which is common in adjuvant trials, MFI2-AS1 is a more potent classifier for ccRCC recurrence risk. Compared to classifications that use multiple genes14, MFI2-AS1 status is easier to determine with common methods such as quantitative PCR, and sill provides high discriminatory power. Using the Leibovich score and MFI2-AS1 status allows for an unprecedented stratification of patients according to their risk of relapse and should be useful in clinical practice.
Detectable expression of MFI2-AS1 may result from amplification of its locus at 3q29, an event that may promote oncogenic mechanisms. Notably, we reported that expression of MFI2-AS1 was associated with expression of acute-phase proteins such as IL6 and SAA1, which might indicate that MFI2-AS1 is involved in the positive regulation of genes responsible for tumor-promoting inflammation, a poor prognosis factor in ccRCC35. In addition, the correlation between MFI2-AS1 expression and metastatic disease indicate that MFI2-AS1 might be involved in tumor progression. Recent data from the literature corroborate these findings, with reported association between expression of MFI2-AS1 and proliferation of osteosarcoma cell lines26.
The results of this study are robust as it was conducted in two large independent cohorts with fresh frozen tissue samples. Modification of the endpoints to another reference classification would have likely led to similar outcomes. Indeed, Leibovich and UISS score are based mostly on similar criteria, notable T stage, nodal involvement and pathological grade. In addition, their concordance index are very similar, which indicate comparable discriminatory power15. This study did not prospectively recruit the two cohorts. However, the characteristics of the patients are similar to those encountered in the literature36. The FU for each patient was not standardized, but patients were monitored according to the standard of care, which included receiving a CT scan every 3–6 months. Samples from ten patients (5.5%) in the validation cohort could not be analyzed for technical reasons (insufficient RNA yield or low quality data), and 27 (13%) patients were excluded due to insufficient follow-up. Our consideration of tumor heterogeneity, an increasing challenge for cancer diagnosis and treatment29, showed that detection of MFI2-AS1 was found recurrently across multiple regions of primary tumors that had documented MFI2-AS1 expression. The differential quantitative expression of MFI2-AS1 could be explained by the variation of the amount of tumor cells in each sample, as we did not perform microdissections, but also by intratumor heterogeneity for MFI2-AS1 expression levels. Dedicated studies will be useful to investigate tumor heterogeneity for MFI2-AS1 expression, not only in the primary tumor, but also between primary and metastatic sites and at different time points, in order to better understand the role of MFI2-AS1 during cancer progression.
Further studies will be important to prospectively validate the impact of MFI2-AS1 stratification in adjuvant clinical trials. Liquid biopsies are under study to detect lncRNA transcripts in circulating blood37, which will vastly improve the availability of prognostic tools. Improved comprehension of the non-coding genome will help determine the oncogenic mechanisms associated with MFI2-AS1 expression, as well as their involvement in tumor immunity. Our work highlights the involvement of the non-coding genome in cancer, an ever-expanding area of research that will help to shape the future of medical oncology.
Electronic supplementary material
Long non-coding RNAs associated with prognosis and tumor stage in the training set
Functional annotation of differentially expressed genes according to MFI2-AS1 expression in the training set
Acknowledgements
Fondation Avec provided laboratory equipment funding and research grants to R Mouawad and GG Malouf.
Author Contributions
R.F., R.M., J.P.S., N.M.T., D.K., X.S. and G.G.M. contributed to the design of the study. X.S. contributed to the analysis of the development study. R.F., X.S., and G.G.M. contributed to the interpretation of the development study data. M.R., M.O.B., J.P., C.V., F.A. and Q.M. contributed to sample collection in the validation study. E.C. contributed to the pathological review of the samples. R.F., R.M. and G.G.M. contributed to the experiments, the analysis and the interpretation of the validation data. R.F., R.M., X.S. and G.GM. contributed to the writing of the article. All authors approved the final version of the article.
Competing Interests
RF, RM, JPS, MR, EC, MOB, JP, CV, FA, QM, DK, XS, and GGM declare no competing interests related to this work. NMT. Honoraria and consulting: BMS, Exelixis, Nektar, Novartis, Pfizer, Argos, Calithera. Research funding: BMS, Exelixis, Epizyme, Novartis, Miranti.
Footnotes
Xiaoping Su and Gabriel G. Malouf contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08363-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25(Suppl 3):iii49–56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 3.Sciarra A, et al. The emerging role of targeted therapy in renal cell carcinoma (RCC): is it time for a neoadjuvant or an adjuvant approach? Crit. Rev. Oncol. Hematol. 2012;81:151–162. doi: 10.1016/j.critrevonc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Haas NB, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passalacqua R, et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-α (IFN-α) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC) J. Immunother. 2014;37:440–447. doi: 10.1097/CJI.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 6.Leibovich BC, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma. Cancer. 2003;98:2566–2575. doi: 10.1002/cncr.11851. [DOI] [PubMed] [Google Scholar]
- 7.Clark PE. The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int. 2009;76:939–945. doi: 10.1038/ki.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugarolas J. PBRM1 and BAP1 as novel targets for renal cell carcinoma. Cancer J. 2013;19:324–332. doi: 10.1097/PPO.0b013e3182a102d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seles, M. et al. Current Insights into Long Non-Coding RNAs in Renal Cell Carcinoma. Int. J. Mol. Sci. 17 (2016). [DOI] [PMC free article] [PubMed]
- 10.Deng M, et al. Identification of novel differentially expressed lncRNA and mRNA transcripts in clear cell renal cell carcinoma by expression profiling. Genomics Data. 2015;5:173–175. doi: 10.1016/j.gdata.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, et al. A serum-circulating long noncoding RNA signature can discriminate between patients with clear cell renal cell carcinoma and healthy controls. Oncogenesis. 2016;5:e192. doi: 10.1038/oncsis.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flippot R, et al. Cancer subtypes classification using long non-coding RNA. Oncotarget. 2016;7:54082–54093. doi: 10.18632/oncotarget.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malouf, G. G. et al. Characterization of long non-coding RNA transcriptome in clear-cell renal cell carcinoma by next-generation deep sequencing. Mol. Oncol., doi:10.1016/j.molonc.2014.07.007 (2014). [DOI] [PMC free article] [PubMed]
- 14.Rini B, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol. 2015;16:676–685. doi: 10.1016/S1470-2045(15)70167-1. [DOI] [PubMed] [Google Scholar]
- 15.Tan M-H, et al. Comparison of the UCLA Integrated Staging System and the Leibovich score in survival prediction for patients with nonmetastatic clear cell renal cell carcinoma. Urology. 2010;75:1365–1370. doi: 10.1016/j.urology.2009.07.1289. [DOI] [PubMed] [Google Scholar]
- 16.Haas, N. B. et al. Initial results from ASSURE (E2805): Adjuvant sorafenib or sunitinib for unfavorable renal carcinoma, an ECOG-ACRIN-led, NCTN phase III trial. J. Clin. Oncol. 33 (2015).
- 17.Ravaud, A. et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N. Engl. J. Med., doi:10.1056/NEJMoa1611406 (2016). [DOI] [PubMed]
- 18.Lam, J. S. et al. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J. Urol. 174, 466–472, discussion 472; quiz 801 (2005). [DOI] [PubMed]
- 19.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature499, 43–49 (2013). [DOI] [PMC free article] [PubMed]
- 20.Delahunt B, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 2013;37:1490–1504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Wierzbicki PM, et al. Identification of a suitable qPCR reference gene in metastatic clear cell renal cell carcinoma. Tumour Biol. 2014;35:12473–12487. doi: 10.1007/s13277-014-2566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupasquier S, et al. Validation of housekeeping gene and impact on normalized gene expression in clear cell Renal Cell Carcinoma: critical reassessment of YBX3/ZONAB/CSDA expression. BMC Mol. Biol. 2014;15:9. doi: 10.1186/1471-2199-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reich HN, et al. Molecular Markers of Injury in Kidney Biopsy Specimens of Patients with Lupus Nephritis. J. Mol. Diagn. JMD. 2011;13:143–151. doi: 10.1016/j.jmoldx.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung M, et al. In search of suitable reference genes for gene expression studies of human renal cell carcinoma by real-time PCR. BMC Mol. Biol. 2007;8:47. doi: 10.1186/1471-2199-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin Z, Ding H, He E, Chen J, Li M. Overexpression of long non-coding RNA MFI2 promotes cell proliferation and suppresses apoptosis in human osteosarcoma. Oncol. Rep. 2016;36:2033–2040. doi: 10.3892/or.2016.5013. [DOI] [PubMed] [Google Scholar]
- 27.Chen S-X, et al. Upregulated expression of long noncoding RNA SNHG15 promotes cell proliferation and invasion through regulates MMP2/MMP9 in patients with GC. Tumour Biol. 2016;37:6801–6812. doi: 10.1007/s13277-015-4404-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J-H, Wei H-W, Yang H-G. Long noncoding RNA SNHG15, a potential prognostic biomarker for hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2016;20:1720–1724. [PubMed] [Google Scholar]
- 29.Gerlinger M, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 2014;46:225–233. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerlinger M, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGranahan N, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin. Oncol. 2013;40:482–491. doi: 10.1053/j.seminoncol.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon TG, et al. ATLAS study: A randomized double-blind phase 3 study of adjuvant axitinib versus placebo in subjects at high risk of recurrent renal cell carcinoma (RCC) J. Clin. Oncol. 2014;32:5s. doi: 10.1200/JCO.2013.52.4827. [DOI] [Google Scholar]
- 34.Choueiri M, Tannir N, Jonasch E. Adjuvant and neoadjuvant therapy in renal cell carcinoma. Curr. Clin. Pharmacol. 2011;6:144–150. doi: 10.2174/157488411797189415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster WS, et al. Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer. 2006;107:46–53. doi: 10.1002/cncr.21951. [DOI] [PubMed] [Google Scholar]
- 36.Heng DYC, et al. Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large, Multicenter Study. J. Clin. Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 37.Arita T, et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–3193. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Long non-coding RNAs associated with prognosis and tumor stage in the training set
Functional annotation of differentially expressed genes according to MFI2-AS1 expression in the training set
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


