Abstract
How do developmental mechanisms evolve to control changing skeletal morphology, the shapes and sizes of individual bones? We address this question with studies of the opercle (OP), a large facial bone that has undergone marked morphological evolution in the ray-finned fish. Attributes for developmental analysis motivated us to examine how OP shape and size evolve and develop in threespine sticklebacks, a model system for understanding vertebrate evolution. We find that when Alaskan anadromous fish take up permanent residence in lakes, they evolve smaller and reshaped OPs. The change is a reduction in the amount of bone laid down along one body axis, and it arises at or shortly after the onset of OP development. A quantitative trait locus is present on linkage group 19 that contributes in a major way to this phenotype.
Keywords: quantitative trait locus, major effect locus, opercle craniofacial patterning, hyoid arch
Akey feature of evolution in vertebrates is the acquisition of new skeletal morphologies, new bone sizes and/or shapes. Changes might occur in size, the overall dimensions of a bone, or shape alone, such that if one dimension increases, another must decrease. Alternatively, size and shape change might be intercoupled. How the underlying developmental determinants of skeletal morphology change during evolution is unknown, but understanding may be within reach (1, 2). Here we take a simplified approach toward understanding skeletal developmental change during evolution, with focus on a single facial bone, the opercle (OP), which has favorable attributes for developmental genetic study (3). We examine this bone in threespine sticklebacks, fish that have excellent qualities for evolutionary analyses (4–6).
The OP is a prominent, flat bone that is roughly triangular shaped in sticklebacks (7) (Fig. 1A). It supports the opercular cover of the gills, its inward–outward movement provides ventilation, and comparative study has shown that the efficiency of opercular pumping depends on its size and shape (8, 9). These morphological features vary markedly among the Actinopterygia or ray-finned fish, of which teleosts represent the largest, most highly derived, and most diverse clade. In basal actinopterygians, the OP is simply the largest and most dorsal member of a series of dermal rays extending from the hyoid arch (10). OP evolution within teleosts in particular has been spectacular and likely adaptive for highly efficient respiration (11).
Fig. 1.
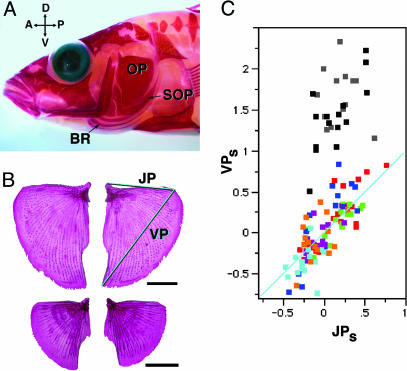
OP morphological variation among Alaskan threespine sticklebacks. (A) A left side view of the head (Alizarin Red staining without clearing). The OP is positioned on the hyoid arch, the most dorsal bone of a DV meristic series (10) that includes the scythe-shaped subopercle (SOP) and three sickle-shaped branchiostegal rays (BR). These bones function in opercular pumping. (B) Dissected, Alizarin Red-stained OPs from anadromous (Anchor River, Upper) and lake (Long Lake, Lower). The examples represent near-extreme high and low cases of variation along the VP axis (C). The sizes are shown as normalized to SL (scale bars, 2 mm). The lines marked JP and VP connect the landmarks J (joint), P (posterior), and V (ventral) that were readily assigned in every case by noting the locations of maximal curvature of the bone. We use the lengths of these lines in shape and size comparisons. (C) A morphospace showing the distribution of OP morphologies from all of the wild-captured Alaskan fish. VPS and JPS are SL-standardized lengths of VP and JP (B), made by taking the residuals of linear regressions of VP length and JP length on standard length (SL) for the lake data set. We excluded the anadromous fish from the regression equation, because the slope of the regression line for the lake fish alone is near 1.0 (Fig. 6). The upper data points are exclusively from anadromous fish (Anchor River, black; Rabbit Slough, gray). The lower data points are exclusively from lake fish (Boot Lake, dark blue; Mud Lake, light blue; Long Lake, orange; Bear Paw Lake, green; Bolo Lake, purple; Whale Lake, red). The theoretical diagonal line passes through the mean of the lake fish, and indicates variation in OP size without a change in shape.
OP development begins early (12–14), and this feature, along with the bone's superficial location, where it can be easily visualized, are attributes that facilitate analysis. In zebrafish, mild reduction of Endothelin1, a signaling protein important for dorsal–ventral (DV) patterning (15–17), prominently expands DV growth of the OP (3). The phenotypic changes in endothelin1 mutants mimic features of OP evolutionary changes among actinopterygians; therefore, changes in the Endothelin1 pathway might be responsible for opercular macroevolution (3).
However, essentially nothing is known about OP microevolutionary change-variation within a single species. Does adaptive genetic variation that can be examined developmentally exist in natural populations? An ideal organism to investigate this question is the threespine stickleback Gasterosteus aculeatus (4, 18, 19). Oceanic (anadromous) sticklebacks can take up permanent residence in fresh water, and when they do, their skeletons evolve, including major reduction of body armor (20–22) during as few as 12 years (<10 generations) (23). Hybrids between anadromous and freshwater sticklebacks are fertile, so that genetic loci responsible for evolution can be identified and mapped (24). Indeed, recent analyses have uncovered genetic loci that may globally underlie skeletal armor loss in this species (25–27). In fact, a single developmental regulatory gene, Pitx1, may be largely responsible for pelvic armor loss (25, 28). Remarkably, in Alaskan populations, both this pelvic armor locus and an unlinked locus underlying lateral plate armor reduction segregate as Mendelian genes (27).
Here we examine OP evolution and development in sticklebacks. We discovered that OPs are smaller and less DV elongated in derived, resident Alaskan lake populations than in ancestral anadromous populations. There is no prominent change along the anterior–posterior (AP) axis. Development is reconfigured, possibly at the very earliest stages of bone patterning, and may be caused by changes at only a few genetic loci. These findings will allow us to examine how naturally segregating genetic variants directly affect developmental programs that control adult facial skeletal morphology.
Materials and Methods
Collections and Crosses. Wild-captured Alaskan anadromous fish from Rabbit Slough and lake fish from Boot Lake, Bear Paw Lake, and a fraction of the fish from Whale Lake were the same as we used previously for a study of armor loss, and methods were as described (27). Furthermore, we used the same genetic crosses for complementation and mapping as described. Brief ly, complementation crosses were made en masse, and mapping crosses were between single pairs of fish. A collection of Bolo Lake (N 61.526, W 149.0489) stickleback was made at the same time as these others (June 2001). Whale Lake fish from two other collections made in the 1990s were added to our data set (three in all wild-captured sets for this lake). Sets of fish from Mud Lake (N 61.563, W 148.9486) and Long Lake (N 61.578, W 149.7639) were collected in 1998. Fish were reared in a laboratory facility for our developmental studies and the genetic crosses as described (27); see ref. 27 for our methods for genotyping and genetic mapping.
Phenotypic Studies and Data Analysis. Adult wild-captured fish and laboratory-reared juvenile fish >1 month old were anesthetized with 0.0016% Finquel, fixed in neutral 4% formaldehyde, and stained with Alizarin Red S (29). For the population studies described below, and for developmental stages >30 days after fertilization (DPF), the OPs were dissected free from both sides of the face of trypsinized fish. The isolated bones were photographed by using a Nikon E4500 digital camera mounted on a Nikon SMZ1500 stereomicroscope. We observed no consistent left–right OP asymmetries (data not shown) and averaged the measurements for both bones in a single fish (variance discarded). For the genetic crosses, the OPs were photographed in situ on only the right side of the face. For the youngest developmental stages (7–30 DPF), live larvae were vitally stained overnight with Alizarin Red (1% in 1 mM Hepes buffer), anesthetized, and mounted between bridged coverslips. Alizarin fluorescence was imaged with a Zeiss Pascal confocal microscope (10× or 20× objective), and z series scans were made (generally, 2-μm steps in the z axis, ≈50 images). Projections were made from these images with Zeiss software (as in Fig. 6, which is published as supporting information on the PNAS web site) and saved. All OP measurements, including the area, were made from digital images with imagej software (National Institutes of Health). Analyses were made with jmp version 5.1 software (SAS Institute, Cary, NC).
Results
OPs Vary in Morphology Between Ancestral and Derived Populations of Alaskan Sticklebacks. Comparing the morphology of the OPs between anadromous (ancestral) and lake (derived) Alaskan populations of stickleback reveals a prominent change in both size normalized to the standard length (SL) of the fish (see Fig. 1) and shape. The bones from an anadromous fish (Fig. 1B Upper) are larger and more DV elongated than are those from a lake fish (Lower). To quantify the difference, we compared wild-captured fish from two anadromous populations and six lake populations that may have evolved independently from anadromous ancestors (27, 30). We measured and normalized to SL the OP area (A) and the two lengths VP and JP (Fig. 1B), yielding AS, VPS, and JPS. All three measures are significantly higher for the anadromous fish than the lake fish (Table 1). Plotting VPS by JPS yields a morphospace “streak” of points, and the anadromous and lake fish do not overlap along this streak (Fig. 1C; see also Fig. 7, which is published as supporting information on the PNAS web site, for a more visual representation). The difference in VPS accounts for nearly all of the size difference; the difference in JPS between anadromous and lake fish is relatively much smaller (Fig. 1C and Table 1). We conclude that, when anadromous fish evolve into lake fish, their OPs become smaller and less ventrally elongated. The change appears very robust; we also observed the change using other length measurements and multivariate analyses, and, for a subset of the data, landmark-based morphometrics (data not shown).
Table 1. OP dimensions for the wild-captured fish.
| Group | n | Areas | VPs | JPs |
|---|---|---|---|---|
| Anadromous | 28 | 2.22 ± 0.42 | 1.53 ± 0.08 | 0.16 ± 0.04 |
| Lake | 96 | 0.00 ± 0.17 | 0.00 ± 0.04 | 0.00 ± 0.03 |
| ANOVA, F1, 122 | 400 | 392 | 8.68 | |
| P | <0.0001 | <0.0001 | 0.004 |
Size standardizations as in Fig. 1. Data are expressed as means ± SE. See Table 2 for a more complete summary. ANOVA statistics, F ratios, and P values are comparisons between Anadromous and Lake fish.
The OPs in fish from different lakes also differ from one another. We see that the points in Fig. 1C approximately fit a line with a slope of 1.0 (the line shown is a diagonal through the mean of the size-standardized lake distribution (0, 0); see Fig. 6 and Table 2, which is published as supporting information on the PNAS web site). A slope of 1 means that both dimensions of the bones are changing in proportion, and therefore that the difference among lake fish OPs is predominately in size, not in shape. All of these populations show degrees of armor loss as well as reduction of OP size, as compared with the anadromous fish, yet the traits are not completely correlated. For example, Whale Lake fish, with the largest OPs (red points), have extreme armor loss (27, 31). In contrast, Mud Lake fish, with the smallest OPs (light blue points), have spines resembling anadromous fish, although their lateral plates are reduced. OP size also does not seem to be correlated with feeding habits: Mud Lake fish feed near the bottom (a “benthic” population) (32), and Long Lake fish (orange points) feed in open water (“limnetic”). These are different feeding styles, but the OPs in these two populations are similar in size.
OP Growth Rates Differ Between Ancestral and Derived Sticklebacks. What changes in development produce the derived OP morphologies of the lake populations? We followed development of laboratory-reared fish obtained from within-population single-pair crosses from anadromous and lake parents. For the earliest stages, from the onset of calcification in early larva until ≈2 weeks thereafter, we used confocal z scans of living, vitally stained preparations.
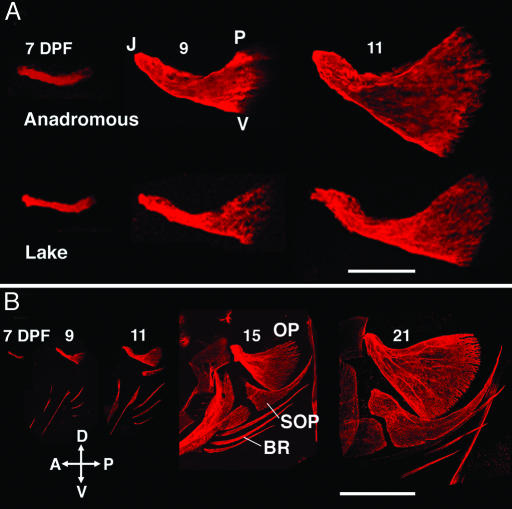
We can first see mineralization of the OP in both morphs at 7 DPF, soon after hatching. At this stage, the OP is only a spicule, a line of calcified matrix beginning where it makes its joint with the hyomandibula and extending posteriorly (Fig. 2A). Hence, in both anadromous and lake fish, initial development is along a single axis, the AP body axis, that forms the eventual JP dimension of the adult bone. As the OP lengthens along the AP axis, its posterior region then begins to grow out secondarily along the DV axis making a triangular shape (and the VP dimension of the bone). DV growth at this stage (9 DPF), and at every stage thereafter, is more prominent in the anadromous fish such that the overall form is more robust in the anadromous fish and more gracile in the lake fish. Both JP and VP continue to lengthen (Fig. 2 A; 11 DPF) and the adult OP morphologies arise gradually (Fig. 2B and data not shown). We note that early development of neighboring facial bones also starts out as simple lines of calcification, which soon transform into recognizable models of the more complex adult shapes (Fig. 2B).
Fig. 2.
An early change in allometric development underlies OP evolution. Confocal microscope z stacks are shown as projections, from Alizarin Red-labeled, living, anesthetized larvae. Left side views, anterior to the left, are shown. (A) Detailed views at 7–11 DPF comparing OP morphologies of representative anadromous and lake larvae. The anadromous bones show relatively more prominent DV expansion. (B) Views including neighboring bones at 7–21 DPF in lake fish (the first three are the same individuals as in A). OP, opercle; SOP, subopercle; BR, branchiostegal ray. At the earliest stage, the OP is the only bone developing in the pharyngeal arches; then, other facial bones appear, including the other members of the OP-branchiostegal meristic series in the hyoid arch shown for the adult in Fig. 1 A. Like the OP, the other dermal bones begin development as lines, which then take on their specific shapes.
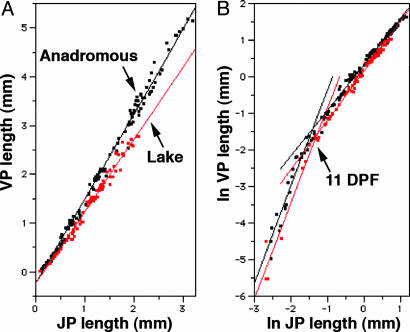
We quantified these observations by measuring VP and JP during development. We observe, strikingly, that during months of growth of both anadromous and lake fish, VP and JP lengths increase in proportion to one another, i.e., the points fit straight lines in a VP by JP plot (Fig. 3A; r2 = 0.99 for both). The slope of the line for the anadromous fish, 1.74, is significantly higher than the slope for the lake fish, 1.48 (see legend to Fig. 3). This difference means that the developing anadromous fish maintain a relatively higher rate of VP elongation. The difference in slopes largely predicts the different shapes of the adult bones (data not shown).
Fig. 3.
Two phases of allometric OP growth differ between anadromous and lake fish. The data are from laboratory-reared fish derived from within-population crosses from Rabbit Slough (anadromous; black points), Bear Paw Lake, and Boot Lake (both lakes combined as red points; we observed no significant development differences between fish from the two lakes). Data were collected from 7 DPF through 260 DPF (anadromous) and through 136 DPF (lake). Statistical comparison (F ratio and P value for anadromous vs. lake) of slopes was performed by analysis of homogeneity of regression (55). (A) Linear–linear VP by JP plots (lengths in mm, not size-standardized). The data from both populations robustly fit straight lines; the slope is significantly higher for the anadromous fish. VPanadromous= –0.23 + 1.72*JPanadromous, r2=0.99. SE = 0.0109. VPlake= –0.24 + 1.48*JPlake, r2= 0.99. SE = 0.0200. Test of slopes: F1, 202= 87.92; P < 0.0001. (B) An early, distinctive, and highly allometric growth phase shows up for both populations in log–log VP by JP plots of the same data. A larger slope means higher allometry (a slope of 1 means isometric growth). We fit two regression lines to each data set: “early period,” <12 DPF, the pair of lines to the left, and “late period,” >11 DPF, pair of lines to the right. Early period: log VPanadromous= 2.31 + 2.66 *log JPanadromous; r2= 0.94; SE = 0.146. Lake: log VPlake= 1.98 + 2.71 *log JPlake; r2= 0.96; SE = 0.217. Test of slopes: F1, 30= 0.052; P = 0.82. Late period: log VPanadromous= 0.32 + 1.25 *log JPanadromous; r2= 0.99; SE = 0.012. Lake: log VPlake= 0.15 + 1.35 *log JPlake; r2= 0.99; S.E. = 0.012. Test of slopes: F1, 197= 21.4; P < 0.0001. The difference in slopes is in the wrong direction to produce the smaller VP length of the adult lake fish by heterochronic acceleration.
By making logarithmic transformations of the same data, another feature of these developmental trajectories becomes clear (Fig. 3B). We can easily fit not one but two straight lines to the data set for each population, suggesting two developmental phases with a transition around 11 DPF for both anadromous and lake fish. The slopes of lines, for both populations, are ≈2.7 for the early phase and diminish to 1.3–1.4 for the late phase. For such plots, larger slopes mean larger positive growth allometry (i.e., VP increases disproportionately higher than JP). The initial high allometry seems to largely reflect the earlier development of JP relative to VP, shown in Fig. 3A.
These data show that the difference in OP morphology is established early, within 7–9 DPF. We find no change in developmental timing between anadromous and lake fish; the change is in amount of bone deposition along the DV axis at any stage.
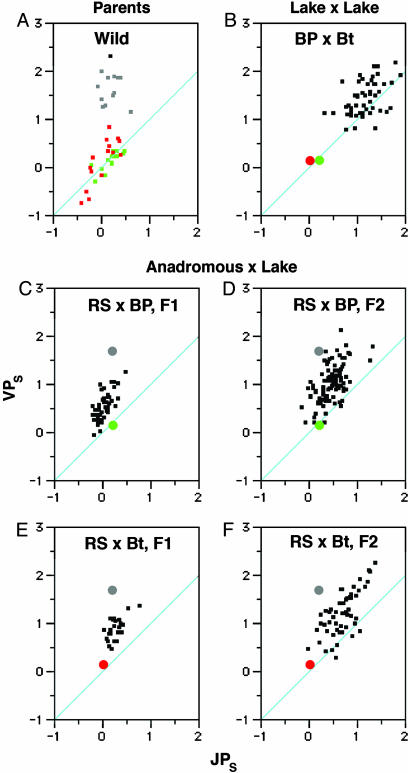
OP Morphology Is Inherited as a Quantitative Trait. Our developmental study shows that laboratory-reared fish develop OPs of approximately the same morphologies as wild-captured fish from the same populations, indicating that genetic variation for these traits is partitioned across populations. To begin to understand this variation, we measured the OPs of young laboratory-reared adults arising from between-population crosses. In a cross between fish from two low-armored lakes, Bear Paw and Boot Lake, the F1 OPs were markedly larger than those of the parental populations (Fig. 4B; the two parental means, shown by the colored circles, are nearly identical). However, in this and another lake-by-lake cross (Table 3, which is published as supporting information on the PNAS web site), we do not observe the ventrally elongated shapes of the ancestral anadromous fish. The upper left region of the morphospace characteristic of the anadromous fish (Fig. 4A, gray points) is empty in Fig. 4B. Rather, in the F1 fish, JPS is increased to nearly the same extent as VPS; the cloud of points are near the diagonal in Fig. 4B. Hence, this distribution suggests genetic complementation, with dominant alleles from at least two loci contributing to OP size but not shape (AAbb × aaBB → AaBb). Alternatively, our results may be explained by a single locus with overdominance. In either case, our data suggest that different alleles affect OP size in the two derived populations exhibiting similar OP morphology.
Fig. 4.
Between-population crosses reveal features of the genetic basis of OP morphology. Morphospace plots are shown as in Figs. 1 and 3. (A) Wild-captured parental populations, a subset of the data also replotted from Fig. 1C (Rabbit Slough, RS, gray; Boot Lake, Bt, red; Bear Paw Lake, BP, green). The means for these distributions of the parental fish OPs are shown by the colored circles in B–F.(B) Laboratory-reared F1 progeny from a lake by lake (BP by Bt) cross. (C–F)F1 (C and E) and F2 (D and F) laboratory-reared progeny from two anadromous by lake crosses, RS by BP (C and D) and RS by Bt (E and F).
Anadromous by lake crosses suggest that genes other than those contributing only to OP size control its ventral elongation. The anadromous by lake F1 hybrids had OPs distributed roughly in between the parents in the morphospace (Fig. 4 C and E). The distributions fall well below that for the anadromous parental population (Rabbit Slough, mean shown by the gray circles). This finding argues forcefully against genes with dominant alleles contributing to shape inheritance, a difference from what we see for size inheritance. For one of the lakes, Bear Paw, the F2 OPs were also approximately intermediate in morphology between the parents (Fig. 4D) and the distribution is broader, as expected because more allelic combinations will be present in F2 progeny than in F1 progeny. As for the F1 OPs, the principal variation is along the VP morphospace axis (Table 3), suggesting a discrete genetic regulation of ventral lengthening (involving change in both size and shape) by a gene set with additive effects.
The F2 hybrid OPs from a second anadromous by lake cross (Rabbit Slough by Boot Lake) deviated from this additive model (Fig. 4F). There is a drift of points along the diagonal of the morphospace. F2 fish from another anadromous by lake cross showed the same deviation, but not so prominently (Table 3). Because neither parental population has the F2 phenotype, the result strongly indicates that new allelic interactions are expanding the size of the bone, either through interactions between alleles within loci (dominance) or interactions of alleles among loci (epistasis).
We calculated the Castle–Wright estimation of the number of effective genetic loci contributing to the morphological changes along the DV axis of the OP when fish evolve from the anadromous to the lake form (33). The method suggests that, for the Rabbit Slough by Bear Paw Lake crosses (which best fit an additive model), about five genes of significant phenotypic effect underlie evolution of the lake OP morphology (4.9 ± 1.6; 95% confidence interval). However, the Castle–Wright estimator is known to be sensitive to deviations from assumptions about the genetic basis of the variation, particularly the effects of epistasis and dominance. Hence, our result should be interpreted as a minimal estimate.
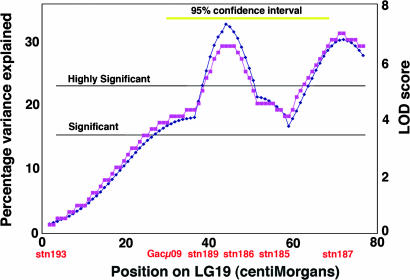
The F2 data can also be used to genetically map the quantitative trait loci (QTLs) responsible for the phenotypes. With available genetic markers (24), we found one or possibly two of these QTLs for the Rabbit Slough by Bear Paw Lake cross: A highly significant major-effect QTL (logarithm of odds score >6) explaining >30% of the variance in VPS is present on LG19 (Fig. 5). Another weakly significant QTL explaining variance in JPS may be present on locus group (LG) 3 (data not shown). These map positions suggest that the genetic basis of OP morphological evolution is different from the genetic basis for the evolution of armor loss; in this same cross, lateral plate and pelvic armor genes map to different linkage groups, LG7 and LG18 (27). Other QTLs must contribute to OP variation between anadromous and lake sticklebacks, as indicated by the Castle–Wright estimate, but our F2 family sizes were not large enough to localize these additional QTLs (34).
Fig. 5.
An OP morphology major-effect locus maps to LG19. LOD score (blue) and percentage of the variance explained (pink) for the size-standardized VP component of OP variation plotted along the entire length of the linkage group are shown. Significant and highly significant marks were determined numerically by using permutation tests of the data across all linkage groups, as was the 95% confidence interval (yellow line) of the main peak by using bootstrap resampling. Data are from the RSxBP F2 progeny shown in Fig. 4D. The presence of two peaks may mean that two QTLs contributing to OP ventral elongation are present on LG19, but also could be due to incorrect assignment of the stn185–187 marker positions. Further analyses will be required.
Discussion
OP evolution from ancestral, anadromous to derived, lake fish occurs as a prominent decrease in the amount of elongation of a single apical region of a triangular bone. Rates of bone growth along a single axis, the DV embryonic axis, appear to be changed early, as we can detect bone mineralization beginning in the young larva. The genetic basis of this developmental change comprises as few as five effective loci, one at the QTL we assigned to LG19. Similar to our previous results on armor loss, a relatively simple genetic basis underlies the evolution of OP morphology. The difference lies in the observation that OP variation seems to be quantitatively distributed in the classic sense.
Evolution of Facial Bone Morphology. Evolution of a smaller facial bone is associated with the prominent skeletal armor reduction that also very consistently characterizes derived freshwater stickleback populations (31, 35). Because the known loci for armor variation and OP variation are unlinked, it seems likely that their coevolution is a functionally correlated response due to natural selection. For example, bone is metabolically much more expensive in fresh water than in the mineral-rich marine environment, providing an explanation for armor loss as well as facial bone loss (31, 36). Alternatively, coevolution of facial bone and armor bone loss might be due to some genetic covariance among bony traits (37–40). The independent segregation of different armor and head bone traits in our crosses indicates that strong genetic covariance is not completely explanatory, but both adaptive and covariance hypotheses need to be tested. Whether reshaping of the OP (as distinguished from resizing) is adaptive also requires further study. Reduction of the ventral apical region might simply be an easy way to make a smaller bone, an example of a developmental bias (41).
We also detected variation in OP size (but not shape) in different lake populations, possibly because of adaptation to different lacustrine environments that we do not presently understand. Genetic drift is also a plausible hypothesis for these differences, because founding of the lake populations may involve bottlenecking that reduces genetic variation. We note that our study has only examined prominent features of OP variation, changes that we could describe simply by length differences along two axes. In fact, the bone's shape is intricate, as is the way it fits with its neighbors along the hyoid arch (Fig. 1). More detailed study of the shapes of these bones could well reveal whether a finer-grained level of morphological variation is present among the stickleback populations that we have examined.
A Change in Bone Deposition Along One Axis Underlies Phyletic Allometry. We examined the developmental basis for the difference between the OPs of anadromous and lake fish, ancestors and descendants, and found a simple biometric change: the rate of OP bone growth along the DV body axis shows evolutionary descent with modification. In both types of fish, early development is prominently allometric. The bone grows first from its joint region along the AP axis before it forms a triangular shape by a second phase of DV-oriented growth. Heterochrony, an almost universally discussed hypothesis to explain phyletic allometry (1, 2, 42–45), does not seem particularly applicable to our findings, in the sense that we can make no simple transposition from one developmental stage of the ancestor to another stage of the descendant. Rather, our findings require that regulatory differences arise at some early stage, possibly the same stage in ancestor and descendant, which would appear to set up the different developmental trajectories played out during the extended periods of larval and juvenile growth. At present, the molecular-genetic mechanisms underlying this kind of change are completely unknown.
How Do Genes Control OP Morphology? A key finding of our study is that we can identify a quantitative genetic component explaining variance associated with DV elongation of the OP. Our crosses also yield evidence for a second genetic system that includes dominant and recessive alleles and regulates OP size without effect on its shape. More studies of the lake fish are required to understand more about this “sizing” system and how it might interact with the DV elongation system.
The DV elongation loci underlie the early developmental change we studied in laboratory-reared fish. Genes functioning along the Endothelin1 signaling pathway are excellent candidates for these loci because they specify early pharyngeal skeletal patterning along the DV axis in zebrafish, including early DV elongation and size of the OP (3, 15); this is the phenotypic change we observe in evolving sticklebacks. It is of major interest to learn whether genes and pathways identified in developmental genetic studies are major players in how morphologies evolve in natural populations. OP evolution in sticklebacks lets us address this issue. The DV elongation loci appear to effect morphology quantitatively, because variance is largely additive in our Bear Paw Lake by anadromous crosses and we locate a QTL on LG19 that explains ≈30% of the variance along this DV axis. There may be only few evolving loci; using the Castle–Wright equation to determine effective gene number suggests that as few as five loci explain the variance in DV elongation. There are caveats associated with this estimation (see Results), but if further work, including mapping with larger numbers of F2 progeny, confirms these findings, our results might mean only a few loci evolved in Bear Paw Lake to regulate DV elongation of the OP. This kind of genetic architecture would seem to match other recent findings. Losses of lateral plates and reduction of the pelvic girdle are controlled by Mendelian genes in the same populations (the same crosses, actually) that we studied (27), and apparently by the same loci worldwide where they also have major effects on the same phenotypes (25, 26, 40, 46). Similarly, Albertson et al. (47) estimate that only few effective loci contribute to differences in particular facial bones between two derived species of African rift lake cichlids. Although standard evolutionary theory predicts that mostly variants of small effect will be used during adaptation (48–50), recent theoretical work shows that, during rapid adaptation to radically new environments, natural selection may commonly act to fix alleles with large phenotypic and fitness effects (51–54). Our findings add experimental support for this theory.
Supplementary Material
Acknowledgments
We are grateful to David Kingsley for sharing unpublished data. John Baker (Clark University, Worcester, MA) supplied a set of Whale Lake fish included in our population studies and gave advice about the analyses. Dolph Schluter provided comments on an earlier version of the manuscript. Alaska Department of Fish and Game assisted with the collections. imagej software was provided by the National Institutes of Health (Bethesda). The research was supported by National Institutes of Health Grants DE13834 and HD22486 (to C.B.K.) and National Science Foundation Grants IBN 023639 (to J.H.P.), EAR9870337, DEB0211391, and DEB0322818 (to M.A.B.). W.A.C. was supported by National Research Service Award Fellowship 5F32GM020892.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OP, opercle; DV, dorsal–ventral; AP, anterior–posterior; DPF, days after fertilization; SL, standard length; QTL, quantitative trait locus.
References
- 1.Wilkins, A. S. (2002) The Evolution of Developmental Pathways (Sinauer, Sunderland, MA).
- 2.Hall, B. K. (1999) Evolutionary Developmental Biology (Kluwer Academic, Dordrecht, The Netherlands).
- 3.Kimmel, C. B., Ullmann, B., Walker, M., Miller, C. T. & Crump, J. G. (2003) Development (Cambridge, U.K.) 130, 1339–1351. [DOI] [PubMed] [Google Scholar]
- 4.Bell, M. A. & Foster, S. A., eds. (1994) The Evolutionary Biology of the Threespine Stickleback (Oxford Univ. Press, Oxford).
- 5.McKinnon, J. S., Mori, S., Blackman, B. K., David, L., Kingsley, D. M., Jamieson, L., Chou, J. & Schluter, D. (2004) Nature 429, 294–298. [DOI] [PubMed] [Google Scholar]
- 6.McKinnon, J. S. & Rundle, H. D. (2002) Trends Ecol. Evol. 17, 480–488. [Google Scholar]
- 7.Browne, P. S. (1994) in The Evolutionary Biology of the Threespine Stickleback., eds. Bell, M. A. & Foster, S. A. (Oxford Univ. Press., Oxford), pp. 207–239.
- 8.Hughes, G. M. (1960) J. Exp. Biol. 37, 11–27. [Google Scholar]
- 9.Liem, K. F. (1993) in The Skull, eds. Hanken, J. & Hall, B. K. (Univ. of Chicago Press, Chicago).
- 10.Hubbs, C. L. (1920) J. Morphol. 33, 61–71. [Google Scholar]
- 11.McAllister, D. E. (1968) Bull. Natl. Mus. Can. 221, 1–239. [Google Scholar]
- 12.Day, L. R. (1963) J. Fish. Res. Bd. Can. 20, 347–372. [Google Scholar]
- 13.Verraes, W. (1977) J. Morphol. 151, 111–120. [DOI] [PubMed] [Google Scholar]
- 14.Cubbage, C. C. & Mabee, P. M. (1996) J. Morphol. 229, 121–160. [DOI] [PubMed] [Google Scholar]
- 15.Miller, C. T., Yelon, D., Stainier, D. Y. & Kimmel, C. B. (2003) Development (Cambridge, U.K.) 130, 1353–1365. [DOI] [PubMed] [Google Scholar]
- 16.Ruest, L.-B., Xiang, X., Lim, K.-C., Levi, G. & Clouthier, D. E. (2004) Development (Cambridge, U.K.) 131, 4413–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozeki, H., Kurihara, Y., Tonami, K., Watatani, S. & Kurihara, H. (2004) Mech. Dev. 121, 387–395. [DOI] [PubMed] [Google Scholar]
- 18.Schluter, D. (2000) The Ecology of Adaptive Radiations (Oxford Univ. Press, Oxford).
- 19.Wootton, R. J. (1976) The Biology of the Sticklebacks (Academic, London).
- 20.Banbura, J. & Bakker, T. C. M. (1995) Behaviour 132, 15–16. [Google Scholar]
- 21.Bell, M. A. (2001) Genetica 112, 445–461. [PubMed] [Google Scholar]
- 22.Reimchen, T. E. (2000) Behaviour 137, 1081–1096. [Google Scholar]
- 23.Bell, M. A., Aguirre, W. E. & Buck, N. J. (2004) Evol. Int. J. Org. Evol. 58, 814–824. [DOI] [PubMed] [Google Scholar]
- 24.Peichel, C. L., Nereng, K. S., Ohgi, K. A., Cole, B. L., Colosimo, P. F., Buerkle, C. A., Schluter, D. & Kingsley, D. M. (2001) Nature 414, 901–905. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro, M. D., Marks, M. E., Peichel, C. L., Blackman, B. K., Nereng, K. S., Jonsson, B., Schluter, D. & Kingsley, D. M. (2004) Nature 428, 717–723. [DOI] [PubMed] [Google Scholar]
- 26.Colosimo, P. F., Peichel, C. L., Nereng, K., Blackman, B. K., Shapiro, M. D., Schluter, D. & Kingsley, D. M. (2004) PLoS Biol. 2, E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cresko, W. A., Amores, A., Wilson, C., Murphy, J., Currey, M., Phillips, P., Bell, M. A., Kimmel, C. B. & Postlethwait, J. H. (2004) Proc. Natl. Acad. Sci. USA 101, 6050–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole, N. J., Tanaka, M., Prescott, A. & Tickle, C. (2003) Curr. Biol. 13, R951–R952. [DOI] [PubMed] [Google Scholar]
- 29.Potthoff, T. (1984) in Ontogeny and Systematics of Fish, ed. Moser, H. G. (Am. Soc. Icthyol. Herpetol., Lawrence, KS), Vol. 1, pp. 35–37. [Google Scholar]
- 30.Bell, M. A., Orti, G., Walker, J. A. & Koenings, J. P. (1993) Evolution (Lawrence, Kans.) 47, 906–914. [DOI] [PubMed] [Google Scholar]
- 31.Bell, M. A. & Orti, G. (1994) Copeia 314–325.
- 32.Walker, J. A. (1997) Biol. J. Linn. Soc. 61, 3–50. [Google Scholar]
- 33.Lynch, M. & Walsh, J. B. (1998) Genetics and Analysis of Quantitative Traits (Sinauer, Sunderland, MA.).
- 34.Beavis, W. D. (1994) in Proceedings of the 49th Annual Corn and Sorghum Research Conference (Am. Seed Trade Assoc., Washington, DC), pp. 252–268.
- 35.Bell, M. A. (1984) in Evolutionary Genetics of Fishes, ed. Turner, B. J. (Plenum, New York), pp. 431–527.
- 36.Giles, N. (1983) J. Zool. 199, 535–544. [Google Scholar]
- 37.Lande, R. (1979) Evolution (Lawrence, Kans.) 33, 402–416. [Google Scholar]
- 38.Lande, R. & Arnold, S. J. (1983) Evolution (Lawrence, Kans.) 37, 1210–1216. [DOI] [PubMed] [Google Scholar]
- 39.Schluter, D. (1996) Evolution (Lawrence, Kans.) 50, 1766–1774. [DOI] [PubMed] [Google Scholar]
- 40.Schluter, D., Clifford, E. A., Nemethy, M. & McKinnon, J. S. (2004) Am. Nat. 163, 809–822. [DOI] [PubMed] [Google Scholar]
- 41.Arthur, W. (2004) Evol. Dev. 6, 282–288. [DOI] [PubMed] [Google Scholar]
- 42.Gould, S. J. (1977) Ontogeny and Phylogeny (Harvard Univ. Press, Cambridge, MA).
- 43.Alberch, P., Gould, S. J., Oster, G. F. & Wake, D. B. (1979) Paleobiology 5, 296–317. [Google Scholar]
- 44.Raff, R. A. (1996) The Shape of Life (Univ. Chicago Press, Chicago).
- 45.Klingenberg, C. P. (1998) Biol. Rev. 73, 79–123. [DOI] [PubMed] [Google Scholar]
- 46.Foster, S. A. & Baker, J. A. (2004) Trends Ecol. Evol. 19, 456–459. [DOI] [PubMed] [Google Scholar]
- 47.Albertson, R. C., Streelman, J. T. & Kocher, T. D. (2003) J. Hered. 94, 291–301. [DOI] [PubMed] [Google Scholar]
- 48.Fisher, R. A. (1930) The Genetical Theory of Natural Selection (Clarendon, Oxford).
- 49.Falconer, D. S. & Mackay, T. F. C. (1996) Introduction to Quantitative Genetics (Longman Scientific and Technical, Essex, U.K.).
- 50.Endler, J. A. (1986) Natural Selection in the Wild (Princeton Univ. Press, Princeton).
- 51.Orr, H. A. (1998) Evolution (Lawrence, Kans.) 52, 935–949. [Google Scholar]
- 52.Orr, H. A. (2002) Evolution (Lawrence, Kans.) 56, 1317–1330. [DOI] [PubMed] [Google Scholar]
- 53.Orr, H. A. (2003) Genetics 163, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orr, H. A. & Betancourt, A. J. (2001) Genetics 157, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokal, R. R. & Rohlf, F. J. (1995) Biometry (Freeman, New York).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.