Abstract
The changes in reproductive phenology (i.e. timing of flowering and fruiting) observed in recent decades demonstrate that tree reproduction has already been altered by climate change. However, understanding the impact of these changes in reproductive success and fitness remains a major challenge for ecologists. We describe here a previously unreported phenomenon: a significant increase in the reproductive effort (seed production) of temperate oaks with increasing spring temperature, observed over the last decade. In contrast, no relationship was found between seed production and precipitation. This sensitivity of seed production to temperature was confirmed by a “space-for-time” substitution based on elevation gradients. Our findings suggest that global warming may enhance oak reproductive effort in temperate ecosystems. Nevertheless, while fitness can be enhanced by higher levels of seed production, it also depends on the frequency and synchronization of mast seeding production, which may also be influenced by climate change.
Introduction
Forests are important for biodiversity and as a terrestrial carbon sink1, and contrasting responses to climate change have been identified. For instance, growth and survival, two of the main components of tree fitness, have been found to be substantially altered by climate change2, 3. In cold and mild areas, such as boreal and temperate forests, global warming is extending tree growing seasons4, 5 and promoting wood production and tree growth3, whereas, in warmer and drier areas, negative impacts on tree growth6 and survival7, 8 have been observed. In addition to the reported impact on growth, and, to a lesser extent, forest dieback, we need to know how tree reproduction, one of the most important components of plant fitness, is being affected by climate change, and its likely response.
Reproduction is critical for the maintenance and demography of populations, and should therefore be assessed carefully when modeling population responses to climate change9. Seedling regeneration and survival are directly linked to variations in seed production10, 11 and the assessment of changes in regeneration from seeds in response to temperature has become a major challenge. There is, therefore, an urgent need to assess the impact of climate change on tree reproduction, to improve our understanding of the likely effects of this phenomenon on tree population dynamics.
An impact of climate change on the timing of reproduction has been reported for numerous organisms12, 13. Indeed, reproductive phenology is known to be sensitive to environmental cues, such as temperature14, 15, so climate change is likely to alter the intensity of seed production. However, the impact of climate change on reproductive effort is difficult to quantify, particularly in forest trees, which display the synchronized, intermittent production of large amounts of seeds. This phenomenon, commonly observed in oak species at the population scale, is called “masting” or “mast-seeding”16, 17. Most studies of tree seed production over long time series have focused on single sites or small numbers of sites in limited areas. The specific features of masting have, thus, made it difficult to assess the sensitivity of seed production to temperature. Moreover, as pointed out by Crone and Rapp18, the large numbers of isolated studies and of weather variables tested have highlighted contradictory correlations with seed production, even for related species. As a result, to avoid artifacts caused by masting, the monitoring of seed production should be replicated in space and time, in ecologically independent forests.
In this study, we analyzed extensive sets of tree reproduction data for two temperate European white oak species (the sessile oak (Quercus petraea) and the pedunculate oak (Q. robur)), to determine whether seed production had changed over the last two decades in response to global warming. Seed production was monitored in 28 forests of Q. petraea and Q. robur distributed throughout France over a period of 14 years. In parallel, a “space-for-time” substitution was used to quantify the temperature sensitivity of acorn production over elevation gradients. These analyses demonstrated significant temperature-induced trends in seed production over the last two decades, suggesting that climate change enhances oak reproductive effort in temperate ecosystems.
Results and Discussion
Temporal trend in seed production
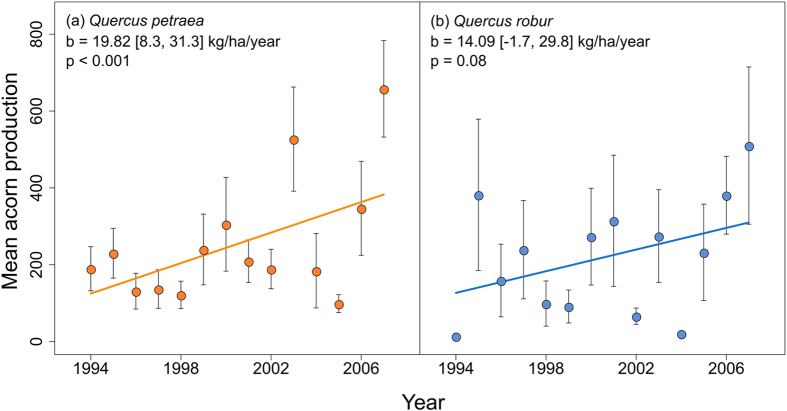
We examined temporal changes in the seed production of two oak species across France over recent decades (1994 to 2007) (Figure S1). We observed an increase over time in reproductive effort for both species (Fig. 1). On average, acorn production (Macorn) in Q. petraea populations increased by 19.8 [8.3, 31.3] kg/ha/year (Table 1) and by 14.1 [−1.7, 29.8] kg/ha/year for Q. robur. However, for Q. robur the regression slope was not significant (Table 1). In addition, the effects of age and diameter have been tested and are reported in Table S1. For both species, growth does not help to explain the variation in seed production (Table S1). However, aging had a significant negative effect on the fructification of Q. petraea (Table S2) while no effect was found for Q. robur. Such a negative effect suggests that older Q. petraea populations produce less acorns than younger ones. This strengthens the positive trend in seed production observed over time. Similar positive temporal trends have been reported in a few other studies. A limited number of reports for Pinus engelmannii 19 and in Nothofagus solandri 20, 21 have demonstrated temporal shifts. In these studies, the monitored populations were located at high elevations, at which reproduction appears to be more sensitive to environmental change20, 21. However, in most cases, no temporal trend in fruit production, for example, was observed22, 23 and such trends have rarely even been sought, due to the scarcity of adequate, long-term datasets. In our study, the many populations surveyed were found in temperate lowland forests located over a large area and at an elevation of between 55 and 330 m above sea level. The mean synchrony of seed production (Spearman correlation coefficient) among the populations was very low for both species (0.11 ± 0.016 for Q. petraea and 0.15 ± 0.052 for Q. robur), demonstrating the lack of synchrony between populations over this large scale (the differences in seed production dynamics between the populations monitored are shown in Figure S2). It is worth noticing that the degree of synchrony changes according to the distance between populations, explaining the low overall synchrony over the large distribution of the populations monitored in our study: the greater the distance between two populations is, the lower the synchrony (Figure S3). As the populations were independent, any common temporal change in reproductive effort can be seen as a robust overall pattern rather than a local trend in a marginal population. Many studies have explored the potential drivers of plant reproduction18, 24, 25, but only a few have investigated changes in reproductive effort in response to global warming, due to a general lack of statistical power26, 27. The large number of asynchronous populations monitored in our study provides a significant advance to assess changes in reproduction though time and according to temperature. In the context of climate change, the temporal trends observed here may reflect the effects of recent warming over the last few decades. Consistent with this view, we observed a significant increase in temperature over time at the sites studied (Figure S4), potentially sufficient to account for the positive temporal trend observed.
Figure 1.
Temporal variation in seed production for Quercus petraea and Quercus robur. Temporal variation in seed production (kg/ha) of 19 and 9 populations of Q. petraea (a) and Q. robur (b) respectively, monitored over 14 years and distributed throughout France. Each dot corresponds to the mean seed production across all populations per year (kilograms per hectare per year averaged over all sites), the standard errors are indicated for each dot. The slope of the regression line and its 95% confidence interval, calculated from a linear mixed-effects model [2], are given for both species, with the coefficient of determination (R 2) between model [2] and mean production.
Table 1.
Temperature sensitivity of reproductive efforts in oaks.
| Species | Temporal | Spatial | ||
|---|---|---|---|---|
| MAcorns/Year | MAcorns/TAp-Ma | MAcorns/Alt100m | MAcorns/TAp-Ma | |
| Q. petraea | 19.82 [8.3, 31.3] | 111.89 [63.1, 146.0] | −83.89 [−149.5, −18.3] | 334.2 [175.6, 589.2] |
| Q. robur | 14.07 [−1.7, 29.8] | 72.66 [19.6, 120.5] | — | — |
Slopes of the linear mixed-effect regression between acorn production in kilograms per hectare (MAcorns) and year (temporal gradient, (MAcorns/Year)), and for every 100 m increase in elevation (spatial gradient, MAcorns/Alt100m), and the mean temperature in April and May in °C (MAcorns/TAp-Ma) in both studies. The 95% confidence intervals are indicated in square brackets. Reproduction in Quercus petraea was monitored in both studies, whereas Quercus robur was monitored in the temporal gradient study only. Significant correlations are indicated in bold.
Reproductive effort in oak is increasing with increasing spring temperatures in temperate areas
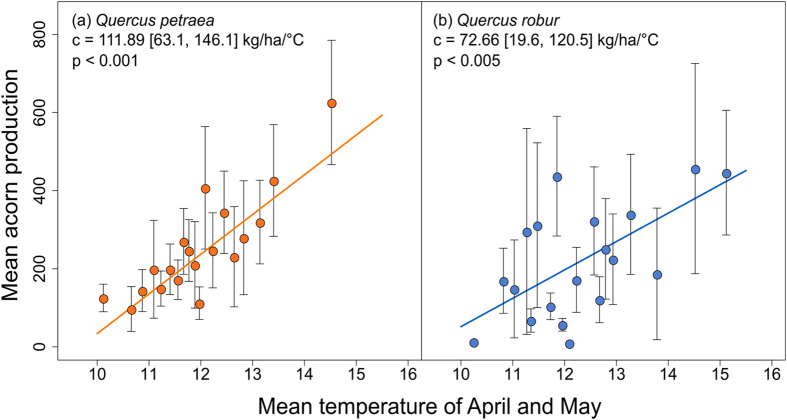
Temperature and rainfall are routinely recorded and are considered the most relevant climatic variables driving seed production24, but their effects seem to differ between tree species and ecosystems23. Tree reproductive effort has been studied mostly in Mediterranean oak species and monitored mostly in Southern Europe and California28. For most of the Southern European species, a warmer, drier summer season results in lower levels of seed production29, 30. Interestingly, the main driver appears to be water deficit rather than temperature per se 31, 32. By contrast, we found that, in both Q. petraea and Q. robur, seed production was positively correlated with spring temperature (Fig. 2a,b and Table 1), which is known to have a strong effect on flowering and pollination14, 15. Moreover, no correlation was found between seed production and annual or seasonal precipitations (Table S3). No study has ever reported positive temporal clines for acorn production, but positive correlations with spring temperature have been found in California for three Mediterranean oak species, Q. lobota, Q. douglasii and Q. kelloggii 33, and three temperate oak species, Q. alba, Q. rubra and Q. velutina 17. In our study, despite the broad distribution of the populations, the positive correlation with spring temperature observed could be explained mostly by temperature variability over time rather than temperature variability over space (Table S4). The trend towards an increase in seed production over time observed for both species was therefore directly correlated with the increase in spring temperature observed over the last decade (Figure S4). Climate change has had a negative impact on reproduction in Mediterranean oaks in Europe32, 34, but we show here that the increase in spring temperature has favored reproduction in temperate oaks. Such a difference could be explained by a water scarcity threshold that is annually reached in Mediterranean ecosystems, while precipitation is currently not limiting for reproduction in European temperate ecosystems.
Figure 2.
Responses of seed production to spring temperature for both Quercus petraea and Quercus robur. Changes in acorn production per population and per year (MAcorns) for Q. petraea (a) and Q. robur (b) according to mean spring temperature. For both species, acorn production data for all populations and all years were binned into 19 temperature classes of approximately the same size, 14 for Q. petraea and 6 or 7 for Q. robur. Mean acorn production per bin is correlated with the temperature class median, the standard errors are indicated for each dot. The slope of the regression line and its 95% confidence interval, calculated from a linear mixed-effects model [4], and the coefficient of determination (R 2) between the model [4] and the binned data are given for each species.
We then examined seed production along elevation gradients in Southern France, to refine the temperature-seed production relationship. Our findings confirm the strong positive correlation between seed production and spring temperature in Q. petraea (Table 1). The gain in acorn production per one-degree rise along the elevation gradient (Macorn/TAp-Ma = 334.2 kg/ha/°C) was three times greater than that along the spatio–temporal gradient (Macorn/TAp-Ma = 111.89 kg/ha/°C). This difference may reflect differences in temperature values and gradients between the two designs. Indeed, the range of spring temperature variation was lower for the spatio-temporal gradient (6.2 °C) than for the elevation gradient (10.8 °C).
What is the impact on tree fitness?
Our observations suggest that climate change may increase the fitness of temperate oaks. An increase in seed production is beneficial to the tree, as it increases seed dispersal35, 36, thereby increasing the number of potential offspring and, consequently, their establishment. In addition, acorn mass increases with increasing temperature, by about 0.15 g per degree [0.09, 0.22] (Fig. 3). This gain may increase the resistance of acorns to environmental stress (predation by insects, frost) and enhance germination37, 38. However, reproduction in many tree species, including oaks, is characterized by masting or mast-seeding, with synchronized large-scale seed production at the population scale (Figure S2). This process is considered to be an adaptive response to the selective pressure exerted by predators16, 17. Masting limits seed predation and promotes seed dispersal, thereby ensuring high rates of offspring survival and optimizing resource allocation to reproduction24, 39. Changes in masting associated with climate change may, therefore, have a negative impact on the fitness of tree populations.
Figure 3.
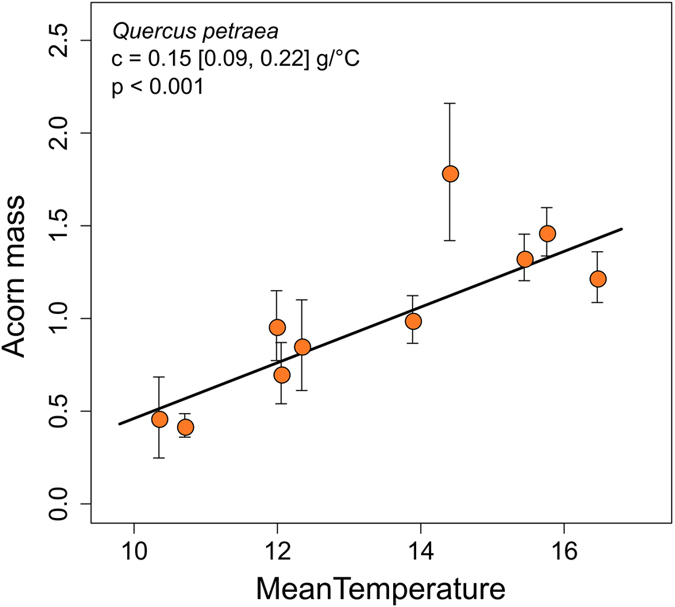
Temperature trends in seed mass for Quercus petraea. Trend in acorn mass (g) with mean temperature from April to November (°C) along the elevation gradient. Each dot corresponds to the mean acorn mass, across years and trees, per population along the elevation gradients. The slope of the regression line and its 95% confidence interval, calculated from a linear mixed-effects model, and the coefficient of determination (R 2) are indicated. April to November corresponds to reproductive cycle length in Q. petraea: from flowering to acorn release.
Nowadays climate change effect on masting frequency is still uncertain and only few studies have investigated change in masting frequency and long-term shifts in allocation to seed production. For instance, Övergaard et al.40 observed, during 30 years of measurement in European beech forest (Fagus sylvatica), an increase in the frequency of mast events directly correlated with an increase in temperature. With increasing periodicity, the temporal variability characterizing mast-seeding and enabling the trees to control predator population size24, 41 might be greatly reduced27. Low inter-annual variability in seed crops may lead to an increase in predator population size, decreasing reduce offspring survival. This paradoxical consequence of climate warming for temperate tree reproduction highlights the need for improvements in our understanding of the proximal mechanisms underlying masting in trees, for prediction of the response of forest ecosystems to climate change.
This study focused on temperate forests dominated by deciduous oak species and cannot be extended to other forest types. However, we can compare the time and temperature trends of acorn production observed in oaks with the patterns reported for whole biomass growth in trees42, 43. There is a clear congruent increase in vegetative growth and reproductive growth (our results) in Q. petraea and Q. robur in recent decades in central Europe. Such trends can be seen as two facets of the overall consequence of the same causes relating to global changes in recent decades. An increase in temperature extends the period of vegetative growth4, 5 and enhances tree growth44. In addition to increasing temperature, increases in the carbon dioxide content of the atmosphere may also promote tree growth in some species, and increases in nitrogen (N) deposition have been shown to stimulate forest growth and carbon sequestration in Europe45. As reproduction in trees is also dependent on resource availability46–49, the combined effects of temperature, carbon dioxide, and nitrogen deposition may also contribute to the increase in seed production. Despite a likely competition for resources between these two processes, a concurrent increase in both is reliable as there seem to be largely independent of each other50. However, the congruent pattern of vegetative and reproductive growth may be negatively affected by extreme events and disturbances, such as firestorms or the spread of insects and diseases, which may also be triggered by global changes51.
Global warming has had a positive effect on temperate oak growth. However, the response of tree reproduction to environmental changes remains unclear, mostly due to our limited understanding of masting processes. Long-term studies of reproductive investment over large areas would be required to assess the global impact of climate change on trees.
Material and Methods
Study sites
We analyzed variations in seed production for two European oak species (Quercus petraea and Quercus robur) along a latitudinal and an elevation gradients. The latitudinal field survey is made of 28 permanent plots that are part of the French intensive forest monitoring network (RENECOFOR) (Figure S1). These plots are widely distributed across France, between latitudes of 43.2° and 50.2°N and longitudes of 0.04° and 3.7°E (Table S5). They correspond to oak tree populations dominated by Q. petraea for 19 of them and by Q. robur for the 9 other ones. All of these populations were already mature when they started to be monitored (mean age of 85.5 years ± 28.7 in 1994). Seed production was assessed for 14 years, from 1994 to 2007. Due to a budget cut, the monitoring program stopped after 2007 and no data of fructification were available after this date. In each forest plot, acorns were collected at the population scale, with ten 0.5 m2-litterfall traps set up under the closed canopy and evenly distributed over an area of about half a hectare. The litter fallen into the traps was collected each season, and sorted by distinguishing the leaves, branches and acorns from oak trees. Acorns were then separated from their cupule and oven-dried. The dry mass of acorns was measured, then divided by the total area of all the traps and expressed in kg/ha. In addition, the mean diameter of trees in each population were assessed every five years from 1991 to 2014. We then estimated it every year using linear regressions. Daily mean, minimal and maximal temperatures (°C) and precipitation (mm) were extracted from the SAFRAN52 spatially explicit database (8 × 8 km grid) for each site.
The elevation gradient survey was set up in the French Pyrenees, along a replicated transect in two parallel valleys: Ossau and Gaves (latitude 42°47′N to 43°45′N; longitude 00°44′W to 00°06′E). Five natural mature populations of Q. petraea were monitored in each valley, at different elevations, from 131 m to 1630 m (Table S5). At each site, nets were set up 1 m above the ground under the whole tree canopy, to collect all the acorns produced by an individual. In total 15, 13, 25 and 30 adult trees were monitored in 2012, 2013, 2014 and 2015, respectively. The 30 trees had a mean height of 19.2 ± 9.4 m and a mean diameter of 37.7 ± 19.6 cm. From 2012 onwards, the organic components (leaves, branches, fruits) fallen from the trees were harvested every two weeks, from the end of September until the beginning of December. For each tree, the projected area of the canopy on the ground (SCOBi) was calculated by first defining the canopy center (O) and then determining the distance from O to the outer limit of the canopy (Bi), at 8 points, 45° apart (OB1–8). The surface area was calculated as:
| 1 |
The harvested litters were sorted in the laboratory, and total acorn production, total dry mass and mean acorn weight per tree and per year (g) were determined. Total seed production was normalized by dividing by the total projected surface area of the tree canopy. Air temperature was measured with a data logger (HOBO Pro RH/Temp, Onset Computer Corporation, Bourne, Massachusetts, USA) at all sites. Data were recorded hourly, from January 1 2012 to December 31 2015.
Statistical analysis
Temporal trend
We evaluated the change in seed production over time separately for the two species, with a linear mixed-effects model:
| 2 |
where a μ and b μ are respectively the overall intercept and the overall regression slope of acorn production regressed over time (Θ) a j and b j are the random population-specific intercept and slope deviations associated with population j,, and ε jΘ are the residuals. For both species, we compared the model [2] with a simpler model not accounting for population random-deviation in slope b j. The fit of the two models was compared with a likelihood-ratio test. No significant variation in slope were detected for both species (Q. petraea: χ2 = 0.18, p = 0.91; Q. robur: χ2 = 0.00, p = 1) indicating that the temporal trends were similar among populations (the variance of b j did not differ from zero). Parameters were thus estimated from the simpler model: .
In addition, we assessed change in seed production over time separately for the two species and compared linear mixed-effects models adding tree age and diameter as fixed effect to the simpler model [2]:
| 3 |
where d μ and g μ are the regression slopes of acorn production regressed respectively over the mean age of the population in 1994 (A) and over the mean diameter of the population estimated for every year (D). To determine which of the two covariates helps to explain better the variation in seed production, we compared models with and without the effects of tree age (A) and diameter (D) using the Akaike Information Criterion corrected for small sample size (AICc) (Table S1). Models with the lowest AICc were selected, however, we consider models with ΔAICc between 0 and 2 to have equivalent support53 and based on the principle of parsimony we selected the simplest one.
For Q. petraea, AICc differences gave support to the model with the fixed effect d μ A, (Table S1), thus, we estimated the effect of age on acorn production (Table S2).
We then evaluated the response of seed production to temperature:
| 4 |
where c μ is the general regression slope of acorn production regressed against temperature (T), a j is the random deviation associated with population j, and c j is the random population-specific deviation in slope, and ε ijT denotes the residuals. As before, we compared the model [4] with a simpler model not accounting for population random-deviation in slope (c j). No significant variation in slope were detected for both species (Likelihood-ratio tests: Q. petraea: χ2 = 2, p = 0.08; Q. robur: χ2 = 2, p = 0.12), indicating that the effect of temperature on acorn production was consistent among populations (Figure S5).
We therefore used the simplified model [4] (i.e. ) to evaluate the effect of temperature on seed production. Model [4] was compared to a model without the temperature fixed effect c μ, using the Akaike Information Criterion corrected for small sample size (AICc). To determine the temperature variable having the strongest influence on seed production, this comparison was performed taking several estimations of annual temperature T: the mean temperature computed for each month and the mean temperature computed for every two month over the year. AICc differences giving support to the more complex model were much higher for the periods of April (AICc = 28.1), March-April (AICc = 17.2), and April-May (AICc = 18.8), for Q. petraea and for April (AICc = 4.9), and April-May (AICc = 5.2) for Q. robur (Figure S6). As the ΔAICc were higher for temperatures recorded during the spring months, we refined the analysis to different spring periods (Table S7). For Q. petraea, a significant regression slope was found with the temperature in April (c μ = 94.8 [61.5, 128.4] kg/ha/°C), March-April (c μ = 103.0 [56.6, 152.4] kg/ha/°C), April-May (c μ = 111.9 [63.1, 146.1] kg/ha/°C) and May-June (c μ = 41.6 [1.1, 86.1] kg/ha/°C). For Q. robur, a significant regression slope was observed only for April (c μ = 54.07 [14.4, 93.7] kg/ha/°C) and April-May (c μ = 72.66 [19.6, 120.5] kg/ha/°C (Tables 1 and S7). The mean temperatures during the periods of April and April-May are determinant for seed production in both species. We represented April-May period in Fig. 2 as it covered a larger period.
To evaluate the effect of precipitation on fructification, we compared model [4] with a similar mixed effect model taking into account the sum of precipitations as a fixed effect in addition to the spring temperature. The inclusion of the five precipitation periods: winter, (January to March), spring (April to June), summer (July to September), autumn (October to December) and the whole year, as predictor were compared with model [4] using AICc.
As spring temperature was found to have increased over the last decades (Figure S4) and seed production was significantly correlated with temperature (Table 1 and Fig. 2), we considered the observed temporal trend in seed production in both species (Table 1 and Fig. 1) to be due principally to the increase in temperature. However, as the populations were distributed over a large area covering a large range of temperatures, we explicitly accounted for variability due to the year and population, with the following multiple regression model:
| 5 |
where b μ denotes the overall slope of the mean population temperature regressed against year (T P) and c μ is the overall slope of mean yearly temperature regressed against population (T Y) for acorn production. a j is the population random intercept and and denotes the residuals. We used model [5], with the mean temperature of April-May (Table S4).
Trend along the elevation gradient
We then evaluated the sensitivity of reproduction to temperature along the elevation gradients in Pyrenees. We used the following mixed-effect model:
| 6 |
where a μ denotes the overall intercept and c μ the overall regression slope of acorn production against temperature (T), p j and n k(j) are the random deviations associated with population j and the individuals k within population j, respectively, and the residuals are denoted ε ijKT. We tested the effect of temperature over the same period as above (Tables 1 and S7).
Finally, using the same model [6], we also evaluated the sensitivity of acorn size to temperature along the same elevation gradient.
All the linear mixed effects models were fitted by the restricted maximum likelihood (REML)54 method in the lme4 R package55.
Electronic supplementary material
Acknowledgements
This research was supported by the European Research Council through the Advanced Grant Project TREEPEACE (#FP7-339728) and the BGF project Potenchene. We are grateful to Jean-Marc Louvet, Benjamin Dencausse and ONF staff for their contribution to the monitoring of seed crops at the forest sites. We thank both the Experimental Unit of Pierroton (UE 0570, INRA, 69 route d’Arcachon, 33612 CESTAS, France) and Toulenne (UE 0393 INRA, Domaine des Jarres 33210 Toulenne, France) for technical support. TC received a PhD grant from TREEPEACE and the Initiative of Excellence program (IdEX) of Bordeaux University.
Author Contributions
T.C. and S.D. conceived the idea for this work. T.C., C.F. and M.N. assembled the dataset and analyzed the data. T.C. and S.D. wrote the manuscript and A.K., S.V., M.N. and C.F. revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09172-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pan Y, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 2.Anderegg WRL, Kane JM, Anderegg LDL. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Change. 2013;3:30–36. doi: 10.1038/nclimate1635. [DOI] [Google Scholar]
- 3.Briceño-Elizondo E, Garcia-Gonzalo J, Peltola H, Matala J, Kellomäki S. Sensitivity of growth of Scots pine, Norway spruce and silver birch to climate change and forest management in boreal conditions. For. Ecol. Manag. 2006;232:152–167. doi: 10.1016/j.foreco.2006.05.062. [DOI] [Google Scholar]
- 4.Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659–659. doi: 10.1038/17709. [DOI] [Google Scholar]
- 5.Vitasse Y, Porté AJ, Kremer A, Michalet R, Delzon S. Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecologia. 2009;161:187–198. doi: 10.1007/s00442-009-1363-4. [DOI] [PubMed] [Google Scholar]
- 6.Jump AS, Hunt JM, Peñuelas J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Change Biol. 2006;12:2163–2174. doi: 10.1111/j.1365-2486.2006.01250.x. [DOI] [Google Scholar]
- 7.Allen CD, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010;259:660–684. doi: 10.1016/j.foreco.2009.09.001. [DOI] [Google Scholar]
- 8.Park Williams A, et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Change. 2012;3:292–297. doi: 10.1038/nclimate1693. [DOI] [Google Scholar]
- 9.Morin X, Viner D, Chuine I. Tree species range shifts at a continental scale: new predictive insights from a process-based model. J. Ecol. 2008;96:784–794. doi: 10.1111/j.1365-2745.2008.01369.x. [DOI] [Google Scholar]
- 10.Moles AT, Westoby M. Seedling survival and seed size: a synthesis of the literature. J. Ecol. 2004;92:372–383. doi: 10.1111/j.0022-0477.2004.00884.x. [DOI] [Google Scholar]
- 11.Turnbull LA, Crawley MJ, Rees M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos. 2000;88:225–238. doi: 10.1034/j.1600-0706.2000.880201.x. [DOI] [Google Scholar]
- 12.Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 13.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006;12:1969–1976. doi: 10.1111/j.1365-2486.2006.01193.x. [DOI] [Google Scholar]
- 14.Sherry RA, et al. Divergence of reproductive phenology under climate warming. Proc. Natl. Acad. Sci. USA. 2007;104:198–202. doi: 10.1073/pnas.0605642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedhly A, Hormaza JI, Herrero M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009;14:30–36. doi: 10.1016/j.tplants.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Kelly D. The evolutionary ecology of mast seeding. Trends Ecol. Evol. 1994;9:465–470. doi: 10.1016/0169-5347(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 17.Sork VL. Evolutionary ecology of mast-seeding in temperate and tropical oaks (Quercus spp.) Vegetatio. 1993;107:133–147. [Google Scholar]
- 18.Crone EE, Rapp JM. Resource depletion, pollen coupling, and the ecology of mast seeding: Mechanisms of mast seeding. Ann. N. Y. Acad. Sci. 2014;1322:21–34. doi: 10.1111/nyas.12465. [DOI] [PubMed] [Google Scholar]
- 19.Buechling A, Martin PH, Canham CD, Shepperd WD, Battaglia MA. Climate drivers of seed production in Picea engelmannii and response to warming temperatures in the southern Rocky Mountains. J. Ecol. 2016;104:1051–1062. doi: 10.1111/1365-2745.12572. [DOI] [Google Scholar]
- 20.Allen RB, Hurst JM, Portier J, Richardson SJ. Elevation-dependent responses of tree mast seeding to climate change over 45 years. Ecol. Evol. 2014;4:3525–3537. doi: 10.1002/ece3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson SJ, et al. Climate and net carbon availability determine temporal patterns of seed production by Nothofagus. Ecology. 2005;86:972–981. doi: 10.1890/04-0863. [DOI] [Google Scholar]
- 22.Abraham ST, Zaya DN, Koenig WD, Ashley MV. Interspecific and intraspecific pollination patterns of valley oak, Quercus lobata, in a mixed stand in coastal Central California. Int. J. Plant Sci. 2011;172:691–699. doi: 10.1086/659646. [DOI] [Google Scholar]
- 23.Koenig WD, Knops JMH. Environmental correlates of acorn production by four species of Minnesota oaks. Popul. Ecol. 2014;56:63–71. doi: 10.1007/s10144-013-0408-z. [DOI] [Google Scholar]
- 24.Kelly, D. & Sork, V. L. Mast seeding in perennial plants: why, how, where? Annu. Rev. Ecol. Syst. 427–447 (2002).
- 25.Pearse IS, Koenig WD, Kelly D. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol. 2016;212:546–562. doi: 10.1111/nph.14114. [DOI] [PubMed] [Google Scholar]
- 26.Kelly D, et al. Of mast and mean: differential-temperature cue makes mast seeding insensitive to climate change. Ecol. Lett. 2013;16:90–98. doi: 10.1111/ele.12020. [DOI] [PubMed] [Google Scholar]
- 27.Mckone MJ, Kelly D, Lee WG. Effect of climate change on mast-seeding species: frequency of mass flowering and escape from specialist insect seed predators. Glob. Change Biol. 1998;4:591–596. doi: 10.1046/j.1365-2486.1998.00172.x. [DOI] [Google Scholar]
- 28.Koenig, W. D. et al. In Mediterranean Oak Woodland Working Landscapes (eds Campos, P. et al.) 181–209 (Springer Netherlands, 2013).
- 29.Cecich RA, Sullivan NH. Influence of weather at time of pollination on acorn production of Quercus alba and Quercus velutina. Can. J. For. Res. 1999;29:1817–1823. doi: 10.1139/x99-165. [DOI] [Google Scholar]
- 30.Fernández-Martínez M, Belmonte J, Maria Espelta J. Masting in oaks: Disentangling the effect of flowering phenology, airborne pollen load and drought. Acta Oecologica. 2012;43:51–59. doi: 10.1016/j.actao.2012.05.006. [DOI] [Google Scholar]
- 31.Pérez-Ramos IM, Ourcival JM, Limousin JM, Rambal S. Mast seeding under increasing drought: results from a long-term data set and from a rainfall exclusion experiment. Ecology. 2010;91:3057–3068. doi: 10.1890/09-2313.1. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Humanes B, Espelta JM. Increased drought reduces acorn production in Quercus ilex coppices: thinning mitigates this effect but only in the short term. Forestry. 2011;84:73–82. doi: 10.1093/forestry/cpq045. [DOI] [Google Scholar]
- 33.Koenig WD, Knops JM, Carmen WJ, Stanback MT, Mumme RL. Acorn production by oaks in central coastal California: influence of weather at three levels. Can. J. For. Res. 1996;26:1677–1683. doi: 10.1139/x26-189. [DOI] [Google Scholar]
- 34.Pérez-Ramos IM, Padilla-Díaz CM, Koenig WD. & Marañón, T. Environmental drivers of mast-seeding in Mediterranean oak species: does leaf habit matter? J. Ecol. 2015;103:691–700. doi: 10.1111/1365-2745.12400. [DOI] [Google Scholar]
- 35.Jansen PA, Bongers F, Hemerik L. Seed mass and mast seeding enhance dispersal by a neotropical scatter-hoarding rodent. Ecol. Monogr. 2004;74:569–589. doi: 10.1890/03-4042. [DOI] [Google Scholar]
- 36.Vander Wall SB. How plants manipulate the scatter-hoarding behaviour of seed-dispersing animals. Philos. Trans. R. Soc. B Biol. Sci. 2010;365:989–997. doi: 10.1098/rstb.2009.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aizen MA, Woodcock H. Effects of acorn size on seedling survival and growth in Quercus rubra following simulated spring freeze. Can. J. Bot. 1996;74:308–314. doi: 10.1139/b96-037. [DOI] [Google Scholar]
- 38.Gómez JM. Bigger is not always better: conflicting selective pressures on seed size in Quercus ilex. Evolution. 2004;58:71–80. doi: 10.1111/j.0014-3820.2004.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 39.Herrera CM, Jordano P, Guitián J, Traveset A. Annual variability in seed production by woody plants and the masting concept: reassessment of principles and relationship to pollination and seed dispersal. Am. Nat. 1998;152:576–594. doi: 10.1086/286191. [DOI] [PubMed] [Google Scholar]
- 40.Ӧvergaard R, Gemmel P, Karlsson M. Effects of weather conditions on mast year frequency in beech (Fagus sylvatica L.) in Sweden. Forestry. 2007;80:555–565. doi: 10.1093/forestry/cpm020. [DOI] [Google Scholar]
- 41.Silvertown JW. The evolutionary ecology of mast seeding in trees. Biol. J. Linn. Soc. 1980;14:235–250. doi: 10.1111/j.1095-8312.1980.tb00107.x. [DOI] [Google Scholar]
- 42.Boisvenue C, Running SW. Impacts of climate change on natural forest productivity – evidence since the middle of the 20th century. Glob. Change Biol. 2006;12:862–882. doi: 10.1111/j.1365-2486.2006.01134.x. [DOI] [Google Scholar]
- 43.McMahon SM, Parker GG, Miller DR, Schlesinger WH. Evidence for a Recent Increase in Forest Growth. Proc. Natl. Acad. Sci. USA. 2010;107:3611–3615. doi: 10.1073/pnas.0912376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxe H, Cannell MG, Johnsen Ø, Ryan MG, Vourlitis G. Tree and forest functioning in response to global warming. New Phytol. 2001;149:369–399. doi: 10.1046/j.1469-8137.2001.00057.x. [DOI] [PubMed] [Google Scholar]
- 45.Magnani F, et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature. 2007;447:849–851. doi: 10.1038/nature05847. [DOI] [PubMed] [Google Scholar]
- 46.Isagi Y, Sugimura K, Sumida A, Ito H. How does masting happen and synchronize? J. Theor. Biol. 1997;187:231–239. doi: 10.1006/jtbi.1997.0442. [DOI] [Google Scholar]
- 47.Ichie T, Kenzo T, Kitahashi Y, Koike T, Nakashizuka T. How does Dryobalanops aromatica supply carbohydrate resources for reproduction in a masting year? Trees. 2005;19:704–711. doi: 10.1007/s00468-005-0434-3. [DOI] [Google Scholar]
- 48.Hoch, G. Carbon Reserves as Indicators for Carbon Limitation in Trees. In Progress in Botany 321–346 (Springer, 2015).
- 49.Sala A, Hopping K, McIntire EJB, Delzon S, Crone EE. Masting in whitebark pine (Pinus albicaulis) depletes stored nutrients. New Phytol. 2012;196:189–199. doi: 10.1111/j.1469-8137.2012.04257.x. [DOI] [PubMed] [Google Scholar]
- 50.Knops JM, Koenig WD, Carmen WJ. Negative correlation does not imply a tradeoff between growth and reproduction in California oaks. Proc. Natl. Acad. Sci. 2007;104:16982–16985. doi: 10.1073/pnas.0704251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seidl R, Schelhaas M-J, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Change. 2014;4:806–810. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidal JP, et al. Multilevel and multiscale drought reanalysis over France with the Safran-Isba-Modcou hydrometeorological suite. Hydrol. Earth Syst. Sci. Discuss. 2010;14:459–478. doi: 10.5194/hess-14-459-2010. [DOI] [Google Scholar]
- 53.Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer Science & Business Media) (2003).
- 54.Zuur, A. F. et al. Mixed effects models and extensions in ecology with R 447–458 (Springer New York) (2009).
- 55.Bates, D., Maechler, M., Bolker, B., Walker, S. & others lme4: Linear mixed-effects models using Eigen and S4. R Package Version1 (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.