Abstract
Central venous oxygen saturation (ScvO2) in the superior vena cava is predominantly determined by cardiac output, arterial oxygen content, and oxygen consumption by the upper body. While abnormal ScvO2 levels are associated with morbidity and mortality in non-uremic populations, ScvO2 has received little attention in hemodialysis patients. From 1/2012 to 8/2015, 232 chronic hemodialysis patients with central venous catheters as vascular access had their ScvO2 monitored during a 6-month baseline period and followed for up to 36 months. Patients were stratified into upper and lower two tertiles by a ScvO2 of 61.1%. Survival analysis employed Kaplan-Meier curves and adjusted Cox proportional hazards models. Patients in the lower tertiles of ScvO2 were older, had longer hemodialysis vintage, lower systolic blood pressure, lower ultrafiltration rates, higher leukocyte counts and neutrophil-to-lymphocyte ratios. Kaplan-Meier analysis indicated a shorter survival time in the lower tertiles of ScvO2 (P = 0.005, log-rank test). In adjusted Cox analysis, a 1 percent point decrease in mean ScvO2 was associated with a 4% increase in mortality (HR 1.04 [95% CI 1.01–1.08], P = 0.044), indicating that low ScvO2 is associated with poor outcomes. Research on the relative contributions of cardiac output and other factors is warranted to further elucidate the pathophysiology underlying this novel finding.
Introduction
The mortality rate of hemodialysis (HD) patients is elevated compared to the normal population1. The primary cause of mortality is cardiovascular disease (CVD), and there is evidence that the mechanism for CVD in HD patients differ from the traditional CVD risk factors in the general population2, 3. High ultrafiltration rates (UFR), episodes of intradialytic hypotension, presence of congestive heart failure (CHF) and left ventricular hypertrophy (LVH) are some of the factors that have been associated with increased mortality4, 5. Additionally nocturnal hypoxemia in HD patients has been demonstrated to be associated with worse cardiovascular outcomes6, 7.
Mixed venous oxygen saturation (SmvO2) and central venous oxygen saturation (ScvO2) have been used in critical care to guide fluid resuscitation8. SmvO2 is the oxygen saturation in the pulmonary artery, which receives blood from the superior vena cava, the inferior vena cava, and the coronary sinus, and therefore reflects – in the absence of arterial venous shunts – the aggregated effects of oxygen delivery to and utilization by the entire body. ScvO2 from upper body central venous catheters (CVC) is the oxygen saturation of blood in the superior vena cava, which reflects the aggregate of oxygen delivery to and utilization by the upper body. Although resting SmvO2 and ScvO2 differ due to the higher oxygen extraction in the upper body, the time trends of SmvO2 and ScvO2 are comparable under most circumstances9–11. While the measurement of SmvO2 requires pulmonary artery catheterization, ScvO2 can be more easily obtained from a CVC.
ScvO2 is determined by oxygen delivery to and oxygen consumption of the arms, head, and upper portion of the torso; the former depends on the arterial blood oxygen content and the cardiac output (CO). At rest with stable arterial oxygen saturation (SaO2), hemoglobin, and tissue oxygen consumption, ScvO2 can serve as a surrogate of CO. Poor oxygen delivery can be caused by decreased CO, e.g. from CHF or reduced cardiac preload, or decreased arterial oxygen content, e.g. due to anemia or hypoxemic states. Oxygen consumption is determined by metabolic status and is altered in sepsis, fever, exercise and sedation12. ScvO2 in the general population is poorly defined, as obtaining this measurement requires a CVC, and patients who require CVC placement are generally significantly ill. One study in healthy subjects found a ScvO2 of 76.8 ± 5.2% during cardiac catheterization13.
Studies in non-uremic populations, have found that abnormal ScvO2 levels are associated with worse morbidity and mortality8, 14–18. ScvO2 levels in HD patients have not been well described. In patients who have ESRD with CVC as vascular access, ScvO2 can be easily and continuously obtained during HD treatments by using the Crit-Line monitorTM (CLM). The CLM is used routinely in Renal Research Institute HD units, which allowed us to investigate the ScvO2 in maintenance HD patients. The goals of our study were to evaluate the baseline characteristics of patients with different levels of intradialytic ScvO2 and to examine the associations between ScvO2 and mortality.
Results
Baseline patient characteristics
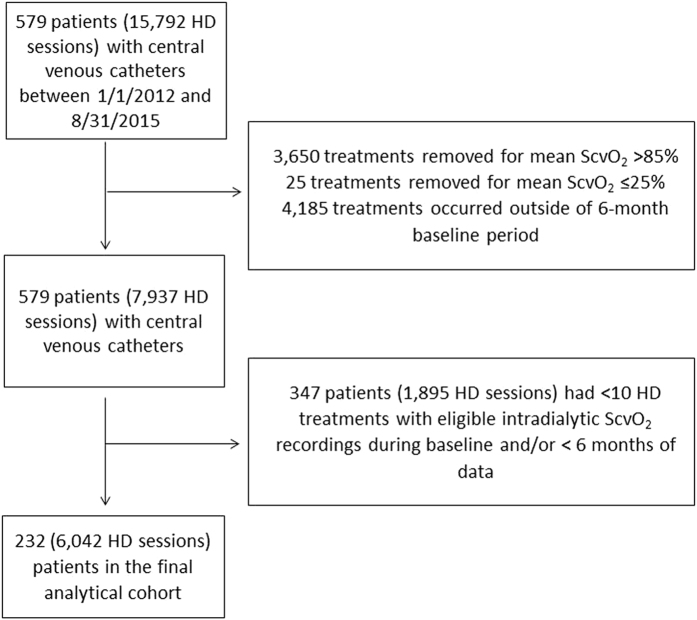
The final analytical cohort comprised of 232 patients with 6,042 HD treatments and was derived after a deliberate step-by-step data cleaning process at the treatment level. Patients were only excluded in the event that they did not contribute sufficient data during baseline, either because of end of study, death, treatment modality change, recovery of renal function, or transfer to another dialysis facility (Fig. 1).
Figure 1.
Study flow chart. HD: hemodialysis, ScvO2: central venous oxygen saturation.
The initial population comprised of 579 patients with CVC as dialysis access, with a total of 15,792 HD treatments with ScvO2 measurements from January 1, 2012 until August 31, 2015. We excluded 3,650 treatments (23%) as they had a mean ScvO2 of greater than 85% and 25 treatments (0.16%) as they had a mean ScvO2 of less than or equal to 25%. We also excluded 4,185 treatments (26.5%) that occurred after the 6-month baseline period. This left us with 579 patients and 7,937 HD sessions, from which we excluded 347 patients with 1,895 HD treatments from the subsequent analysis because they had less than the required 10 HD treatments with ScvO2 recordings and/or less than 6 months of follow up (Fig. 1 ). Out of the 155 patients excluded for not having 6 months of follow up time, 79 were due to death.
In our study population, the mean age was 62.7 ± 15.7 years, dialysis vintage was 2.9 ± 4.6 years, 56% were white, 48.3% were male, 59% had diabetes mellitus (DM), 22% had CHF, and 10.3% had chronic obstructive pulmonary disease (COPD) (Table 1). Median follow-up time was 431 days.
Table 1.
Baseline characteristics of all patients, lower tertiles and upper tertile.
| Variables | All patients Mean ± SD | Lower Two Tertiles Mean ± SD | Upper Tertile Mean ± SD | Group Difference Mean (95% CI) | P- value |
|---|---|---|---|---|---|
| Patients [N] | 232 | 154 | 78 | ||
| Number of eligible HD treatments during baseline [per patient] | 26.1 ± 13.3 | 26.1 ± 13.1 | 26.1 ± 13.8 | 0.0 (−3.8 to 3.6) | 0.953a |
| Demographics | |||||
| Age [years] | 62.7 ± 15.7 | 66.0 ± 13.8 | 56.2 ± 17.3 | 9.8 (5.3 to 14.2) | 0.001a |
| Race [% white] | 56.0 | 53.9 | 60.3 | −6.4 | 0.357b |
| Gender [% male] | 48.3 | 48.1 | 48.7 | −0.6 | 0.924b |
| Vintage [years] | 2.9 ± 4.6 | 3.3 ± 5.1 | 2.0 ± 3.6 | 1.3 | 0.0136c |
| BMI [kg/m2] | 28.1 ± 6.9 | 28.6 ± 7.0 | 27.3 ± 6.5 | 1.2 (−0.7 to 3.2) | 0.207a |
| ScvO 2 saturation [%] | |||||
| Mean ScvO2 | 58.7 ± 7.3 | 54.9 ± 5.3 | 66.3 ± 4.2 | −11.4 (−12.6 to −10.1) | n.a. |
| Median ScvO2 | 59.1 ± 7.3 | 55.2 ± 5.3 | 66.6 ± 4.2 | −11.4 (−12.7 to −10.1) | n.a. |
| Minimum ScvO2 | 48.4 ± 9.7 | 44.4 ± 8.9 | 56.3 ± 5.7 | −11.9 (−13.8 to −10.0) | n.a. |
| Maximum ScvO2 | 65.2 ± 6.2 | 62.0 ± 4.6 | 71.5 ± 3.6 | −9.5 (−10.6 to −8.4) | n.a. |
| SD ScvO2 | 3.4 ± 1.1 | 3.6 ± 1.1 | 2.9 ± 0.8 | 0.7 (0.4 to 0.9) | <0.001a |
| Start ScvO2 | 59.1 ± 7.4 | 55.4 ± 5.6 | 66.5 ± 4.4 | −11.1 (−12.4 to −9.8) | n.a. |
| End ScvO2 | 57.3 ± 7.8 | 53.5 ± 6.2 | 64.8 ± 4.8 | −11.3 (−12.7 to −9.8) | n.a. |
| End – Start ScvO2 | −1.8 ± 3.6 | −1.9 ± 3.7 | −1.7 ± 3.5 | −0.2 (−1.2 to 0.8) | 0.62 |
| Comorbidities [%] | |||||
| Diabetes | 59.0 | 60.4 | 56.4 | 4.0 | 0.560b |
| CHF | 22.0 | 21.4 | 23.1 | −1.7 | 0.775b |
| COPD | 10.3 | 11.0 | 9.0 | 2.0 | 0.626b |
| Treatment parameters | |||||
| Pre-dialysis SBP [mmHg] | 146.4 ± 22.0 | 143.7 ± 22.9 | 151.7 ± 19.1 | −8.0 (−14.0 to −2.1) | 0.009a |
| Post-dialysis SBP [mmHg] | 140.3 ± 20.1 | 137.8 ± 20.5 | 145.5 ± 18.2 | −7.6 (−13.0 to −2.2) | 0.006a |
| Peridialytic SBP change [mmHg] | −6.1 ± 11.9 | −6.0 ± 11.7 | −6.4 ± 12.4 | 0.4 (−2.9 to 3.7) | 0.820a |
| IDWG [kg] | 2.0 ± 0.8 | 1.9 ± 0.8 | 2.1 ± 0.8 | −0.12 (−0.3 to 0.1) | 0.249a |
| IDWG relative to post-dialysis weight [%] | 2.6 ± 0.9 | 2.5 ± 0.9 | 2.8 ± 1.0 | −0.3 (−0.6 to −0.1) | 0.007a |
| UFV [L] | 1.9 ± 0.8 | 1.9 ± 0.79 | 2.0 ± 0.8 | −0.1 (−0.4 to 0.1) | 0.173a |
| Normalized UFV [mL/kg] | 25.3 ± 9.7 | 24 ± 8.9 | 28 ± 10.7 | −4 (−6.6 to −1.4) | 0.003a |
| Post-dialysis weight [kg] | 77.4 ± 20.4 | 79.0 ± 21.4 | 74.3 ± 18.1 | 4.6 (−0.9 to 10.2) | 0.102a |
| Treatment time [minutes] | 219.0 ± 23 | 217.7 ± 23.8 | 221.5 ± 21.1 | −3.7 (−10.1 to 2.5) | 0.235a |
| Equilibrated Kt/V | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.2 | 0.0 (−0.1 to 0.1) | 0.610a |
| Laboratory parameters | |||||
| Hgb [g/dL] | 10.6 ± 0.9 | 10.6 ± 0.9 | 10.6 ± 0.96 | 0.0 (−0.3 to 0.3) | 0.962a |
| Serum sodium [mmol/L] | 138.6 ± 3.1 | 138.6 ± 3.2 | 138.7 ± 2.8 | −0.1 (−0.9 to 0.7) | 0.782a |
| Serum potassium [mmol/L] | 4.7 ± 0.6 | 4.6 ± 0.6 | 4.7 ± 0.4 | −0.1 (−0.2 to 0.1) | 0.292a |
| Intact PTH [pg/mL] | 518.3 ± 481.1 | 538.3 ± 498.3 | 478.6 ± 445.4 | 59.7 (−72.9 to 192.2) | 0.376a |
| Serum bicarbonate [mmol/L] | 23.4 ± 2.2 | 23.3 ± 2.3 | 23.7 ± 2.2 | −0.4 (−1.0 to 0.2) | 0.165a |
| Leukocytes [1000/µL] | 7.0 ± 2.0 | 7.2 ± 2.1 | 6.6 ± 1.7 | 0.6 (0.1 to 1.1) | 0.019a |
| Platelets [1000/µL] | 212.9 ± 63.9 | 216.9 ± 65.1 | 205.1 ± 61.3 | 11.8 (−6.5 to 30.1) | 0.204a |
| NLR | 4.4 ± 2.6 | 4.6 ± 2.8 | 3.8 ± 2.0 | 0.79 (0.2 to 1.4) | 0.015a |
| Serum albumin [g/dL] | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.8 ± 0.4 | −0.1 (−0.2 to 0.04) | 0.165a |
| Ferritin [ng/mL] | 780.5 ± 510.2 | 798.8 ± 487.9 | 744.7 ± 552.9 | 54.1 (−86.8 to 195.0) | 0.45a |
| Transferrin saturation [%] | 30.7 ± 9.5 | 29.8 ± 8.9 | 32.6 ± 10.5 | −2.8 (−5.4 to −0.2) | 0.036a |
95% CI, 95% confidence interval; SD, standard deviation; ScvO2, central venous oxygen saturation; BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure; UFV, ultrafiltration volume; IDWG, interdialytic weight gain; Hgb, hemoglobin; PTH, parathyroid hormone; NLR, neutrophil-to-lymphocyte ratio; n.a., not applicable.
a t test.
bChi-square test.
cWilcoxon test.
During baseline, ScvO2 was recorded in 26 ± 13.3 HD treatments per patient. On a population level the ScvO2 was normally distributed with a mean of 58.7 ± 7.3%. Analysis of intradialytic ScvO2 dynamics across all patients indicated that on average ScvO2 slightly increased over the first 60 minutes of treatment, and then progressively declined below starting levels towards the end of HD (Fig. 2).
Figure 2.
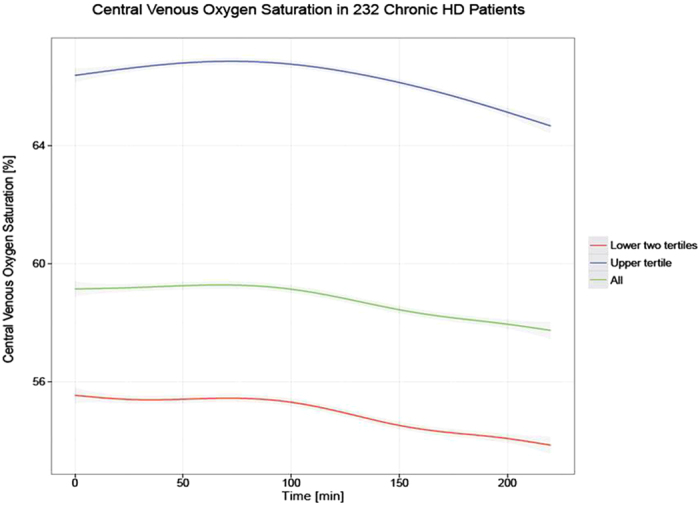
Time course of mean ScvO2 during hemodialysis in all patients (green), lower two tertiles (red) and upper tertile (blue). The respective 95% confidence intervals are indicated in gray.
ScvO2 as a dichotomous outcomes
Comparison of baseline characteristics between upper and lower tertiles
Patients were stratified into upper tertile (N = 78) and lower two tertiles (N = 154); a mean ScvO2 level below 61.1% during baseline period separated the two groups. A comparison of baseline characteristics between upper and lower tertiles is presented in Table 1. The patients in the lower tertiles were older (66.0 ± 13.8 years vs 56.2 ± 17.3 years, P < 0.001), had longer dialysis vintage (3.3 ± 5.1 years vs 2.0 ± 3.6 years, P = 0.031), lower pre-dialysis systolic blood pressure (SBP) (143.7 ± 22.9 mmHg vs 151.7 ± 19 mmHg, P = 0.009), lower post-dialysis SBP (137.8 ± 20.5 mmHg vs 145.5 ± 18.2 mmHg, P = 0.006), and had lower normalized UFR (6.8 ± 2.4 mL/kg/hr vs 7.7 ± 2.9 mL/kg/hr, P = 0.015). Furthermore, lower tertile subjects had higher leukocyte counts (7.2 ± 2.1 * 1000/µL vs 6.6 ± 1.7 * 1000/µL, P = 0.019) and higher neutrophil-to-lymphocyte ratios (NLR) (4.6 ± 2.8 vs 3.8 ± 2.0, P = 0.015). There was no statistically significant difference in comorbidities of DM, CHF or COPD.
Mortality between upper and lower tertiles
During the 36-month follow-up period, there were a total of 54 deaths, 45 in the lower two tertiles and 9 in the upper tertile. Mortality rate was 24.1/100 patient years in lower two tertiles and 9.0/100 patient years in upper tertile (P = 0.005). Univariate Kaplan-Meier analysis indicated a significantly shorter survival among lower tertile patients (P = 0.0051, log-rank test) (Fig. 3).
Figure 3.
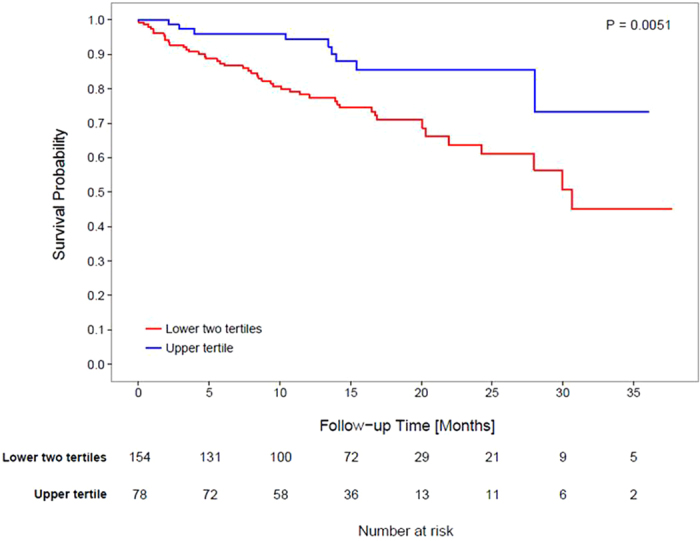
Kaplan-Meier estimates for survival probabilities in the lower two tertiles (red) and the upper tertile (blue), respectively. Median follow up for the lower two tertiles was 428 days while the median follow up time for the upper tertile was 432 days. The number of patients at risk is indicated in the table below the graph. The time to death differs significantly between the two groups (P = 0.0051, log-rank test).
ScvO2 as a continuous variable
In unadjusted Cox analysis, for every 1 percent point decrease in mean ScvO2 there was an associated 6% increase in mortality (HR 1.06 (1.03–1.10)). There was no material change in the results after adjustment for age, gender, comorbidities (COPD and CHF), log vintage, inflammatory markers (albumin, NLR), hemoglobin and erythropoietin dose (HR 1.04 (1.01–1.08)) (Table 2 ).
Table 2.
Crude and adjusted hazard ratios for all-cause mortality for a 1% decrease in central venous oxygen saturation.
| Outcome | Events | Crudea | Minimally Adjustedb | Fully Adjustedc | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| All-cause mortality | 54 | 1.06 (1.03 to 1.10) | <0.001 | 1.05 (1.02 to 1.09) | 0.003 | 1.04 (1.01 to 1.08) | 0.0437 |
HR, hazard ratio.
aUnadjusted model.
bAdjusted for age, gender, chronic obstructive pulmonary disease and congestive heart failure.
cAdjusted for age, gender, chronic obstructive pulmonary disease, congestive heart failure, albumin, hemoglobin, erythropoietin dose, neutrophil to lymphocyte ratio, and log vintage.
Correlates of ScvO2
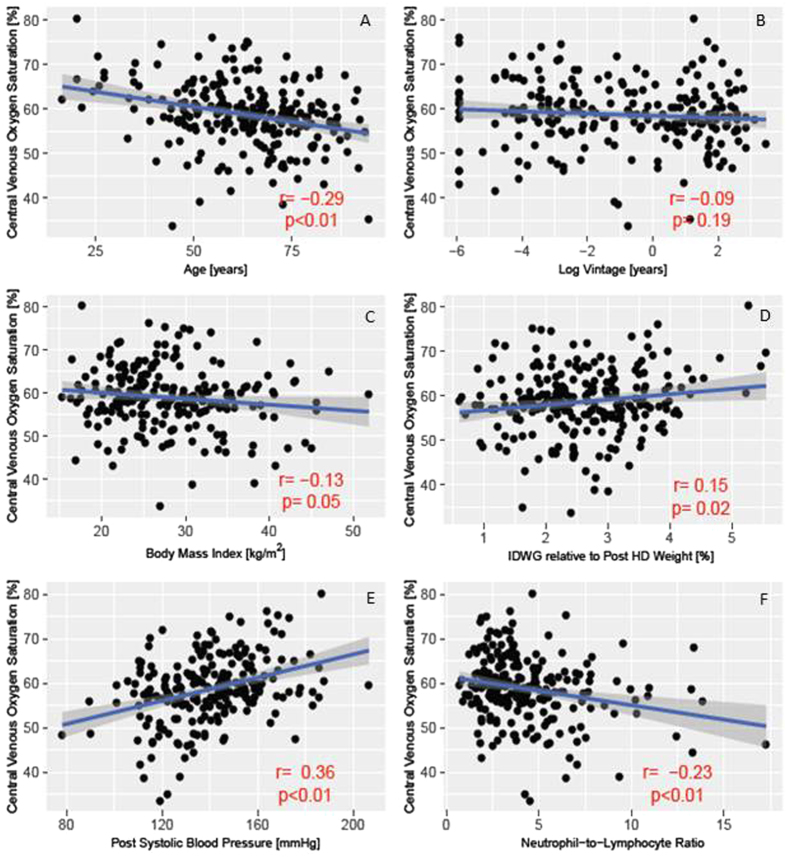
Figure 4 depicts the relationship between ScvO2 and patient characteristics that were found to differ between the two groups. Mean ScvO2 across patients was plotted against age, log vintage, body mass index (BMI), interdialytic weight gain (IDWG) relative to post-HD weight, post-HD SBP, and NLR. As vintage was not normally distributed, it was log transformed. Age, BMI, log vintage and NLR were negatively associated with ScvO2, while post-HD SBP and IDWG were positively correlated with ScvO2. While all correlates were statistically significant except for ScvO2 and log vintage (P = 0.19), correlation coefficients were relatively low.
Figure 4.
Correlates of central venous oxygen saturation with respect to patient characteristics. Each point represents one patient; the depicted data points represent the respective parameter averages during the 6-month baseline period. (A) Age; (B) Log vintage; (C) Body mass index; (D) Interdialytic weight gain relative to post-dialysis body weight; (E) Post-dialysis systolic blood pressure; (F) Neutrophil-to-lymphocyte ratio.
Discussion
Our study indicates that in chronic HD patients with CVC as vascular access, lower ScvO2 levels are associated with poorer survival.
Despite the relative ease with which ScvO2 can be obtained in HD patients with CVC as access, to date only small studies have examined this key indicator of cardiac function. Cordtz et al.19 in 2008 evaluated 20 HD patients and classified them as either hypotension prone or hypotension resistant and measured their ScvO2 at treatment initiation and end. The authors found a significant decrease in ScvO2 in hypotension prone patients. Harrison et al.20 investigated 18 HD patients and found a strong inverse correlation between ScvO2 at the end of dialysis and ultrafiltration volume normalized to post-HD body weight. A recent review of intradialytic oxygen saturation did not identify any previous research examining the association between ScvO2 and patient survival21.
In the study by Harrison et al. the mean ScvO2 was 63.5 ± 13% pre-HD and 56.4 ± 8% post-HD20, whereas in the study by Cordtz et al. the initial ScvO2 was 52.2 ± 6.7% in hypotension prone and 49.7 ± 6.9% in hypotension resistant patients19. While these studies focused on ScvO2 at HD start and end, we examined ScvO2 continuously throughout the HD session. The ScvO2 levels found in our study are below the levels of ~70% observed in healthy subjects13, but consistent with those reported in HD patients. The exact etiology of low intradialytic ScvO2 in HD patients is not well established, but may be partially explained by the lower hemoglobin levels, and the higher prevalence of cardiac dysfunction and pulmonary hypertension in HD patients.
In our cohort, when ScvO2 was assessed throughout the entire HD treatment, on average ScvO2 increased slightly over the first hour and then progressively declined during the remaining treatment time. The determinants of ScvO2 can be visualized by rearrangement of the familiar form of Fick’s law and replacement of SmvO2 with ScvO2, and CO with upper body blood flow (UBBF), which results in the following equation
| 1 |
with ScvO2 and SaO2 in %, upper body oxygen consumption in mL/min, Hgb in g/L, UBBF in L/min, and K being 1.34, the amount of oxygen (in mL) bound per g of hemoglobin.
Therefore, there are four components which may change during HD and that will cause a decrease in ScvO2; (i) increased tissue oxygen consumption; (ii) a decrease in SaO2, (iii) a decrease in hemoglobin concentration, and (iv) a decrease in upper body blood flow. An increase in oxygen consumption can occur due to an increase in metabolic rate. A small study done in maintenance HD patients found that whole body energy expenditure, measured by indirect calorimetry, increased during HD22. However, to what extent the upper body energy expenditure changes during HD is currently unknown. It would be of interest to know if oxygen consumption by the brain, by far the largest consumer of oxygen in the upper body, changes during HD. Intradialytic SaO2 has been demonstrated to decrease during the first hour of HD; unfortunately we do not have SaO2 levels in our patients23. While HD patients have lower hemoglobin levels than the general population, during HD as UF occurs and the relative blood volume decreases, hemoglobin levels generally rise due to hemoconcentration24. We suspect that a reduction in CO and consequently a decrease in upper body blood flow is the predominant driving factor leading to a drop in ScvO2. The almost linear relation between cerebral perfusion and CO has been recently reviewed25. When faced with any of the other possibilities in a patient with intact cardiac function, there should be a compensatory response in CO26. There is growing literature on depressed CO during HD treatment due to poor vascular refill and regional wall motion abnormalities (RWMA)27, 28. In fact, a recent study using intradialytic magnetic resonance imaging of the heart demonstrated that systolic contractile function fell during HD, with all 12 patients experiencing some degree of segmental left ventricular dysfunction along with evidence of decreased intravascular volume and an inadequate heart rate response29.
ScvO2 in patients with sepsis, post-surgery, and trauma has been examined, with studies finding that abnormal ScvO2 levels are associated with increased morbidity and mortality15, 16, 18. However, the ESRD population is unique in many aspects, and the results of prior studies in other populations therefore may not be fully applicable.
We and others have observed a left shift of the ScvO2 distribution in HD patients compared to healthy subjects, possibly related to anemia and lower CO. Therefore we refrained from defining comparison groups based on ScvO2 levels obtained in healthy subjects but rather used ScvO2 tertiles obtained from our large HD population, where a ScvO2 of 61.1% separated the top from the bottom two tertiles13, 30. In a review of literature, ScvO2 levels below 64.4% were associated with morbidity post-surgery, and values below 62% were associated with mortality in patients with pulmonary hypertension18, 31. In the trauma setting, ScvO2 < 65% on initial evaluation in the emergency room predicted higher blood loss and greater severity of injuries16. We complemented this binary analysis with a continuous spline analysis of the association between ScvO2 and hazard ratio for all-cause mortality; that analysis indicated that mean ScvO2 levels below 63% were associated with increased mortality.
Our finding that patients with lower ScvO2 were older may reflect the poorer cardiac function expected in older subjects. Of note, the prevalence of CHF increases with age, as does CHF mortality32. On univariate analysis, age was an independent risk factor for mortality. However, even after adjustment for age in our analysis, ScvO2 as a continuous variable remained a significant predictor of mortality.
While the correlation coefficients were low, we identified several significant correlations between ScvO2 and patient variables such as the association between lower ScvO2 levels and longer dialysis vintage. We speculate that this finding may be related to recurrent hemodynamic stress and cardiac injury caused by HD. McIntyre et al. demonstrated that HD induced RWMA in a subset of maintenance HD patients. While at baseline there was no difference in left ventricular ejection fraction (LVEF) between patients who developed RWMA and those that did not, at 1 year follow-up, the group of patients that developed RWMA during HD had significantly lower resting LVEF5.
In our study, lower tertile patients had lower pre-HD and lower post-HD SBP, a finding possibly related to low CO. Of note, an association between low pre-HD SBP and mortality has been repeatedly shown33, 34. It is interesting to note that in our study the prevalence of CHF did not differ between lower and upper tertiles. Unfortunately, no routine echocardiography assessments were available in our patients, so we cannot comment on the possibility of deficient documentation, classification or misdiagnosis of CHF. One intriguing possibility is that we may be identifying a group of patients without clinically overt signs and symptoms of CHF at rest, who however have reduced cardiac reserve or autonomic dysfunction and are unable to mount the necessary increase in sympathetic response and CO when faced with the hemodynamic stress of HD27, 35.
The main limitation of our study is its observational nature, which prevents any conclusions related to causality. As mentioned earlier, routine echocardiograms are unfortunately not available in our study population, making potentially very insightful correlational analyses of ScvO2 and cardiac structure and function impossible. Lastly, we appreciate that ScvO2 measurements may be altered by changes in catheter tip position due to changes in body position; however, we have no indication that this may affect one of the two groups disproportionally and created any bias.
Considering a recent review of this topic, we believe that this is the largest study to date examining the epidemiology of ScvO2 in maintenance HD patients21. CVC are used as vascular access in the majority of U.S. patients starting HD1. While this situation is certainly not desirable, the presence of a CVC allows us to measure ScvO2, a vitally important physiological parameter. This additional diagnostic opportunity may be particularly important in the incident period, the time with the highest cardiovascular morality rate1. In fact, a recent study published by Mancini et al. demonstrates that variability in SaO2 is associated with intradialytic hypotension36. This supports the potential role of oxygen saturation monitoring during dialysis.
In conclusion, our research shows that routine measurement of ScvO2 during HD provides a novel window into patients’ biology that may help to improve our care for this vulnerable patient population.
Method
Population and study design
This is a retrospective multi-center study of a cohort of maintenance HD patients from 17 facilities of the Renal Research Institute (RRI) across the United States between January 2012 and August 2015. In these clinics, CLM use is part of standard care. All patients were treated with bicarbonate dialysate and polysulfone membranes. Over 80% of patients had a prescribed dialysate temperature of 37 °C. All patients who received HD via a CVC and had at least 6 months of clinical data and 10 dialysis treatments with eligible ScvO2 recordings (definition of eligibility see below) were eligible for inclusion into the study. Therefore our study included both incident and prevalent HD patients. The CLM was rolled out into dialysis units in a staggered manner, and we used the first treatment with CLM data as start date of the patients’ 6 month baseline period. Since eligible patients had to contribute 6 months’ worth of data, by design only those patients who survived for at least 6 months were included into the study (Fig. 1). Patient characteristics were assessed over the baseline period, and mortality was assessed during a follow-up period for a maximum duration of three years. Figure 5 summarizes the study design. For group comparison patients were stratified based on the population ScvO2 that separated the top tertile from the bottom two tertiles. Descriptive statistics of the ScvO2 distribution showed a ScvO2 of 61.1% to be the cut-off between these two groups. Patients were censored in the event of kidney transplantation, transfer to a non-RRI facility, dialysis treatment modality change, recovery of kidney function, or end of follow-up.
Figure 5.
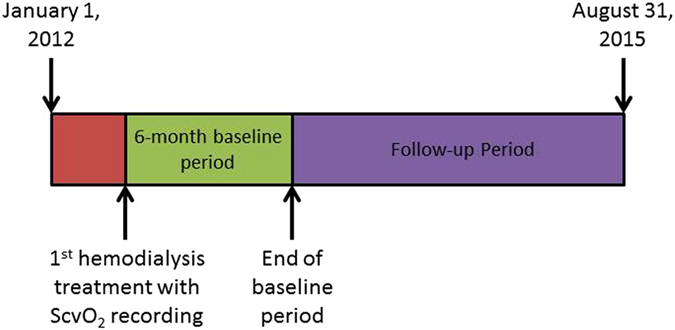
Data were reviewed starting from January 1, 2012. Due to the staggered deployment of Crit-line monitors to dialysis units, patients were enrolled into the study on a rolling basis. The first hemodialysis treatment with ScvO2 measurements marked the beginning the 6-month baseline period. Follow-up ended with either end of study (August 31, 2015), death, treatment modality change, recovery of renal function, or transfer to another dialysis facility. ScvO2: central venous oxygen saturation.
The study was approved by the New England Institutional Review Board (14–446) and conducted in accordance with the Declaration of Helsinki. Informed consent was not obtained as this was determined not to be human subject research, and we were working with de-identified data.
This study has been registered at clinicaltrials.gov (NCT02501044).
Measurement of ScvO2
Intradialytic ScvO2 measurements were obtained by the CLM. The CLM has been approved by the U.S. Food and Drug Administration (FDA) for the measurement of hematocrit, relative blood volume, and oxygen saturation in the extracorporeal dialysis circuit. The CLM measures oxygen saturation 9,000 times per minute and reports the mean of these measurements every minute. The manufacturer reported accuracy for oxygen saturation measurement is 2%. Patients’ mean, median, minimum, maximum, standard deviation, start-HD, end-HD ScvO2 was calculated per treatment and then averaged across all treatments per patient and subsequently across patients. We chose to do our analysis using the mean ScvO2 as there was low variability across treatments for each patient (mean coefficient of variability of 7.5 ± 4%).
Clinical and laboratory data
Laboratory measurements were done at Spectra East Laboratories (Rockleigh, NJ, USA). The results were downloaded to the RRI data warehouse and extracted to the study database. Continuous variables were averaged during the baseline period. BMI was calculated using post-HD dry weight.
Data eligibility
To ensure appropriate data quality, we included only treatments where mean ScvO2 was below 85%, as higher values are incompatible with central venous blood13. Mean ScvO2 measurements less than 25% were excluded because they are considered incompatible with life37. Additionally, data points with relative blood volume measurements above 102% were considered very unlikely, potentially due to saline administration, and hence excluded. This constituted 3% of all data points.
Comorbidities
CHF, DM, and COPD were defined using International Classification of Diseases - 9 (ICD-9) codes.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) if normally distributed and as median (25th, 75th percentile) otherwise. Categorical variables are presented as percentages of the respective group. Statistics of ScvO2 variables were calculated on a HD treatment level and then aggregated on a patient level.
Baseline characteristics of exposed and unexposed were compared using chi-square test for categorical variables and two-sample t test for continuous variables, Wilcoxon Rank-Sum test were used for non-parametric variables. Survival characteristics were compared using Kaplan-Meier plots, log-rank test, and Cox proportional hazards models.
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and R 3.0.2 (libraries ggplot2, splines, survival, pspline; R Foundation for Statistical Computing, Vienna, Austria).
Disclosure
P.K. holds stock in Fresenius Medical Care. L.C. is supported in part by the NIH (5T32DK007757-18). The other authors declared no competing interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Data Availability
Consolidated data may be shared with other scientists at their request.
Acknowledgements
We thank Dr. Jeroen Kooman and Frank van der Sande (both Maastricht University, The Netherlands) and Drs. Steven Coca and Girish Nadkarni (both from Mount Sinai Hospital, New York) for discussions related to this research. We also thank the staff of the Renal Research Institute clinics for facilitating the recording of the oxygen saturation measurements. This work was funded by the Renal Research Institute.
Author Contributions
P.K., S.T., and H.Z. designed the study. L.C., A.M.W., I.C., and D.F. were instrumental in the interpretation of the results. L.C. wrote the main manuscript text. H.Z. acquired data and performed all the statistical analysis. All authors contributed to the manuscript and approved the final version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United States Renal Data System. 2015 USRDS annual data report: Epidemiology of kidney disease in the United States. (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2015).
- 2.Ragosta M, et al. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. American heart journal. 2004;147:1017–1023. doi: 10.1016/j.ahj.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Schneider A, et al. Determinants of cardiovascular risk in haemodialysis patients: post hoc analyses of the AURORA study. Am J Nephrol. 2013;37:144–151. doi: 10.1159/000346710. [DOI] [PubMed] [Google Scholar]
- 4.Stefansson BV, et al. Intradialytic hypotension and risk of cardiovascular disease. Clinical journal of the American Society of Nephrology: CJASN. 2014;9:2124–2132. doi: 10.2215/CJN.02680314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clinical journal of the American Society of Nephrology: CJASN. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoccali C, Mallamaci F, Tripepi G. Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. Journal of the American Society of Nephrology: JASN. 2002;13:729–733. doi: 10.1097/01.ASN.0000032548.18973.0F. [DOI] [PubMed] [Google Scholar]
- 7.Zoccali C, et al. Nocturnal hypoxemia, night-day arterial pressure changes and left ventricular geometry in dialysis patients. Kidney international. 1998;53:1078–1084. doi: 10.1111/j.1523-1755.1998.00853.x. [DOI] [PubMed] [Google Scholar]
- 8.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 9.Schou H, Perez de Sa V, Larsson A. Central and mixed venous blood oxygen correlate well during acute normovolemic hemodilution in anesthetized pigs. Acta anaesthesiologica Scandinavica. 1998;42:172–177. doi: 10.1111/j.1399-6576.1998.tb05104.x. [DOI] [PubMed] [Google Scholar]
- 10.Chawla LS, et al. Lack of equivalence between central and mixed venous oxygen saturation. Chest. 2004;126:1891–1896. doi: 10.1378/chest.126.6.1891. [DOI] [PubMed] [Google Scholar]
- 11.Scheinman MM, Brown MA, Rapaport E. Critical assessment of use of central venous oxygen saturation as a mirror of mixed venous oxygen in severely ill cardiac patients. Circulation. 1969;40:165–172. doi: 10.1161/01.CIR.40.2.165. [DOI] [PubMed] [Google Scholar]
- 12.Vincent, J. L. Ebrary Inc. Yearbook of intensive care and emergency medicine (Springer, Berlin, 2005).
- 13.Barratt-Boyes BG, Wood EH. The oxygen saturation of blood in the venae cavae, right-heart chambers, and pulmonary vessels of healthy subjects. The Journal of laboratory and clinical medicine. 1957;50:93–106. [PubMed] [Google Scholar]
- 14.Balzer F, et al. High central venous saturation after cardiac surgery is associated with increased organ failure and long-term mortality: an observational cross-sectional study. Critical care. 2015;19 doi: 10.1186/s13054-015-0889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Textoris J, et al. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Critical care. 2011;15 doi: 10.1186/cc10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scalea TM, et al. Central venous oxygen saturation: a useful clinical tool in trauma patients. The Journal of trauma. 1990;30:1539–1543. doi: 10.1097/00005373-199012000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Futier E, et al. Central venous O(2) saturation and venous-to-arterial CO(2) difference as complementary tools for goal-directed therapy during high-risk surgery. Critical care. 2010;14 doi: 10.1186/cc9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearse R, et al. Changes in central venous saturation after major surgery, and association with outcome. Crit Care. 2005;9:R694–699. doi: 10.1186/cc3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordtz J, Olde B, Solem K, Ladefoged SD. Central venous oxygen saturation and thoracic admittance during dialysis: new approaches to hemodynamic monitoring. Hemodial Int. 2008;12:369–377. doi: 10.1111/j.1542-4758.2008.00283.x. [DOI] [PubMed] [Google Scholar]
- 20.Harrison LE, Selby NM, McIntyre CW. Central venous oxygen saturation: a potential new marker for circulatory stress in haemodialysis patients? Nephron. Clinical practice. 2014;128:57–60. doi: 10.1159/000362557. [DOI] [PubMed] [Google Scholar]
- 21.Campos I, et al. Intradialytic Hypoxemia in Chronic Hemodialysis Patients. Blood purification. 2016;41:177–187. doi: 10.1159/000441271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikizler TA, et al. Increased energy expenditure in hemodialysis patients. Journal of the American Society of Nephrology: JASN. 1996;7:2646–2653. doi: 10.1681/ASN.V7122646. [DOI] [PubMed] [Google Scholar]
- 23.Meyring-Wösten A. Intradialytic Hypoxemia and Clinical Outcomes in Patients on Hemodialysis. Clinical Journal of the American Society of Nephrology. 2016;11:616–625. doi: 10.2215/CJN.08510815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wink J, Vaziri ND, Barker S, Hyatt J, Ritchie C. The effect of hemodialysis on whole blood, plasma and erythrocyte viscosity. The International journal of artificial organs. 1988;11:340–342. [PubMed] [Google Scholar]
- 25.Meng L, Hou W, Chui J, Han R, Gelb AW. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology. 2015;123:1198–1208. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg A, Keller G. Oxygen consumption during maintenance hemodialysis. Nephron. 1979;23:276–281. doi: 10.1159/000181650. [DOI] [PubMed] [Google Scholar]
- 27.Daugirdas JT. Dialysis hypotension: a hemodynamic analysis. Kidney international. 1991;39:233–246. doi: 10.1038/ki.1991.28. [DOI] [PubMed] [Google Scholar]
- 28.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW. Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;47:830–841. doi: 10.1053/j.ajkd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan, C. et al. Intradialytic Cardiac Magnetic Resonance Imaging to Assess Cardiovascular Responses in a Short-Term Trial of Hemodiafiltration and Hemodialysis. Journal of the American Society of Nephrology (2016). [DOI] [PMC free article] [PubMed]
- 30.Madsen P, Iversen H, Secher NH. Central venous oxygen saturation during hypovolaemic shock in humans. Scandinavian journal of clinical and laboratory investigation. 1993;53:67–72. doi: 10.1080/00365519309092533. [DOI] [PubMed] [Google Scholar]
- 31.Sitbon O, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. Journal of the American College of Cardiology. 2002;40:780–788. doi: 10.1016/S0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 32.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. Journal of the American College of Cardiology. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;48:606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Port FK, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1999;33:507–517. doi: 10.1016/S0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 35.Chesterton LJ, et al. Categorization of the hemodynamic response to hemodialysis: the importance of baroreflex sensitivity. Hemodialysis international. International Symposium on Home Hemodialysis. 2010;14:18–28. doi: 10.1111/j.1542-4758.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- 36.Mancini, E. et al. Intra-dialytic blood oxygen saturation (SO2): association with dialysis hypotension (the SOGLIA Study). Journal of nephrology (2016). [DOI] [PubMed]
- 37.Marx G, Reinhart K. Venous oximetry. Current opinion in critical care. 2006;12:263–268. doi: 10.1097/01.ccx.0000224872.09077.dc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Consolidated data may be shared with other scientists at their request.