Abstract
Purpose of the Review
This review will provide an overview of the microcephalic primordial dwarfism (MPD) class of disorders and provide the reader comprehensive clinical review with suggested care guidelines for patients with microcephalic osteodysplastic primordial dwarfism, type II (MOPDII).
Recent Findings
Over the last 15 years, significant strides have been made in the diagnosis, natural history, and management of MOPDII.
Summary
MOPDII is the most common and well described form of MPD. The classic features of the MPD group are severe pre- and postnatal growth retardation, with marked microcephaly. In addition to these features, individuals with MOPDII have characteristic facies, skeletal dysplasia, abnormal dentition, and an increased risk for cerebrovascular disease and insulin resistance. Biallelic loss-of-function mutations in the pericentrin gene cause MOPDII, which is inherited in an autosomal recessive manner.
Keywords: MOPDII, Primordial dwarfism, Pericentrin
Introduction
Dr. Helmut Seckel was a professor of pediatrics in The University of Chicago School of Medicine when, in 1960, he published his book, Bird-Headed Dwarfs, Studies on Developmental Anthropology [1]. In this book, he described 15 patients (13 historical and 2 personal) with severe intrauterine and proportionate postnatal dwarfism, severe microcephaly, “bird-headed” profile with receding forehead and chin, large and beaked nose, and severe mental retardation [2, 3]. It was clear to Majewski and his colleagues that the Seckel syndrome, as it had come to be known, was a heterogeneous group of disorders [2]. In 1982, in a series of papers, Dr. Majewski detailed the similarities and essential features of Seckel syndrome while noting that distinct forms of microcephalic primordial dwarfism could be delineated [2, 4•, 5]. Still today, there remains variation and lack of clarity in the usage of the term Seckel syndrome. For some, Seckel syndrome denotes a specific condition, and for others, it is a more global term to describe the group of conditions which Seckel initially described [6]. For clarity’s sake, and while recognizing his seminal work in this field, we will attempt to avoid this confusion and the use of the term Seckel syndrome. Instead, we will use the term microcephalic primordial dwarfism (MPD) to describe the group of related disorders characterized by severe pre- and postnatal growth failure together with microcephaly [6].
Based upon clinical and radiographic characteristics, by 1982, Majewski et al. were able to characterize what we now call microcephalic osteodysplastic primordial dwarfism (MOPD) types I, II, and III [2, 4•, 5]. Over time, it was recognized that MOPD I and MOPD III were variants of the same disorder that had previously been described by Drs. Taybi and Linder as cephalo-skeletal dysplasia [7–12]. It is now known that this condition is caused by mutations in RNU4ATAC [13–16]. Today, many other distinct forms of MPD are recognized, some clinically and others through molecular diagnosis. These include Meier-Gorlin syndrome (caused by mutations in the genes: ORC1, ORC4, ORC6, CDC6, CDT1, and CDC45); Ligase IV deficiency; XRCC4 deficiency; and MPD, TRAIP Type [6, 17–27]. There are also several molecular forms of MPD with more significant intellectual impairment as was part of Seckel’s original description. These include mutations in CEP152, ATR, ATRIP, CTiP, PLK4, and CENPJ genes [28–32]. In addition, there are other monogenic syndromes where microcephaly and dwarfism of sufficient severity may occur to become a differential diagnosis for MPD. These include IMAGe syndrome (CDKN1C), Schimke immunosseous dysplasia (SMARCAL1), SOFT syndrome (PCO1), and SHORT syndrome (PIK3R1) [33–38]. As it is the most common and therefore most well described, the remainder of this review will focus on the clinical manifestations of MOPDII (OMIM #210720).
Overview
In addition to the classic features of the MPD group (i.e., severe pre- and postnatal growth retardation, with marked microcephaly), individuals with MOPDII have a characteristic skeletal dysplasia, abnormal dentition, an increased risk for cerebrovascular disease, and insulin resistance [39••, 40–44, 45••]. Distinct craniofacial features include simple or slightly dysplastic pinnae with attached lobes; a broad, elevated nasal root with a wide bridge and a columella lying below the alae nasi (see Fig. 1) [39••]. Additional hallmarks of MOPD II include a high-pitched nasal voice, café-au-lait spots, and elevated platelet counts in some [39••, 46]. MOPDII is caused by mutations in the pericentrin (PCNT) gene and is inherited in an autosomal recessive manner [47•]. MOPDII is one of the most common forms of MPD with more than 150 cases identified worldwide [48]. Diagnosis relies on clinical features and radiographic examinations and can be confirmed by molecular genetic testing of PCNT. Life expectancy is generally decreased, but we are aware of individuals currently in their 30s. [39••, 48]
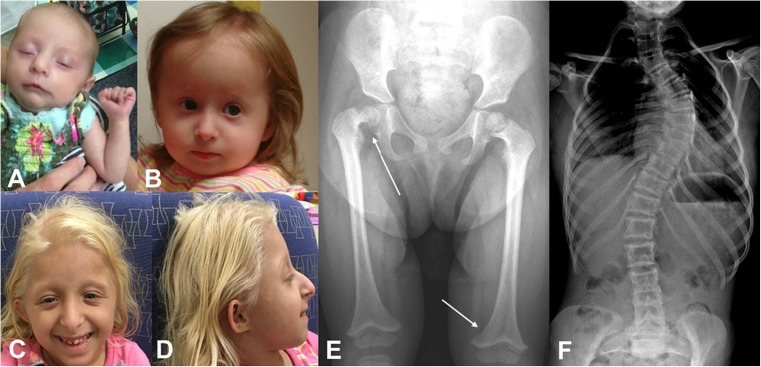
Fig. 1.
Characteristic features of MOPDII. a Patient 1. A 7-week-old female with MOPDII. Mesomelic shortening, mild widening of the nasal bridge, and abroad nasal root are present. b Patient 2. An 18-month-old female with MOPDII. The nasal bridge is widened and the columella lies below the alae nasi. c Patient 3. A 7-year-old female with MOPDII. In the frontal view, the nose is prominent, with a wide bridge and broad root. The nasal tip is full and the columella lies below the alae nasi. The palpebral fissures are down slanting. d Patient 3. In profile, the absence of a sloping forehead, simple pinna with attached lobes, and nasal prominence can be seen. e AP radiograph of a 26-month-old female demonstrating the distal femoral metaphyses inverted V-shape (bottom arrow) and coxa vara on the right (top arrow). f AP radiograph of a 13-year-old female demonstrating scoliosis. Photographs were provided by the authors with written permission
Molecular Basis
In 2008, Rauch et al. and Griffith et al. concurrently recognized that biallelic loss-of-function mutations in the pericentrin gene (PCNT) cause MPD. Rauch et al. suggested MOPDII as the form caused by these mutations while phenotypically similar cases were classified by Griffith et al. as a form of Seckel syndrome [47•, 49•]. In subsequent work by Willems in 2010, all patients with a clinical diagnosis of MOPDII (8 of 8) were identified to have PCNT mutations and 5 of 16 patients with a clinical diagnosis of Seckel syndrome were identified to have PCNT mutations [40]. Retrospective analysis of the five Seckel patients with PCNT mutations suggested that they all belong to the MOPDII spectrum [40]. It is now recognized that MOPDII is genetically homogeneous and caused by loss-of-function mutations in PCNT [40, 50].
The PCNT gene is located on 21q22.3 and encodes PCNT protein, a giant coiled-coil protein (∼370 kDa) that localizes to centrosomes throughout the cell cycle [47•, 51]. The centrosome organizes cytoplasmic organelles and primary cilia in interphase cells and mitotic spindle microtubules to ensure proper chromosome segregation during cell division [47•, 51]. PCNT anchors regulatory and structural molecules to centrosomes, specifically the γ-tubulin ring complex which initiates the assembly of the mitotic spindle apparatus [52]. Absence of PCNT results in disorganized mitotic spindles and missegregation of chromosomes [47•]. Additionally, a role in the ataxia telangiectasia and Rad3-related protein (ATR)-mediated DNA damage-dependent signaling has been reported [49•].
Growth
One of the primary features of MOPDII is extreme growth failure from early fetal life onwards. These differences can be observed by ultrasound at some of the earliest stages of pregnancy. Sonographic recognition of growth deficiency occurs somewhere between 12 and 14 weeks of the pregnancy, and the growth deficiency becomes progressively more severe over the remainder of pregnancy [39••]. Bober et al. have documented growth in the most detail in a study of 26 patients with molecularly confirmed MOPDII [53••]. They noted the average gestational age at birth was 34.8 weeks. Birth lengths in this group, when corrected for gestational age, were on average 7.0 ± 2.4 SD below the population mean or the average length of a 28–29-week neonate [53••]. The birth weights, when corrected for gestational age, were on average 3.9 ± 1.1 SD below the mean or the average weight of a 31–32-week neonate [53••]. The birth head circumference measurements, when corrected for gestational age, were on average 8.5 ± 2.1 SD below the mean or the average size of a 30–31-week neonate [53••]. These parameters differed only slightly from those of Hall et al. who suggested that at term, these children were on average the size of a 28-week premature infant for height, weight, and head circumference [39••, 53••]. While the similarity of the size is remarkable, it is also important to note that Hall’s cohort is now recognized to have included patients with other forms of MPD. At skeletal maturity, individuals with MOPDII had heights which were on average −10.3 ± 2.3 SD below the population mean for the height, weights were on average 14.3 ± 7.7 SD below the population mean and head circumferences were 8.5 ± 2.1 SD below the population mean [53••]. Using the growth parameter SDs at skeletal maturity, it could be determined the age which that parameter was the 50th centile. The skeletally mature male is the height of an average 3-year-10-month-old boy, the weight of an average 5-year-2-month-old boy with the head circumference of an almost 5-month-old male infant. The skeletally mature female is the height of an average 3-year-11-month-old girl, the weight of an average 5-year-3-month-old girl with the head circumference of a 6-month-old female infant [53••]. Overall, the body habitus of an individual with MOPDII changes over time [39••, 45••, 47•, 53••]. In the first few years of life, children are typically lean (if not overfed) with decreased subcutaneous fat. From the age of 5 or 6 years and onwards, particularly through puberty, truncal obesity develops.
Many patients with MOPDII have been treated with growth hormone (hGH) [39••, 53••]. Hall et al. noted that there appeared to be very little response to hGH therapy, although in some cases, an initial increase in growth velocity was seen [39••]. No statistically significant differences were seen when comparing the average SDs of height, weight, or OFC in hGH-treated versus non-treated patients [53••]. Most recently, Faienza et al. discussed a child with MOPDII and acquired IGF-1 deficiency (IGFlD) most likely caused by liver cirrhosis and subsequent decline of hepatic IGF-1 output [54]. On the basis of the severe growth failure and low IGF-1 levels, treatment with recombinant human IGF-1 (rhIGF-1) was initiated. The rhIGF-1 treatment was stopped after 6 months from the start due to the worsening of skeletal dysplasia [54]. The authors concluded that rhIGF-1 treatment did not seem to improve the final stature in patients having both MOPD II and acquired IGFlD; however, it did seem to preserve the typical growth pattern of MOPD II subjects, avoiding a further worsening of the growth deficiency [54].
Craniofacial Features
Distinctive craniofacial features are present most notably in the nose, ears, mouth, and jaw of individuals with MOPD II. These features can be recognized at birth and evolve over time (see Fig. 1) [39••]. A very comprehensive description of all of the features is given by Dr. Hall and her colleagues, and we will summarize these features and add to them with our personal observations [39••]. The nose is prominent, with a wide bridge and broad root. This is not always obvious in the newborn period, but it becomes quite distinctive over the first year. The nasal tip is full and the columella often lies below the alae nasi. The ears may be simple or mildly dysplastic with attached lobules. Positioning is typically normal. The ears are usually not small for the head size, but are small for age. The mouth is proportioned to the size of the face. In infancy, micrognathia is mild to moderate, and this may persist throughout life. The eyes usually appear prominent during childhood and become less so with age. There may be a down slanting to the palpebral fissure.
Dentition
One of the most distinctive features of MOPDII is the teeth, specifically the secondary teeth [39••, 41, 55–57]. The primary teeth are small for age but may appear normal in size with relationship to the mouth. There is often increased space between the primary teeth and enamel is deficient. Hypodontia can be present [39••]. The secondary teeth tend to be more distinctive. The teeth present are both absolutely and relatively small. They are typically dysplastic and have enamel hypoplasia. The roots of the teeth can be absent, short, or single (for molars) [39••, 41, 55–57]. Due to the combination of poorly rooted teeth or poor quality, chewing can be adversely affected. In many individuals, by the second and third decades, teeth are lost naturally or removed [39••]. Dentures or implants are often used to help with chewing.
Skeletal Features
As recognized by Majewski, MOPDII is a form of skeletal dysplasia [4•]. In general, the long bones are thin, with progressive widening of the metaphyses. There is epiphyseal ossification delay, dislocation, or subluxation of the radial heads and hips and small iliac wings with flat acetabular angles [4•, 39••, 58••]. Mesomelia, most significantly in the upper extremity, can be seen in infancy and becomes more apparent over time [4•, 39••]. At birth, skeletal changes are minor and include a high and narrow pelvis with small iliac wings and flat acetabulum [39••]. As the distal femoral epiphyses ossify, they become triangular and the distal femoral metaphyses develop an inverted V-shape (see Fig. 1) [4•, 39••, 59, 60]. The patellae are present and appear to be of proportionate size [39••]. Although Hall has described the knees as becoming more prominent and dislocating laterally because of loose lateral ligaments, this is not something that we have observed in our population [39••]. As ossification is delayed, the bone age always appears to be delayed [4•, 39••]. Radial head subluxation or dislocation can be seen with subsequent decrease range of motion in the elbow [4•, 39••]. As the flaring of the distal radius and ulna metaphyses increases, often a bow in these bones develops and the mesomelic shortening becomes more prominent [39••]. In the hand, cone and ivory phalangeal epiphyses have been described [39••, 60]. Pseudoepiphyses of the metacarpals can be seen with the first and fifth brachymetacarpalia [39••, 60]. Fifth digit clinodactyly is often present, and less commonly, fusion of the phalanges is observed [39••]. Scoliosis has been observed, particularly in girls in late childhood or at puberty, and can progress rapidly (see Fig. 1) [39••]. Fractures do not appear to be more common and fracture healing does not appear to be affected [61].
The most detailed study of MOPDII hips was done by Karatas et al. and published in 2014 [58••]. They reviewed radiographic and clinical records of 12 patients with molecularly confirmed MOPDII. Eight of the 12 patients and 13 of 24 hips (54%) had hip pathology. Four patients had coxa vara (three unilateral and one bilateral; 19% of observed hips). One patient had bilateral hip dysplasia and subluxation. One patient had coxa valga with subluxation of the right hip and degenerative arthritis of the left hip, one had developmental hip dislocation, and one patient had bilateral AVN of the hips [58••]. These observations were very much in keeping with the description by Hall et al. [39••]. It is hypothesized that as the epiphyses begin to manifest, the femoral head is small and hypoplastic and begins to slip down along the femoral shaft leading to a progressive coxa vara [39••, 58••]. Once the initial coxa vara was observed in children between 2 and 5 years of age, the slipping progressed rapidly to become severe within 6 to 10 months. Several patients underwent in situ pinning to prevent severe permanent coxa vara deformity, with good results [58••].
Central Nervous System Vascular Anomalies
Central nervous system (CNS) vascular anomalies are a significant cause of morbidity and mortality in patients with MOPDII. Many case reports and series have been published which have helped to document the natural history and treatment of these vascular anomalies [39••, 42–44, 60, 62–66••]. Moyamoya disease and aneurysms can develop over time and, if untreated, can lead to stroke, disability, and even death [39••, 44, 66••]. The term moyamoya is Japanese for “puff of smoke” and is used to describe the appearance of collateral vascular networks which develop in response to stenosis of the adjacent larger arteries. The lifelong prevalence of CNS vascular disease has been estimated to be between 19 and 52%, with more recent estimates toward the upper end of this range [39••, 44, 66••]. The number of affected males and females appears to be the same [44, 66••]. Moyamoya angiopathy appears more likely to occur in childhood, while aneurysms seem to occur more frequently in older individuals. Some patients have moyamoya only, some have aneurysm(s) only, and some have both in combination [39••, 44, 66••]. In the youngest child observed to have strokes, they began in utero, were ischemic, and related to moyamoya [39••]. One child with MOPDII was noted to progress from virtually undetectable stenosis to complete occlusion and moyamoya disease over a 2-year period [44]. The typical moyamoya pathogenesis in these patients seems to begin with vessel narrowing in the internal carotid artery, A1 or M1 segments. The narrowing may first predominate on one side and progress to bilateral stenosis, with subsequent occlusion of the vessels and collateral formation [44]. Aneurysmal disease most likely occurs as a response of an abnormal vessel to long-term hemodynamic stresses [43, 44, 66••]. The cerebrovascular disease of MOPDII resulted in the death in 23% of patients with MOPDII described to have CNS vascular disease [66••].
Based upon their own observations and literature review, multiple authors have advocated for routine screening for CNS vascular anomalies [39••, 42–44, 65, 66••]. Currently, there is general agreement on several points. The preferred imaging modality is magnetic resonance imaging (MRI) of the brain with magnetic resonance angiography (MRA) of the circle of Willis, cervical arteries, and major cerebral vessels [44, 66••]. Initial MRI/MRA neuroimaging should be performed at diagnosis [44, 66••]. Subsequent MRI/MRA neuroimaging is recommended on a yearly basis throughout the first decade [44, 66••]. In the second decade, Perry et al. propose increasing the screening interval to every other year, while Bober et al. propose continued screening every 12 to 18 months [44, 66••]. There are no recommendations for the cessation of screening at any age [44, 66••]. Patients can have multiple cerebrovascular disorders at different time points, and therefore, identification of initial disease should not exclude them from future screening [66••]. Perry et al. point out that MRI and MRA are sensitive for the detection of clinically significant moyamoya. Although MRA may not be the optimal modality for the detection of all intracranial aneurysms, it is unlikely that aneurysms below the resolution of magnetic resonance angiography would merit treatment [66••]. In addition to routine surveillance, neuroimaging studies should be considered during times when acute neurological symptoms are suggestive of intracranial hemorrhage or ischemia [44, 66••]. Cognitive decline can be a presenting feature of angiopathy. Mental deterioration may be due to multiple infarcts, hemorrhage, or (potentially reversible) chronic ischemia [44].
Following identification of moyamoya disease, several surgical approaches to these patients have been attempted to minimize long-term sequelae. The most commonly used revascularization procedure is encephalodurosynangiosis (EDAS) [43, 44, 60, 64]. Revascularization surgery in patients with MOPDII is technically feasible and strongly indicated to prevent long-term sequelae [43, 44, 64]. Based upon their anatomic location, aneurysms have been treated with endovascular coil occlusion as well and microsurgical clipping [39••, 43, 44]. Non-surgical treatments with various types of anticoagulants have been attempted [65].
Insulin Resistance
In 2011, Huang-Doran et al. demonstrated that insulin resistance is common in MOPDII [45••]. Twenty-one patients with molecularly confirmed MOPDII were studied, and 81% (18/21) were insulin resistant with 48% (10/21) having diabetes (mean age of onset 15 years [range 5–28]) [45••]. The patients without insulin resistance were all younger than 4 years of age. The data suggested that insulin resistance is not congenital but most commonly acquired between 5 and 10 years of age [45••]. In at least three patients, insulin resistance was first noted during growth-hormone therapy [45••]. Given this and the fact that patients with MOPDII are not hGH deficient, the authors suggest that hGH should not be used [45••, 53••]. Since insulin resistance is so common in MOPDII, the routine management of patients with MOPDII should include measurements of fasting glucose, lipid profile, and liver function [45••].
Development
Intellectual development is surprisingly typical in most individuals given their markedly reduced brain size [39••, 53••]. Only individuals who have had strokes have been noted to have moderate or severe intellectual impairment [39••]. Furthermore, acute cognitive decline may be a presenting sign of the development of moyamoya [44]. Overall, sexual maturation and development appears to be typical in its progression [39••]. Adult woman have normal menses [39••]. No documented pregnancies have occurred in adult women, and no adult men are documented to have fathered children [39••]. The onset of puberty in 29% (6/21) of girls over 8 years of age was precocious, and polycystic ovaries were noted in another individual [39••]. As pointed out by Huang-Duran et al., both precocious puberty and polycystic ovaries can be caused by severe insulin resistance and may explain this medical problem [45••].
Cardiac
At birth, the heart has typically normal structure although bicuspid aortic valve, atrial septal defect, and patent ductus arteriosus have been noted infrequently [39••]. There is also documented cardiac pathology which can develop in individuals with MOPDII at older ages. A 17-year-old male patient developed narrowing of the left anterior descending coronary artery (LAD) and a narrowing of the first diagonal branch; angioplasty of the LAD and stenting of the diagonal with two stents was performed [44]. Five years later, these stents failed and he underwent open cardiac bypass surgery. He died of an acute myocardial infarction about 18 months postoperatively after initially doing well (personal communication). A female patient at 23 years of age underwent stenting for mid circumflex and right coronary stenosis, with good success (personal communication). Two patients, one reported by Hall and one known to the authors have died from a myocarditis and myocardial infarct [39••]. The latter male died at 18 years of age.
Renal Disease/Hypertension
There is no clear pattern of renal abnormality seen, but infrequent structural abnormalities as well as nephrolithiasis have been described [39••]. At least two individuals have undergone a renal transplant for chronic renal failure of uncertain etiology (personal communication, Craig Langman). Although most patients with MOPDII have normal blood pressure [39••], there are others with significant hypertension [43, 62, 63]. The etiology of hypertension is not well understood and may be due to renovascular causes or perhaps is centrally mediated [64]. All of the hypertensive MOPDII patients described in the literature have had moyamoya, and in one individual, the bypass procedure which treated the moyamoya led to a reduction in systemic blood pressure [64]. Many of the hypertensive patients also have proteinuria (personal communication, Craig Langman). It is well understood that age, sex, and height are all factors which affect blood pressure norms [67]. As height is so significantly affected in individuals with MOPDII, it is unclear what systolic and diastolic pressures are pathologic in this population. However, moderately elevated blood pressures by age-related norms are likely pathologic in this group and could contribute to the formation, progression, and rupture of aneurysms as well as other end organ damage [64]. Complicating this issue is that controlling hypertension in patients with moyamoya can be a challenge with even a moderate reduction in cerebral perfusion possibly leading to ischemic stroke [64].
Dermatologic
There are many dermatologic changes which have been described in MOPDII. Perhaps the most common are cafe-au-lait spots (CALs) that are ovoid, smooth-edged, and become more apparent with age. CALs have been reported up to 2 cm [39••, 60, 62, 63, 68]. Cutis marmorata [60] and mottling is seen frequently, especially in infancy [39••]. Multiple creases develop on the hands and feet with aging [39••]. In school-aged children, there is increased dark pigmentation around the neck, on the trunk, and in the axilla. Initially, it was not clear if these changes were classic acanthosis nigricans or acanthosis nigricans-like [39••, 45••]. Given the subsequent recognition of insulin resistance in this population, and the fact that these areas do darken with sun exposure or hGH treatment, true acanthosis nigricans is probable [45••]. Later in life, areas of hypopigmentation and dry skin can also develop [39••]. The hair tends to be fine and mildly sparse such that the scalp can frequently be seen through the hair. The eyebrows may be thin [39••].
Hematologic
Asymptomatic thrombocytosis, leukocytosis, and anemia are often seen in MOPDII [43, 46]. We have also seen mild iron-deficient anemia develop in post-menarche in women with MOPDII.
Conclusions
In addition to the classic features of the MPD group (i.e. severe prenatal and postnatal growth retardation, with marked microcephaly), individuals with MOPDII have a characteristic skeletal dysplasia, abnormal dentition, an increased risk for cerebrovascular disease and insulin resistance [39••, 40–44, 45••]. Since cerebrovascular disease appears to only be a feature of MOPD II and not of other forms of MPD, establishing the correct diagnosis of MOPDII in an individual with primordial dwarfism is therefore essential for care [52, 65]. This can be done by confirming the presence of biallelic loss-of-function mutations in PCNT. A summary of recommendations can be seen in Table 1.
Table 1.
Recommended care guidelines
| Medical issues | At diagnosis | Yearly screening | Yearly screening to begin at age 5 |
|---|---|---|---|
| Growth | Plot on MOPDII curves | Monitor growth on MOPDII curves | |
| Dental | Begin dental carea | ||
| Skeletal | Hip and spine radiographs | Hip evaluation and radiographs with spine evaluation and radiographs as indicatedb | |
| CNS Vascular | MRI/MRA brain | MRI/MRA brainc | |
| Insulin Resistance | Studies of lipids, hepatic function, and glucose homeostasis | ||
| Renal/blood pressure | Renal ultrasoundd
BP measurement |
BP measurement | Assessments of renal function |
| Cardiac | Echocardiogramd | ||
| Hematologic | Complete blood count |
aAs soon as teeth are present
bUntil skeletal maturity
cAfter the age of 10 years, there is uncertainty about screening yearly vs every other year
dIf not previously performed and there is any clinical suspicion
One of the primary features of MOPDII is the marked differences in growth. Height, weight, and head circumference for age growth charts exist for MOPDII and should be used to track and monitor growth [53••]. Once a diagnosis is established, initial MRI/MRA neuroimaging should be performed [44, 66••]. Subsequent MRI/MRA neuroimaging should be done on a yearly basis throughout the first decade and between 12 and 24 months in the second decade [44, 66••]. There are no recommendations for the cessation of screening at any age [44, 66••]. As an adjunct, routine blood pressure monitoring should be performed [64]. Values should be interpreted carefully with norms for age, height, and sex considered with awareness that slightly or moderately increased pressures could be pathologic in these individuals. Patients can have multiple cerebrovascular disorders at different time points, and therefore, identification of initial disease should not exclude them from future screening [66••]. Skeletal radiographs should be performed at diagnosis, specifically focusing on the spine and hips with serial repeat examinations to monitor for scoliosis and coxa vara [39••, 58••]. Coxa vara appears to be more prevalent at younger ages. Scoliosis may develop before puberty but seems to be most closely related to the pubertal growth spurt and may progress very rapidly [39••, 58••]. Routine dental care should be initiated early to maximize the function of existing dentition. Based upon the work of Huang-Doran et al. [45••], assessments for signs of insulin resistance, diabetes, and liver disease at 5 years of age is appropriate. We believe this screening should continue on a yearly basis and should also include complete blood counts and renal function testing [48]. Based on the review of Hall et al. [39••], obtaining renal and cardiac ultrasounds at diagnosis should be considered. Life expectancy is generally decreased, but individuals can live at least into their 30s (oldest molecularly confirmed patient is 39 years old (personal communication)). Since many individuals have had severe complications before management and treatment plans were able to be put into place, we hope that with these strategies, overall quality and length of life for these individuals can be improved.
Acknowledgements
The authors wish to thank the Potentials Foundation and the Walking With Giants Foundation for their support and all primordial families for their participation in research throughout the world. Without their contribution, knowledge about this rare condition would not be as robust as it currently is. The authors also wish to thank Angela Duker, CGC, for her tireless work with patients and families affected by MOPDII and her critical reading of this manuscript.
Compliance with Ethical Standards
Conflict of Interest
Michael Bober reports grants and other from Walking With Giants Foundation, grants from Potentials Foundation, outside the submitted work. Andrew Jackson’s work is supported by funding from MRC and ERC (281847).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rare Bone Disease
An erratum to this article is available at https://doi.org/10.1007/s11914-017-0389-5.
Contributor Information
Michael B. Bober, Phone: 302 651 5916, Email: Michael.Bober@nemours.org
Andrew P. Jackson, Phone: +44 (0) 131 651 8500, Email: Andrew.jackson@igmm.ed.ac.uk
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Grossman BJ, Helmut GP. Seckel, 1900-1960. J Pediatr. 1960;57(4):638. doi: 10.1016/S0022-3476(60)80108-4. [DOI] [Google Scholar]
- 2.Majewski F, Goecke T. Studies of microcephalic primordial dwarfism I: approach to a delineation of the Seckel syndrome. Am J Med Genet. 1982;12(1):7–21. doi: 10.1002/ajmg.1320120103. [DOI] [PubMed] [Google Scholar]
- 3.Seckel HPG. Bird-headed dwarfs. Springfield, Ill.,: C. C. Thomas; 1960. 241 p. p.
- 4.Majewski F, Ranke M, Schinzel A. Studies of microcephalic primordial dwarfism II: the osteodysplastic type II of primordial dwarfism. Am J Med Genet. 1982;12(1):23–35. doi: 10.1002/ajmg.1320120104. [DOI] [PubMed] [Google Scholar]
- 5.Majewski F, Stoeckenius M, Kemperdick H. Studies of microcephalic primordial dwarfism III: an intrauterine dwarf with platyspondyly and anomalies of pelvis and clavicles—osteodysplastic primordial dwarfism type III. Am J Med Genet. 1982;12(1):37–42. doi: 10.1002/ajmg.1320120105. [DOI] [PubMed] [Google Scholar]
- 6.Klingseisen A, Jackson AP. Mechanisms and pathways of growth failure in primordial dwarfism. Genes Dev. 2011;25(19):2011–2024. doi: 10.1101/gad.169037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haan EA, Furness ME, Knowles S, Morris LL, Scott G, Svigos JM, et al. Osteodysplastic primordial dwarfism: report of a further case with manifestations similar to those of types I and III. Am J Med Genet. 1989;33(2):224–227. doi: 10.1002/ajmg.1320330216. [DOI] [PubMed] [Google Scholar]
- 8.Winter RM, Wigglesworth J, Harding BN. Osteodysplastic primordial dwarfism: report of a further patient with manifestations similar to those seen in patients with types I and III. Am J Med Genet. 1985;21(3):569–574. doi: 10.1002/ajmg.1320210318. [DOI] [PubMed] [Google Scholar]
- 9.Meinecke P, Passarge E. Microcephalic osteodysplastic primordial dwarfism type I/III in sibs. J Med Genet. 1991;28(11):795–800. doi: 10.1136/jmg.28.11.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meinecke P, Schaefer E, Wiedemann HR. Microcephalic osteodysplastic primordial dwarfism: further evidence for identity of the so-called types I and III. Am J Med Genet. 1991;39(2):232–236. doi: 10.1002/ajmg.1320390228. [DOI] [PubMed] [Google Scholar]
- 11.Taybi H. Cephalo-skeletal dysplasia and microcephalic osteodysplastic primordial dwarfism. Pediatr Radiol. 1992;22(6):476. doi: 10.1007/BF02013522. [DOI] [PubMed] [Google Scholar]
- 12.Taybi H. Microcephalic osteodysplastic primordial dwarfism and cephalo-skeletal dysplasia (Taybi-Linder syndrome) Am J Med Genet. 1992;43(3):628–629. doi: 10.1002/ajmg.1320430326. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Salam GM, Miyake N, Eid MM, Abdel-Hamid MS, Hassan NA, Eid OM, et al. A homozygous mutation in RNU4ATAC as a cause of microcephalic osteodysplastic primordial dwarfism type I (MOPD I) with associated pigmentary disorder. Am J Med Genet A. 2011;155A(11):2885–2896. doi: 10.1002/ajmg.a.34299. [DOI] [PubMed] [Google Scholar]
- 14.Nagy R, Wang H, Albrecht B, Wieczorek D, Gillessen-Kaesbach G, Haan E, et al. Microcephalic osteodysplastic primordial dwarfism type I with biallelic mutations in the RNU4ATAC gene. Clinical genetics 2011. [DOI] [PMC free article] [PubMed]
- 15.Edery P, Marcaillou C, Sahbatou M, Labalme A, Chastang J, Touraine R, et al. Association of TALS developmental disorder with defect in minor splicing component U4atac snRNA. Science. 2011;332(6026):240–243. doi: 10.1126/science.1202205. [DOI] [PubMed] [Google Scholar]
- 16.He H, Liyanarachchi S, Akagi K, Nagy R, Li J, Dietrich RC, et al. Mutations in U4atac snRNA, a component of the minor spliceosome, in the developmental disorder MOPD I. Science. 2011;332(6026):238–240. doi: 10.1126/science.1200587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boles RG, Teebi AS, Schwartz D, Harper JF. Further delineation of the ear, patella, short stature syndrome (Meier-Gorlin syndrome) Clin Dysmorphol. 1994;3(3):207–214. doi: 10.1097/00019605-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Murray JE, Bicknell LS, Yigit G, Duker AL, van Kogelenberg M, Haghayegh S, et al. Extreme growth failure is a common presentation of ligase IV deficiency. Hum Mutat. 2014;35(1):76–85. doi: 10.1002/humu.22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray JE, van der Burg M, IJ H, Carroll P, Wu Q, Ochi T, et al. Mutations in the NHEJ component XRCC4 cause primordial dwarfism. Am J Hum Genet. 2015;96(3):412–424. doi: 10.1016/j.ajhg.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ijspeert H, Warris A, van der Flier M, Reisli I, Keles S, Chishimba S, et al. Clinical spectrum of LIG4 deficiency is broadened with severe dysmaturity, primordial dwarfism, and neurological abnormalities. Hum Mutat. 2013;34(12):1611–1614. doi: 10.1002/humu.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harley ME, Murina O, Leitch A, Higgs MR, Bicknell LS, Yigit G, et al. TRAIP promotes DNA damage response during genome replication and is mutated in primordial dwarfism. Nat Genet. 2015. [DOI] [PMC free article] [PubMed]
- 22.Alkuraya FS. Primordial dwarfism: an update. Current opinion in endocrinology, diabetes, and obesity. 2015;22(1):55–64. doi: 10.1097/MED.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 23.Khetarpal P, Das S, Panigrahi I, Munshi A. Primordial dwarfism: overview of clinical and genetic aspects. Mol Genet Genomics 2015. [DOI] [PubMed]
- 24.Bicknell LS, Bongers EM, Leitch A, Brown S, Schoots J, Harley ME, et al. Mutations in the pre-replication complex cause Meier-Gorlin syndrome. Nat Genet. 2011;43(4):356–359. doi: 10.1038/ng.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bicknell LS, Walker S, Klingseisen A, Stiff T, Leitch A, Kerzendorfer C, et al. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat Genet. 2011;43(4):350–355. doi: 10.1038/ng.776. [DOI] [PubMed] [Google Scholar]
- 26.Fenwick AL, Kliszczak M, Cooper F, Murray J, Sanchez-Pulido L, Twigg SR, et al. Mutations in CDC45, encoding an essential component of the pre-initiation complex, cause Meier-Gorlin syndrome and craniosynostosis. Am J Hum Genet. 2016;99(1):125–138. doi: 10.1016/j.ajhg.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guernsey DL, Matsuoka M, Jiang H, Evans S, Macgillivray C, Nightingale M, et al. Mutations in origin recognition complex gene ORC4 cause Meier-Gorlin syndrome. Nat Genet. 2011;43(4):360–364. doi: 10.1038/ng.777. [DOI] [PubMed] [Google Scholar]
- 28.Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, Bicknell LS, et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet. 2011;43(1):23–26. doi: 10.1038/ng.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogi T, Walker S, Stiff T, Hobson E, Limsirichaikul S, Carpenter G, et al. Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel syndrome. PLoS Genet. 2012;8(11):e1002945. doi: 10.1371/journal.pgen.1002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntyre RE, Lakshminarasimhan Chavali P, Ismail O, Carragher DM, Sanchez-Andrade G, Forment JV, et al. Disruption of mouse Cenpj, a regulator of centriole biogenesis, phenocopies Seckel syndrome. PLoS Genet. 2012;8(11):e1003022. doi: 10.1371/journal.pgen.1003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qvist P, Huertas P, Jimeno S, Nyegaard M, Hassan MJ, Jackson SP, et al. CtIP mutations cause Seckel and Jawad syndromes. PLoS Genet. 2011;7(10):e1002310. doi: 10.1371/journal.pgen.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin CA, Ahmad I, Klingseisen A, Hussain MS, Bicknell LS, Leitch A, et al. Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy. Nat Genet. 2014;46(12):1283–1292. doi: 10.1038/ng.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avila M, Dyment DA, Sagen JV, St-Onge J, Moog U, Chung BH, et al. Clinical reappraisal of SHORT syndrome with PIK3R1 mutations: towards recommendation for molecular testing and management. Clin Genet. 2015. [DOI] [PubMed]
- 34.Bennett J, Schrier Vergano SA, Deardorff MA. IMAGe syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al., editors. GeneReviews(R). Seattle (WA) 2014.
- 35.Deguchi K, Clewing JM, Elizondo LI, Hirano R, Huang C, Choi K, et al. Neurologic phenotype of Schimke immuno-osseous dysplasia and neurodevelopmental expression of SMARCAL1. J Neuropathol Exp Neurol. 2008;67(6):565–577. doi: 10.1097/NEN.0b013e3181772777. [DOI] [PubMed] [Google Scholar]
- 36.Barraza-Garcia J, Ivan Rivera-Pedroza C, Salamanca L, Belinchon A, Lopez-Gonzalez V, Sentchordi-Montane L, et al. Two novel POC1A mutations in the primordial dwarfism, SOFT syndrome: clinical homogeneity but also unreported malformations. Am J Med Genet A 2015. [DOI] [PubMed]
- 37.Min Ko J, Jung S, Seo J, Ho Shin C, Il Cheong H, Choi M, et al. SOFT syndrome caused by compound heterozygous mutations of POC1A and its skeletal manifestation. J Hum Genet 2016. [DOI] [PubMed]
- 38.Shaheen R, Faqeih E, Shamseldin HE, Noche RR, Sunker A, Alshammari MJ, et al. POC1A truncation mutation causes a ciliopathy in humans characterized by primordial dwarfism. Am J Hum Genet. 2012;91(2):330–336. doi: 10.1016/j.ajhg.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall JG, Flora C, Scott CI, Jr, Pauli RM, Tanaka KI. Majewski osteodysplastic primordial dwarfism type II (MOPD II): natural history and clinical findings. Am J Med Genet A. 2004;130A(1):55–72. doi: 10.1002/ajmg.a.30203. [DOI] [PubMed] [Google Scholar]
- 40.Willems M, Genevieve D, Borck G, Baumann C, Baujat G, Bieth E, et al. Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families. J Med Genet. 2010;47(12):797–802. doi: 10.1136/jmg.2009.067298. [DOI] [PubMed] [Google Scholar]
- 41.Kantaputra P, Tanpaiboon P, Porntaveetus T, Ohazama A, Sharpe P, Rauch A, et al. The smallest teeth in the world are caused by mutations in the PCNT gene. Am J Med Genet A. 2011;155A(6):1398–1403. doi: 10.1002/ajmg.a.33984. [DOI] [PubMed] [Google Scholar]
- 42.Brancati F, Castori M, Mingarelli R, Dallapiccola B. Majewski osteodysplastic primordial dwarfism type II (MOPD II) complicated by stroke: clinical report and review of cerebral vascular anomalies. Am J Med Genet A. 2005;139(3):212–215. doi: 10.1002/ajmg.a.31009. [DOI] [PubMed] [Google Scholar]
- 43.Waldron JS, Hetts SW, Armstrong-Wells J, Dowd CF, Fullerton HJ, Gupta N, et al. Multiple intracranial aneurysms and moyamoya disease associated with microcephalic osteodysplastic primordial dwarfism type II: surgical considerations. J Neurosurg Pediatr. 2009;4(5):439–444. doi: 10.3171/2009.6.PEDS08137. [DOI] [PubMed] [Google Scholar]
- 44.Bober MB, Khan N, Kaplan J, Lewis K, Feinstein JA, Scott CI, Jr, et al. Majewski osteodysplastic primordial dwarfism type II (MOPD II): expanding the vascular phenotype. Am J Med Genet A. 2010;152A(4):960–965. doi: 10.1002/ajmg.a.33252. [DOI] [PubMed] [Google Scholar]
- 45.Huang-Doran I, Bicknell LS, Finucane FM, Rocha N, Porter KM, Tung YC, et al. Genetic defects in human pericentrin are associated with severe insulin resistance and diabetes. Diabetes. 2011;60(3):925–935. doi: 10.2337/db10-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unal S, Alanay Y, Cetin M, Boduroglu K, Utine E, Cormier-Daire V, et al. Striking hematological abnormalities in patients with microcephalic osteodysplastic primordial dwarfism type II (MOPD II): a potential role of pericentrin in hematopoiesis. Pediatr Blood Cancer. 2014;61(2):302–305. doi: 10.1002/pbc.24783. [DOI] [PubMed] [Google Scholar]
- 47.Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 2008;319(5864):816–819. doi: 10.1126/science.1151174. [DOI] [PubMed] [Google Scholar]
- 48.Bober MB, Duker AL, Jackson AP, Murray J. Microcephalic osteodysplastic primordial dwarfism Type II. Orphanet. 2014.
- 49.Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40(2):232–236. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piane M, Della Monica M, Piatelli G, Lulli P, Lonardo F, Chessa L, et al. Majewski osteodysplastic primordial dwarfism type II (MOPD II) syndrome previously diagnosed as Seckel syndrome: report of a novel mutation of the PCNT gene. Am J Med Genet A. 2009;149A(11):2452–2456. doi: 10.1002/ajmg.a.33035. [DOI] [PubMed] [Google Scholar]
- 51.Sillibourne JE, Delaval B, Redick S, Sinha M, Doxsey SJ. Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity. Mol Biol Cell. 2007;18(9):3667–3680. doi: 10.1091/mbc.E06-07-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch A. The shortest of the short: pericentrin mutations and beyond. Best Pract Res Clin Endocrinol Metab. 2011;25(1):125–130. doi: 10.1016/j.beem.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 53.•• Bober MB, Niiler T, Duker AL, Murray JE, Ketterer T, Harley ME, et al. Growth in individuals with Majewski osteodysplastic primordial dwarfism type II caused by pericentrin mutations. American journal of medical genetics Part A. 2012. This paper provides detailed information regarding growth of individuals with MOPDII. [DOI] [PubMed]
- 54.Faienza MF, Acquafredda A, D'Aniello M, Soldano L, Marzano F, Ventura A, et al. Effect of recombinant insulin-like growth factor-1 treatment on short-term linear growth in a child with Majewski osteodysplastic primordial dwarfism type II and hepatic insufficiency. J Pediatr Endocr Met 2013. [DOI] [PubMed]
- 55.Kantaputra PN. Apparently new osteodysplastic and primordial short stature with severe microdontia, opalescent teeth, and rootless molars in two siblings. Am J Med Genet. 2002;111(4):420–428. doi: 10.1002/ajmg.10589. [DOI] [PubMed] [Google Scholar]
- 56.Kantaputra PN, Tanpaiboon P, Unachak K, Praphanphoj V. Microcephalic osteodysplastic primordial dwarfism with severe microdontia and skin anomalies: confirmation of a new syndrome. Am J Med Genet A. 2004;130A(2):181–190. doi: 10.1002/ajmg.a.30079. [DOI] [PubMed] [Google Scholar]
- 57.Hall JG. Re: Microcephalic osteodysplastic primordial dwarfism with severe microdontia and skin anomalies [Kantaputra et al. 2004. Am J Med Genet 130A:181–190]. American journal of medical genetics Part A. 2005;135(1):114; author reply 5. [DOI] [PubMed]
- 58.Karatas AF, Bober MB, Rogers K, Duker AL, Ditro CP, Mackenzie WG. Hip pathology in Majewski osteodysplastic primordial dwarfism type II. J Pediatr Orthop. 2014;34(6):585–590. doi: 10.1097/BPO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 59.Fukuzawa R, Sato S, Sullivan MJ, Nishimura G, Hasegawa T, Matsuo N. Autopsy case of microcephalic osteodysplastic primordial “dwarfism” type II. Am J Med Genet. 2002;113(1):93–96. doi: 10.1002/ajmg.10716. [DOI] [PubMed] [Google Scholar]
- 60.Kannu P, Kelly P, Aftimos S. Microcephalic osteodysplastic primordial dwarfism type II: a child with cafe au lait lesions, cutis marmorata, and moyamoya disease. Am J Med Genet A. 2004;128A(1):98–100. doi: 10.1002/ajmg.a.30086. [DOI] [PubMed] [Google Scholar]
- 61.Rigter LS, El Moumni M, Ten Duis HJ, Wendt KW. Fracture healing of an osteodysplastic femur in a microcephalic osteodysplastic primordial dwarfism II (MOPD II) patient: a case report. Eur J Pediatr Surg 2012. [DOI] [PubMed]
- 62.Nishimura G, Hasegawa T, Fujino M, Hori N, Tomita Y. Microcephalic osteodysplastic primordial short stature type II with cafe-au-lait spots and moyamoya disease. Am J Med Genet A. 2003;117A(3):299–301. doi: 10.1002/ajmg.a.10230. [DOI] [PubMed] [Google Scholar]
- 63.Young ID, Barrow M, Hall CM. Microcephalic osteodysplastic primordial short stature type II with cafe-au-lait spots and moyamoya disease: another patient. Am J Med Genet A. 2004;127A(2):218–220. doi: 10.1002/ajmg.a.20647. [DOI] [PubMed] [Google Scholar]
- 64.Moftakhar P, Smith ER, Choulakian A, Scott RM, Danielpour M. Moyamoya disease in children with congenital dwarfing conditions. Pediatr Neurosurg. 2010;46(5):373–380. doi: 10.1159/000322017. [DOI] [PubMed] [Google Scholar]
- 65.Kilic E, Utine E, Unal S, Haliloglu G, Oguz KK, Cetin M, et al. Medical management of moyamoya disease and recurrent stroke in an infant with Majewski osteodysplastic primordial dwarfism type II (MOPD II). Eur J Pediatr 2012. [DOI] [PubMed]
- 66.Perry LD, Robertson F, Ganesan V. Screening for cerebrovascular disease in microcephalic osteodysplastic primordial dwarfism type II (MOPD II): an evidence-based proposal. Pediatr Neurol. 2013;48(4):294–298. doi: 10.1016/j.pediatrneurol.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 67.Rao G. Diagnosis, epidemiology, and management of hypertension in children. Pediatrics. 2016;138(2). [DOI] [PubMed]
- 68.Webber N, O'Toole EA, Paige DG, Rosser E. Cutaneous features associated with microcephalic osteodysplastic primordial dwarfism type II. Pediatr Dermatol. 2008;25(3):401–402. doi: 10.1111/j.1525-1470.2008.00698.x. [DOI] [PubMed] [Google Scholar]